

Chin J Plant Ecol ›› 2019, Vol. 43 ›› Issue (2): 119-130.DOI: 10.17521/cjpe.2018.0269
• Research Articles • Previous Articles Next Articles
ZHU Wei1,YU Li-Xuan1,ZHAO De-Hai2,JIA Li-Ming1,*( )
)
Received:2018-10-30
Accepted:2019-01-30
Online:2019-02-20
Published:2019-06-04
Contact:
JIA Li-Ming
Supported by:ZHU Wei, YU Li-Xuan, ZHAO De-Hai, JIA Li-Ming. Architectural analysis of root systems of mature trees in sandy loam soils using the root development classification[J]. Chin J Plant Ecol, 2019, 43(2): 119-130.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2018.0269
| 林型 Stand type | 树种 Tree species | 平均胸径 Average DBH (cm) | 平均树高 Average tree height (m) | 株行距 Plant & row spacing (m) | 平均冠幅 Average crown width (m) | 间伐措施 Thinning treatment |
|---|---|---|---|---|---|---|
| 毛白杨纯林 Populus tomentosa stand | 毛白杨 P. tomentosa | 36.1 | 35.5 | 6 × 6 | 7.0 | 造林后第十年间伐除去刺槐, 以前的混交林成为现有纯林 All R. pseudoacacia trees were removed from mixed-species stand after 10 years of planting, resulting in P. tomentosa pure stand |
| 毛白杨×刺槐混交林 Mixtures with P. tomentosa and Robinia pseudoacacia | 毛白杨 P. tomentosa | 37.0 | 26.4 | 3 × 6 | 6.6 | 无 None |
| 毛白杨×刺槐混交林 Mixtures with P. tomentosa and R. pseudoacacia | 刺槐 R. pseudoacacia | 25.1 | 21.1 | 3 × 6 | 8.6 | 无 None |
Table 1 General information of sample plots in Populus tomentosa stand and mixtures with P. tomentosa and Robinia pseudoacacia
| 林型 Stand type | 树种 Tree species | 平均胸径 Average DBH (cm) | 平均树高 Average tree height (m) | 株行距 Plant & row spacing (m) | 平均冠幅 Average crown width (m) | 间伐措施 Thinning treatment |
|---|---|---|---|---|---|---|
| 毛白杨纯林 Populus tomentosa stand | 毛白杨 P. tomentosa | 36.1 | 35.5 | 6 × 6 | 7.0 | 造林后第十年间伐除去刺槐, 以前的混交林成为现有纯林 All R. pseudoacacia trees were removed from mixed-species stand after 10 years of planting, resulting in P. tomentosa pure stand |
| 毛白杨×刺槐混交林 Mixtures with P. tomentosa and Robinia pseudoacacia | 毛白杨 P. tomentosa | 37.0 | 26.4 | 3 × 6 | 6.6 | 无 None |
| 毛白杨×刺槐混交林 Mixtures with P. tomentosa and R. pseudoacacia | 刺槐 R. pseudoacacia | 25.1 | 21.1 | 3 × 6 | 8.6 | 无 None |
| 纯林毛白杨 Populus tomentosa in pure stand | 混交林毛白杨 P. tomentosa in mixed stand | 混交林刺槐 Robinia pseudoacacia in mixed stand | |||||
|---|---|---|---|---|---|---|---|
| 追踪根系编号 Code of the tracked roots | 距离/深度 Distance/ depth (m) | 终点状态 End state | 距离/深度 Distance/ depth (m) | 终点状态 End state | 距离/深度 Distance/ depth (m) | 终点状态 End state | |
| 水平根 Lateral root | 1 | 3.3 | 萌生幼树 Sapling from root sprouting | 8.6 | 与邻株连生 Root grafted with neighbor tree | 6.5 | 垂直向下生长 Vertically downward growth |
| 2 | 5.1 | 与邻株连生 Root grafted with neighbor tree | 11.1 | 分叉 Branching | 8.2 | 分叉 Branching | |
| 3 | 6.1 | 垂直向下生长 Vertically downward growth | 13.5 | 垂直向下生长 Vertically downward growth | 8.5 | 垂直向下生长 Vertically downward growth | |
| 4 | 6.4 | 垂直向下生长 Vertically downward growth | |||||
| 5 | 7.6 | 垂直向下生长 Vertically downward growth | |||||
| 垂直根 Vertical root | 1 | 3.1 | 到头, 末端直径0.3 mm To the end, root tip diameter 0.3 mm | 4.0 | 到头, 末端长瘤 To the end, with tumor | 2.7 | 竖直向上生长 Vertically upward growth |
| 2 | 4.3 | 到头, 末端直径0.3 mm To the end, root tip diameter 0.3 mm | 4.3 | 到头, 末端直径0.5 mm To the end, root tip diameter 0.5 mm | 3.7 | 到头, 末端直径0.3 mm To the end, root tip diameter 0.3 mm | |
