

Chin J Plant Ecol ›› 2018, Vol. 42 ›› Issue (4): 498-507.DOI: 10.17521/cjpe.2017.0320
• Research Articles • Previous Articles Next Articles
Zi-Piao YE1,Shi-Hua DUAN2,Ting AN1,Hua-Jing KANG3,*( )
)
Online:2018-04-20
Published:2018-03-21
Contact:
Hua-Jing KANG
Supported by:Zi-Piao YE, Shi-Hua DUAN, Ting AN, Hua-Jing KANG. Determination of maximum electron transport rate and its impact on allocation of electron flow[J]. Chin J Plant Ecol, 2018, 42(4): 498-507.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2017.0320
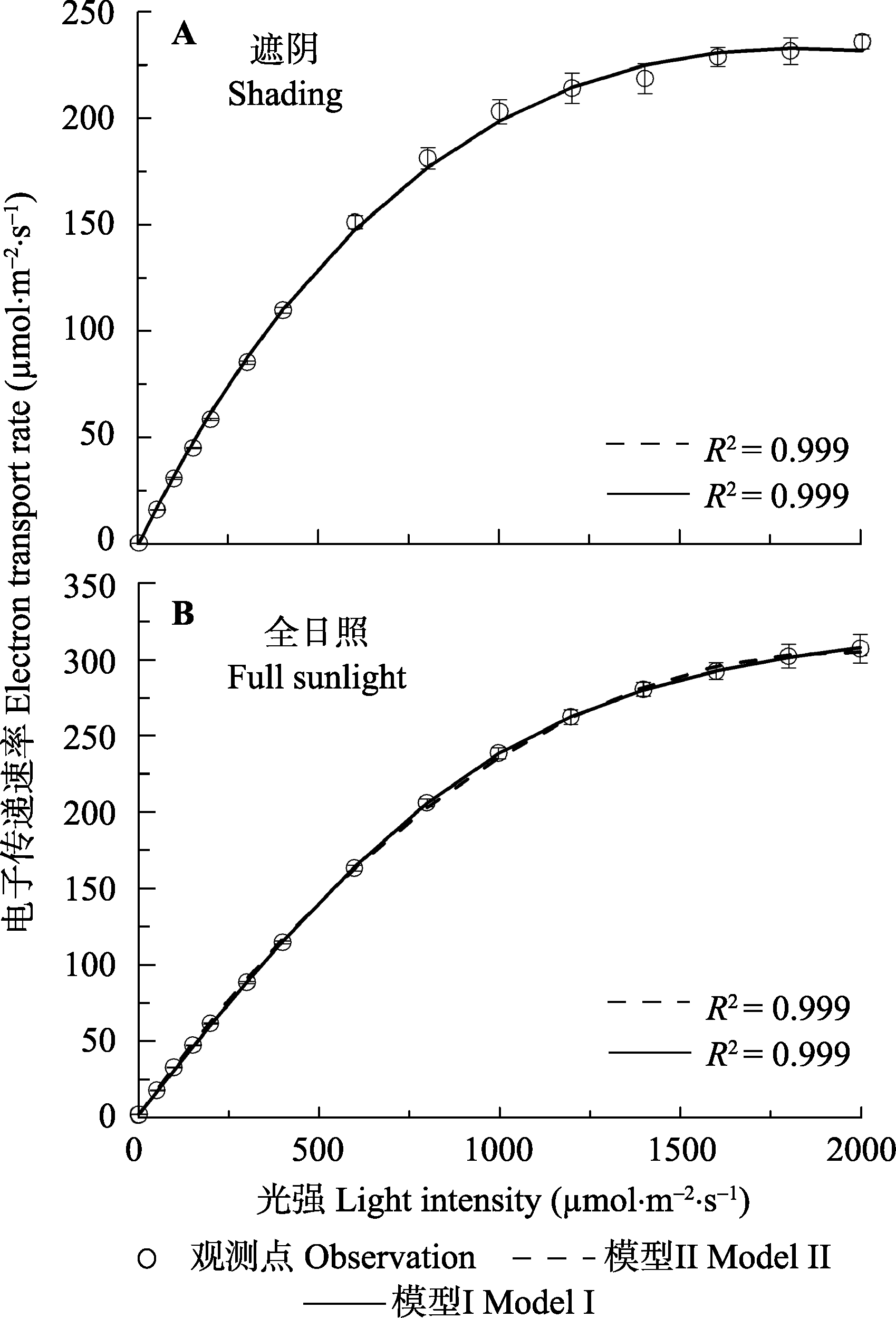
Fig. 1 Light response of electron transport rate for leaves of Glycine max under shading (A) and full sunlight (B) environments (mean ± SE, n = 5). Model I, non-rectangular hyperbola model; Model II, mechanistic model of light-response of electron transport rate.
| 参数 Parameter | 处理 Treatment | |||||
|---|---|---|---|---|---|---|
| 遮阴 Shading | 全日照 Full sunlight | |||||
| 模型I Model I | 模型II Model II | 测量值 Observed value | 模型I Model I | 模型II Model II | 测量值 Observed value | |
| 最大电子传递速率 Maximum electron transport rate (Jmax, mmol·m-2·s-1) | 269.13 ± 5.22a | 236.68 ± 1.39b | ?236.29 | 354.26 ± 17.73a | 307.91 ± 8.95b | ?306.43 |
| 饱和光强 Saturated light intensity (PARsat, mmol·m-2·s-1) | — | 1 839.98 ± 50.53 | ?1 800 | — | 1 967.69 ± 110.64 | ?2 000 |
| 确定系数 Determination coefficient (R2) | 0.999 | 0.999 | — | 0.999 | 0.999 | — |
Table 1 Observed data and results fitted by non-rectangular hyperbola model (model I) and the mechanistic model of light-response of electron transport rate (model II) for light-response curves of electron transport rate (J-I curves) of soybean under two light environments (mean ± SE, n = 5)
| 参数 Parameter | 处理 Treatment | |||||
|---|---|---|---|---|---|---|
| 遮阴 Shading | 全日照 Full sunlight | |||||
| 模型I Model I | 模型II Model II | 测量值 Observed value | 模型I Model I | 模型II Model II | 测量值 Observed value | |
| 最大电子传递速率 Maximum electron transport rate (Jmax, mmol·m-2·s-1) | 269.13 ± 5.22a | 236.68 ± 1.39b | ?236.29 | 354.26 ± 17.73a | 307.91 ± 8.95b | ?306.43 |
| 饱和光强 Saturated light intensity (PARsat, mmol·m-2·s-1) | — | 1 839.98 ± 50.53 | ?1 800 | — | 1 967.69 ± 110.64 | ?2 000 |
| 确定系数 Determination coefficient (R2) | 0.999 | 0.999 | — | 0.999 | 0.999 | — |
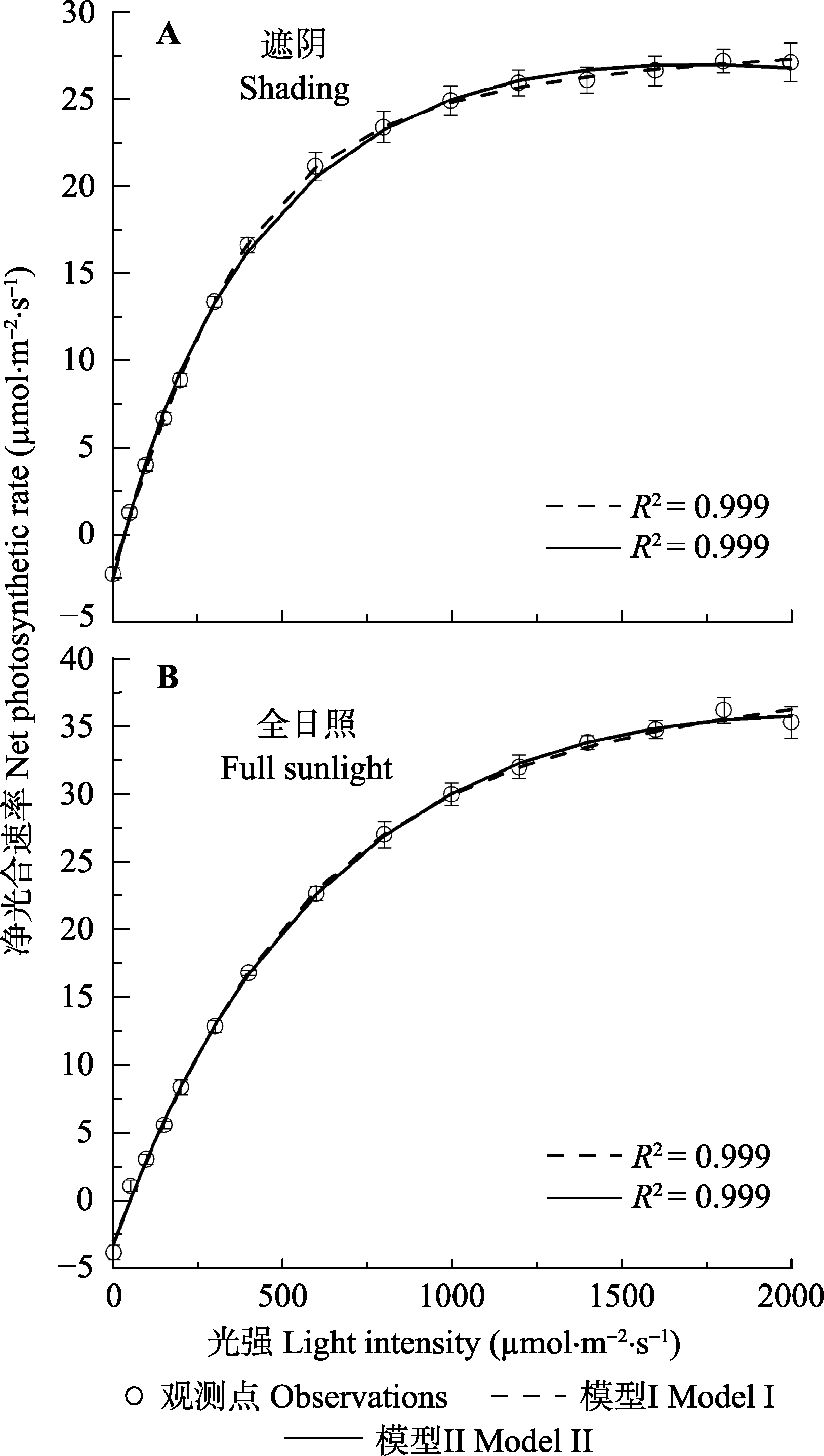