| 3 | 5.7 | 到头, 末端直径0.25 mm To the end, root tip diameter 0.25 mm | 4.3 | 到头, 末端直径0.76 mm To the end, root tip diameter 0.76 mm | 5.9 | 到头, 末端直径0.02 mm To the end, root tip diameter 0.02 mm | |
| 4 | 6.4 | 向西转弯 Bend to the west | |||||
Table 2 Description of end states of the tracked roots in three root systems
| 纯林毛白杨 Populus tomentosa in pure stand | 混交林毛白杨 P. tomentosa in mixed stand | 混交林刺槐 Robinia pseudoacacia in mixed stand | |||||
|---|---|---|---|---|---|---|---|
| 追踪根系编号 Code of the tracked roots | 距离/深度 Distance/ depth (m) | 终点状态 End state | 距离/深度 Distance/ depth (m) | 终点状态 End state | 距离/深度 Distance/ depth (m) | 终点状态 End state | |
| 水平根 Lateral root | 1 | 3.3 | 萌生幼树 Sapling from root sprouting | 8.6 | 与邻株连生 Root grafted with neighbor tree | 6.5 | 垂直向下生长 Vertically downward growth |
| 2 | 5.1 | 与邻株连生 Root grafted with neighbor tree | 11.1 | 分叉 Branching | 8.2 | 分叉 Branching | |
| 3 | 6.1 | 垂直向下生长 Vertically downward growth | 13.5 | 垂直向下生长 Vertically downward growth | 8.5 | 垂直向下生长 Vertically downward growth | |
| 4 | 6.4 | 垂直向下生长 Vertically downward growth | |||||
| 5 | 7.6 | 垂直向下生长 Vertically downward growth | |||||
| 垂直根 Vertical root | 1 | 3.1 | 到头, 末端直径0.3 mm To the end, root tip diameter 0.3 mm | 4.0 | 到头, 末端长瘤 To the end, with tumor | 2.7 | 竖直向上生长 Vertically upward growth |
| 2 | 4.3 | 到头, 末端直径0.3 mm To the end, root tip diameter 0.3 mm | 4.3 | 到头, 末端直径0.5 mm To the end, root tip diameter 0.5 mm | 3.7 | 到头, 末端直径0.3 mm To the end, root tip diameter 0.3 mm | |
| 3 | 5.7 | 到头, 末端直径0.25 mm To the end, root tip diameter 0.25 mm | 4.3 | 到头, 末端直径0.76 mm To the end, root tip diameter 0.76 mm | 5.9 | 到头, 末端直径0.02 mm To the end, root tip diameter 0.02 mm | |
| 4 | 6.4 | 向西转弯 Bend to the west | |||||

Fig. 1 Photos of root systems of Populus tomentosa in pure stand (A), P. tomentosa and Robinia pseudoacacia in mixed stand (B) (Lens position to the east).
| 根系系统 Root system | a (A) | Pe | V0 (M) | b | qa | qb | TI |
|---|---|---|---|---|---|---|---|
| 纯林毛白杨 Populus tomentosa in pure forest | 29 | 8 367 | 793 | 10.551 | 0.02 | -0.000 2 | 0.50 |
| 混交林毛白杨 P. tomentosa in mixed forest | 61 | 21 322 | 1 456 | 14.644 | 0.03 | 0.004 0 | 0.56 |
| 混交林刺槐 Robinia pseudoacacia in mixed forest | 21 | 3 990 | 465 | 8.581 | 0.02 | -0.006 0 | 0.50 |
Table 3 The values of the topological indices for three root systems
| 根系系统 Root system | a (A) | Pe | V0 (M) | b | qa | qb | TI |
|---|---|---|---|---|---|---|---|
| 纯林毛白杨 Populus tomentosa in pure forest | 29 | 8 367 | 793 | 10.551 | 0.02 | -0.000 2 | 0.50 |
| 混交林毛白杨 P. tomentosa in mixed forest | 61 | 21 322 | 1 456 | 14.644 | 0.03 | 0.004 0 | 0.56 |
| 混交林刺槐 Robinia pseudoacacia in mixed forest | 21 | 3 990 | 465 | 8.581 | 0.02 | -0.006 0 | 0.50 |
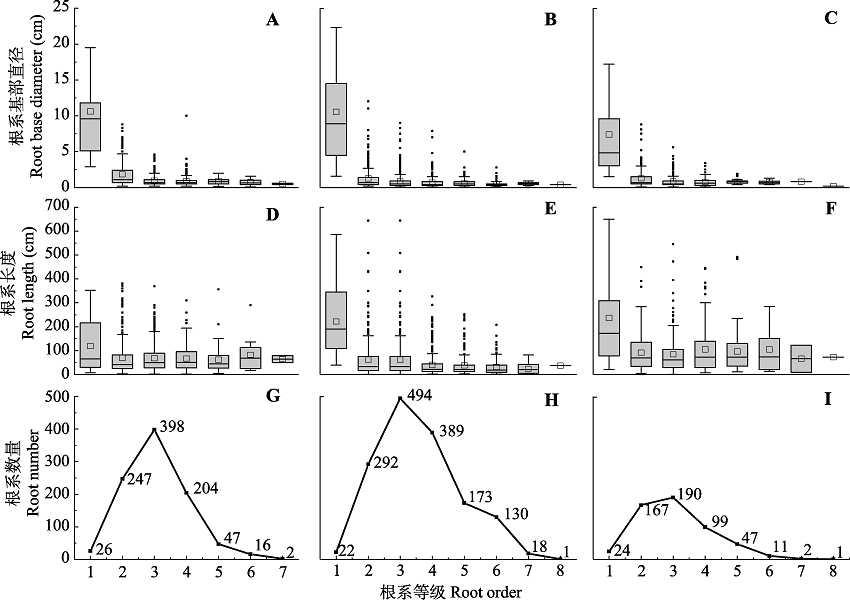
Fig. 2 Variations of basal diameter, length, and number of roots for different order roots in root systems of Populus tomentosa in pure forest (A, D, G), P. tomentosa in mixed forest (B, E, H), and Robinia pseudoacacia in mixed forest (C, F, I). The individual points on the box plots represent outliers, the squares on the box plots represent the average.
| 根形态指标