Fig. 2 Light-response curves of photosynthesis for shade (A) and sun (B) leaves of soybean (mean ± SE, n = 5). Model I, non-rectangular hyperbola model; Model II, mechanistic model of light-response of electron transport rate.
| 参数 Parameter | 处理 Treatment | |||||
|---|---|---|---|---|---|---|
| 遮阴 Shading | 全日照 Full sunlight | |||||
| 模型I Model I | 模型II Model II | 测量值 Observed value | 模型I Model I | 模型II Model II | 测量值 Observed value | |
| 初始斜率 Initial slope of A-I curve, α (mmol·mol-1) | 0.061 ± 0.044b | 0.081 ± 0.032a | - | 0.064 ± 0.025a | 0.069 ± 0.025a | - |
| 最大净光合速率 Maximum net photosynthetic rate, Anmax (mmol·m-2·s-1) | 31.28 ± 1.33a | 26.92 ± 1.23b | ?27.23 | 45.56 ± 1.41a | 35.52 ± 1.26b | ?36.17 |
| 饱和光强 Saturated irradiance, Isat (mmol ·m-2·s-1) | - | 1 569.96 ± 24.89 | ?1 600 | - | 1 998.36 ± 36.45 | ?1 800 |
| 光补偿点 Light compensation point, Ic (mmol·m-2·s-1) | 40.65 ± 2.85a | 40.83 ± 2.74a | ?41.59 | 51.49 ± 3.52a | 51.62 ± 3.45a | ?51.96 |
| 暗呼吸速率 Dark respiration, Rd (mmol·m-2 ·s-1) | 2.42 ± 0.87b | 2.99 ± 0.58a | ?3.12 | 3.19 ± 0.56a | 3.45 ± 0.42a | ?3.51 |
| 确定系数 Determination coefficient, R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
Table 2 Observed data and photosynthetic parameters fitted by non-rectangular hyperbola model (model I) and the mechanistic model of light-response of electron transport rate (model II) for light-response curves of photosynthesis (An-I curves) of soybean under two light environments, respectively (mean ± SE, n = 5).
| 参数 Parameter | 处理 Treatment | |||||
|---|---|---|---|---|---|---|
| 遮阴 Shading | 全日照 Full sunlight | |||||
| 模型I Model I | 模型II Model II | 测量值 Observed value | 模型I Model I | 模型II Model II | 测量值 Observed value | |
| 初始斜率 Initial slope of A-I curve, α (mmol·mol-1) | 0.061 ± 0.044b | 0.081 ± 0.032a | - | 0.064 ± 0.025a | 0.069 ± 0.025a | - |
| 最大净光合速率 Maximum net photosynthetic rate, Anmax (mmol·m-2·s-1) | 31.28 ± 1.33a | 26.92 ± 1.23b | ?27.23 | 45.56 ± 1.41a | 35.52 ± 1.26b | ?36.17 |
| 饱和光强 Saturated irradiance, Isat (mmol ·m-2·s-1) | - | 1 569.96 ± 24.89 | ?1 600 | - | 1 998.36 ± 36.45 | ?1 800 |
| 光补偿点 Light compensation point, Ic (mmol·m-2·s-1) | 40.65 ± 2.85a | 40.83 ± 2.74a | ?41.59 | 51.49 ± 3.52a | 51.62 ± 3.45a | ?51.96 |
| 暗呼吸速率 Dark respiration, Rd (mmol·m-2 ·s-1) | 2.42 ± 0.87b | 2.99 ± 0.58a | ?3.12 | 3.19 ± 0.56a | 3.45 ± 0.42a | ?3.51 |
| 确定系数 Determination coefficient, R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| 参数 Parameter | 处理 Treatment | |||||
|---|---|---|---|---|---|---|
| 遮阴 Shading | 全日照 Full sunlight | |||||
| 模型I Model I | 模型II Model II | 测量或计算值 Observed value | 模型I Model I | 模型II Model II | 测量或计算值 Observed value | |
| 最大电子传递速率 Maximum electron transport rate, Jmax (mmol·m-2·s-1) | 269.13a | 236.68b | 236.29b | 354.26a | 307.91b | 306.43b |
| 最大净光合速率 Maximum net photosynthetic rate, Anmax (mmol·m-2·s-1) | 31.28a | 26.92b | 27.23b | 45.56a | 35.52b | 36.17b |
| 碳同化电子流 Electron flow of partitioning C assimilation, JC-max (mmol·m-2·s-1) | 166.48a | 155.67a | 155.54a | 219.23a | 203.78a | 203.26a |
| 光呼吸电子流 Electron flow of partitioning photorespiration assimilation, JO-max (mmol·m-2·s-1) | 102.65a | 81.01b | 80.75b | 135.03a | 104.13b | 103.17b |
Table 3 Photosynthetic electron flows of partitioning C assimilation and photorespiration pathway
| 参数 Parameter | 处理 Treatment | |||||
|---|---|---|---|---|---|---|
| 遮阴 Shading | 全日照 Full sunlight | |||||
| 模型I Model I | 模型II Model II | 测量或计算值 Observed value | 模型I Model I | 模型II Model II | 测量或计算值 Observed value | |
| 最大电子传递速率 Maximum electron transport rate, Jmax (mmol·m-2·s-1) | 269.13a | 236.68b | 236.29b | 354.26a | 307.91b | 306.43b |
| 最大净光合速率 Maximum net photosynthetic rate, Anmax (mmol·m-2·s-1) | 31.28a | 26.92b | 27.23b | 45.56a | 35.52b | 36.17b |
| 碳同化电子流 Electron flow of partitioning C assimilation, JC-max (mmol·m-2·s-1) | 166.48a | 155.67a | 155.54a | 219.23a | 203.78a | 203.26a |
| 光呼吸电子流 Electron flow of partitioning photorespiration assimilation, JO-max (mmol·m-2·s-1) | 102.65a | 81.01b | 80.75b | 135.03a | 104.13b | 103.17b |
| 1 |
Baker NR ( 2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89-113.
DOI URL PMID |
| 2 |
Bellucco V, Marras S, Grimmond CSB, J?rvi L, Sirca C, Spano D ( 2017). Modelling the biogenic CO2 exchange in urban and non-urban ecosystems through the assessment of light-response curve parameters. Agricultural and Forest Meteorology, 236, 113-122.
DOI URL |
| 3 |
Brading P, Warner ME, Davey P, Smith DJ, Achterberg EP, Suggett DJ ( 2011). Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). Limnology and Oceanography, 56, 927-938.
DOI URL |
| 4 |
Buckley TN, Diaz-Espejo A ( 2015). Reporting estimates of maximum potential electron transport rate. New Phytologist, 205, 14-17.
DOI URL PMID |
| 5 |
Calama R, Puértolas J, Madrigal G, Pardos M ( 2013). Modeling the environmental response of leaf net photosynthesis in Pinus pinea L. natural regeneration. Ecological Modelling, 251, 9-21.
DOI URL |
| 6 |
Cheng LL, Fuchigami LH, Breen PJ ( 2001). The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves. Journal of Experimental Botany, 52, 1865-1872.
DOI URL |
| 7 |
Cruz JA, Avenson TJ, Kanazawa A, Takizawa K, Edwards GE, Kramer DM ( 2005). Plasticity in light reactions of photosynthesis for energy production and photoprotection. Journal of Experimental Botany, 56, 395-406.
DOI URL PMID |
| 8 |
dos Santos JUM, de Carvalho GJF, Fearnside PM ( 2013). Measuring the impact of flooding on Amazonian trees: Photosynthetic response models for ten species flooded by hydroelectric dams. Trees, 27, 193-210.
DOI URL |
| 9 |
Dubois JJB, Fiscus EL, Booker FL, Flowers MD, Reid CD ( 2007). Optimizing the statistical estimation of the parameters of the Farquhar-von Caemmerer-Berry model of photosynthesis. New Phytologist, 176, 402-414.
DOI URL PMID |
| 10 |
Epron D, Godard D, Cornic G, Genty B ( 1995). Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Fagus sylvatica L. and Castanea sativa Mill.). Plant,Cell & Environment, 18, 43-51.
DOI URL |
| 11 |
Farquhar GD, Busch FA ( 2017). Changes in the chloroplastic CO2 concentration explain much of the observed Kok effect: A model. New Phytologist, 214, 570-584.
DOI URL PMID |
| 12 |
Farquhar GD, Caemmerers S, Berry JA ( 1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
DOI URL PMID |
| 13 |
Fila G, Badeck FW, Meyer S, Cerovic Z, Ghashghaie J ( 2006). Relationships between leaf conductance to CO2 diffusion and photosynthesis in micropropagated grapevine plants, before and after ex vitro acclimatization. Journal of Experimental Botany, 57, 2687-2695.
DOI URL PMID |
| 14 |
Gao S, Yan Q, Chen L, Song Y, Li J, Fu C, Dong M ( 2017 a). Effects of ploidy level and haplotype on variation of photosynthetic traits: Novel evidence from two Fragaria species. PLOS ONE, 12, e0179899. DOI: 10.1371/journal.? pone.0179899.
DOI URL PMID |
| 15 |
Gao Y, Xia JB, Chen YP, Zhao YY, Kong QX, Lang Y ( 2017b). Effects of extreme soil water stress on photosynthetic efficiency and water consumption characteristics of Tamarix chinensis in China’s Yellow River Delta. Journal of Forestry Research, 28, 491-501.
DOI URL |
| 16 |
Guo W, Zhan SY, Yin H, Li XY, Lü X, Yang L, Wang Y ( 2016). Effect of enhanced UV-B radiation on photosynthetic electron transport and light response characteristics of japonica. Journal of Nanjing Agricultural University, 39, 603-610.
DOI URL |
|
[ 郭巍, 战莘晔, 殷红, 李雪莹, 吕晓, 杨璐, 王一 ( 2016). UV-B辐射增强对粳稻光合电子传递与光响应特性的影响. 南京农业大学学报, 39, 603-610.]
DOI URL |
|
| 17 |
Harley PC, Sharkey TD ( 1991). An improved model of C3 photosynthesis at high CO2: Reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynthesis Research, 27, 169-178.
DOI URL PMID |
| 18 | Hu WH, Ye ZP, Yan XH, Yang XS ( 2017). PSII function and intrinsic characteristics of light-harvesting pigment molecules for sun- and shading-leaf in Magnolia grandiflora during overwintering. Bulletin of Botanical Research, 37, 281-287. |
| [ 胡文海, 叶子飘, 闫小红, 杨旭升 ( 2017). 越冬期广玉兰阳生叶和阴生叶PSII功能及捕光色素分子内禀特性的比较研究. 植物研究, 37, 281-287.] | |
| 19 |
Je?ilová E, No?ková-Hlavá?ková V, Duchoslav M ( 2015). Photosynthetic characteristics of three ploidy levels of Allium oleraceum L. (Amaryllidaceae) differing in ecological amplitude. Plant Species Biology, 30, 212-224.
DOI URL |
| 20 |
Kang HJ, Li H, Tao YL, Zhang HL, Quan W, Ouyang Z ( 2015). Discussion on simultaneous measurements of leaf gas exchange and chlorophyll fluorescence for estimating photosynthetic electron allocation. Acta Ecologica Sinica, 35, 1217-1224.
DOI URL |
|
[ 康华靖, 李红, 陶月良, 张海利, 权伟, 欧阳竹 ( 2015). 气体交换与荧光同步测量估算植物光合电子流的分配. 生态学报, 35, 1217-1224.]
DOI URL |
|
| 21 |
Kirchhoff H, Haase W, Wegner S, Danielsson R, Ackermann R, Albertsson P ( 2007). Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry, 46, 11169-11176.
DOI URL |
| 22 | Leng HB, Qin J, Ye K, Feng SC, Gao K ( 2014). Comparison of light response models of photosynthesis in Nelumbo nucifera leaves under different light conditions. Chinese Journal of Applied Ecology, 25, 2855-2860. |
| [ 冷寒冰, 秦俊, 叶康, 奉树成, 高凯 ( 2014). 不同光照环境下荷花叶片光合光响应模型比较. 应用生态学报, 25, 2855-2860.] | |
| 23 |
Li XN, Brestic M, Tan DX, Zivcak M, Zhu XC, Liu SQ, Song FB, Reiter RJ, Liu FL ( 2018). Melatonin alleviates low PSI-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. Journal of Pineal Research, 64, DOI: 10.1111/jpi.12453.
DOI URL PMID |
| 24 |
Li XN, Hao CL, Zhong JW, Liu FL, Cai J, Wang X, Zhou Q, Dai TB, Cao WX, Jiang D ( 2015). Mechano-stimulated modifications in the chloroplast antioxidant system and proteome changes are associated with cold response in wheat. BMC Plant Biology, 15, 1-13.
DOI URL PMID |
| 25 |
Liang XY, Liu SR ( 2017). A review on the FvCB biochemical model of photosynthesis and the measurement of A-Ci curves. Chinese Journal of Plant Ecology, 41, 693-706.
DOI URL |
|
[ 梁星云, 刘世荣 ( 2017). FvCB生物化学光合模型及A-Ci曲线测定. 植物生态学报, 41, 693-706.]
DOI URL |
|
| 26 |
Long SP, Bernacchi CJ ( 2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany, 54, 2393-2401.
DOI URL PMID |
| 27 |
Mayoral C, Calama R, Sánchez-González M, Pardos M ( 2015). Modelling the influence of light, water and temperature on photosynthesis in young trees of mixed Mediterranean forests. New Forests, 46, 485-506.
DOI URL |
| 28 | Miao Z, Xu M, Lathrop RG, Wang Y ( 2009). Comparison of the A-Cc curve fitting methods in determining maximum ribulose-1,5-bisphosphate carboxylase/oxygenase carboxylation rate, potential light saturated electron transport rate and leaf dark respiration. Plant, Cell & Environment, 32, 109-122. |
| 29 |
Niyogi KK, Truong TB ( 2013). Evolution of flexible non-?photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Current Opinion in Plant Biology, 16, 307-314.
DOI URL PMID |
| 30 |
Park KS, Bekhzod K, Kwon JK, Son JE ( 2016). Development of a coupled photosynthetic model of sweet basil hydroponically grown in plant factories. Horticulture, Environment and Biotechnology, 57, 20-26.
DOI URL |
| 31 |
Quiroz R, Loayza H, Barreda C, Gavilán C, Posadas A, Ramírez DA ( 2017). Linking process-based potato models with light reflectance data: Does model complexity enhance yield prediction accuracy? European Journal of Agronomy, 82, 104-112.
DOI URL |
| 32 |
Ralph PJ, Gademann R ( 2005). Rapid light curves: A powerful tool to assess photosynthetic activity. Aquatic Botany, 82, 222-237.
DOI URL |
| 33 |
Ser?dio J, Ezequiel J, Frommlet J, Laviale M, Lavaud J ( 2013). A method for the rapid generation of nonsequential light-response curves of chlorophyll fluorescence. Plant Physiology, 163, 1089-1102.
DOI URL PMID |
| 34 |
Shimada A, Kubo T, Tominaga S, Yamamoto M ( 2017). Effect of temperature on photosynthesis characteristics in the passion fruits ‘Summer Queen’ and ‘Ruby Star’. The Horticulture Journal, 86, 194-199.
DOI URL |
| 35 |
Smith E ( 1937). The influence of light and carbon dioxide on photosynthesis. Journal of General Physiology, 20, 807-830.
DOI URL PMID |
| 36 |
Sun J, Sun J, Feng Z ( 2015). Modelling photosynthesis in flag leaves of winter wheat (Triticum aestivum) considering the variation in photosynthesis parameters during developpment. Functional Plant Biology, 42, 1036-1044.
DOI URL |
| 37 |
Takahashi S, Badger M ( 2011). Photoprotection in plants: A new light on photosystem II damage. Trends in Plant Sciences, 16, 53-59.
DOI URL PMID |
| 38 | Tang XL, Cao YH, Gu LH, Zhou BZ ( 2017a). Advances in photo-physiological responses of leaves to environmental factors based on the FvCB model. Acta Ecologica Sinica, 37, 6633-6645. |
| [ 唐星林, 曹永慧, 顾连宏, 周本智 ( 2017a). 基于FvCB模型的叶片光合生理对环境因子的响应研究进展. 生态学报, 37, 6633-6645.] | |
| 39 | Tang XL, Zhou BZ, Zhou Y, Ni X, Cao YH, Gu LH ( 2017b). Photo-physiological and photo-biochemical characteristics of several herbaceous and woody species based on FvCB model. Chinese Journal of Applied Ecology, 28, 1482-1488. |
| [ 唐星林, 周本智, 周燕, 倪霞, 曹永慧, 顾连宏 ( 2017b). 基于FvCB模型的几种草本和木本植物光合生理生化特性. 应用生态学报, 28, 1482-1488.] | |
| 40 | Thornley JHM ( 1976). Mathematical Models in Plant Physiology. Academic Press, London. 86-110. |
| 41 |
Valentini R, Epron D, de Angelis P, Matteucci G, Dreyer E ( 1995). In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Tukey oak (Q. cerris L.) leaves: Diurnal cycles under different levels of water supply. Plant, Cell & Environment, 18, 631-640.
DOI URL |
| 42 | von Caemmerer S ( 2000). Biochemical Models of Leaf Photosynthesis. Techniques in Plant Sciences No. 2. Collingwood. CSIRO Publishing, Australia, Victoria. |
| 43 |
von Caemmerer S ( 2013). Steady-state models of photosynthesis. Plant, Cell & Environment, 36, 1617-1630.
DOI URL PMID |
| 44 |
von Caemmerer S, Farquhar GD ( 1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta, 153, 376-387.
DOI URL |
| 45 |
Wang HZ, Han L, Xu YL, Niu JL, Yu J ( 2017). Simulated photosynthetic responses of Populus euphratica during drought stress using light-response models. Acta Ecologica Sinica, 37, 2315-2324.
DOI URL |
|
[ 王海珍, 韩路, 徐雅丽, 牛建龙, 于军 ( 2017). 干旱胁迫下胡杨光合光响应过程模拟与模型比较. 生态学报, 37, 2315-2324.]
DOI URL |
|
| 46 |
Wang RR, Xia JB, Yang JH, Zhao YY, Liu JT, Sun JK ( 2013). Comparison of light response models of photosynthesis in leaves of Periploca sepium under drought stress in sand habitat formed from seashells. Chinese Journal of Plant Ecology, 37, 111-121.
DOI URL |
|
[ 王荣荣, 夏江宝, 杨吉华, 赵艳云, 刘京涛, 孙景宽 ( 2013). 贝壳砂生境干旱胁迫下杠柳叶片光合光响应模型比较. 植物生态学报, 37, 111-121.]
DOI URL |
|
| 47 |
White AJ, Critchley C ( 1999). Rapid light curves: A new fluorescence method to asses the state of the photosynthetic apparatus. Photosynthesis Research, 59, 63-72.
DOI URL |
| 48 |
Ye ZP ( 2007). A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica, 45, 637-640.
DOI URL |
| 49 |
Ye ZP, Hu WH, Xiao YA, Fan DY, Yi JH, Duan SH, Yan XH, He L, Zhang SS ( 2014). A mechanistic model of light-response of photosynthetic electron flow and its application. Chinese Journal of Plant Ecology, 38, 1241-1249.
DOI URL |
|
[ 叶子飘, 胡文海, 肖宜安, 樊大勇, 尹建华, 段世华, 闫小红, 贺俐, 张斯斯 ( 2014). 光合电子流对光响应的机理模型及其应用. 植物生态学报, 38, 1241-1249.]
DOI URL |
|
| 50 |
Ye ZP, Robakowski P, Suggett JD ( 2013a). A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules. Planta, 237, 837-847.
DOI URL PMID |
| 51 |
Ye ZP, Suggett JD, Robakowski P, Kang HJ ( 2013b). A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of PSII in C3 and C4 species. New Phytologist, 152, 1251-1262.
DOI URL PMID |
| 52 | Yin XY, Struik PC, Romero P, Harbinson J, Evers JB, van der Putten PEL, Vos J ( 2009). Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: A critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell & Environment, 32, 448-464. |
| [1] | LI Li-Yuan, LI Jun, TONG Xiao-Juan, MENG Ping, ZHANG Jin-Song, ZHANG Jing-Ru. Simulation on the light-response curves of electron transport rate of Quercus variabilis and Robinia pseudoacacia leaves in the Xiaolangdi area, China [J]. Chin J Plant Ecol, 2018, 42(10): 1009-1021. |
| [2] | YE Zi-Piao, DUAN Shi-Hua, AN Ting, KANG Hua-Jing. Construction of CO2-response model of electron transport rate in C4 crop and its application [J]. Chin J Plant Ecol, 2018, 42(10): 1000-1008. |
| [3] | Dan WANG, Yun-Zhou QIAO, Bao-Di DONG, Jing GE, Ping-Guo YANG, Meng-Yu LIU. Differential effects of diurnal asymmetric and symmetric warming on yield and water utilization of soybean [J]. Chin J Plant Ecol, 2016, 40(8): 827-833. |
| [4] | Zi-Piao YE, Wen-Hai HU, Xiao-Hong YAN. Comparison on light-response models of actual photochemical efficiency in photosystem II [J]. Chin J Plant Ecol, 2016, 40(11): 1208-1217. |
| [5] | YE Zi-Piao,HU Wen-Hai,XIAO Yi-An,FAN Da-Yong,YIN Jian-Hua,DUAN Shi-Hua,YAN Xiao-Hong,HE Li,ZHANG Si-Si. A mechanistic model of light-response of photosynthetic electron flow and its application [J]. Chin J Plant Ecol, 2014, 38(11): 1241-1249. |
| [6] | ZHANG Xu-Cheng, YU Xian-Feng, GAO Shi-Ming. Effects of nitrogen application rates on photosynthetic energy utilization in wheat leaves under elevated atmospheric CO2 concentration [J]. Chin J Plant Ecol, 2010, 34(10): 1196-1203. |
| [7] | SONG Kai-Shan, ZHANG Bai, WANG Zong-Ming, LIU Dian-Wei, LIU Huan-Jun. SOYBEAN CHLOROPHYLL A CONCENTRATION ESTIMATION MODELS BASED ON WAVELET-TRANSFORMED, IN SITU COLLECTED, CANOPY HYPERSPECTRAL DATA [J]. Chin J Plant Ecol, 2008, 32(1): 152-160. |
| [8] | Zhou San, Zhou Ming, Zhang Shuo, Liu Zhan-Tao, Zhao Yong-Juan, Yu Tian-Zhen, Yue Wang. ISOFLAVONE ACCUMULATION IN WILD SOYBEAN UNDER SALINE CONDITIONS AND ITS ECOLOGICAL SIGNIFICANCE [J]. Chin J Plant Ecol, 2007, 31(5): 930-936. |
| [9] | ZU Yuan-Gang, ZHANG Zhong-Hua, WANG Wen-Jie, YANG Feng-Jian, HE Hai-Sheng. DIFFERENT CHARACTERISTICS OF PHOTOSYNTHESIS IN STEMS AND LEAVES OF MIKANIA MICRANTH [J]. Chin J Plant Ecol, 2006, 30(6): 998-1004. |
| [10] | QIANG Wei-Ya, CHEN Tuo, TANG Hong-Guan, FENG Hu-Yuan, AN Li-Zhe, WANG Xun-Ling. Effect of Cadmium and Enhanced UV-B Radiation on Soybean Root Excretion [J]. Chin J Plan Ecolo, 2003, 27(3): 293-298. |
| [11] | LI Han-Bing, BAI Ke-Zhi, HU Yu-Xi, KUANG Ting-Yun, LIN Jin-Xing. Stomatal Frequency on some Non-Leaf Organs of Four Crop Species and Their Significance in Photosynthesis(in English) [J]. Chin J Plan Ecolo, 2002, 26(3): 351-354. |
| [12] | CHEN You-Jian, HUANG Yi, TAO Shu. Dynamic Change of Soil Properties in the Rhizosphere of Maize and Soybean. [J]. Chin J Plan Ecolo, 2002, 26(3): 283-287. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn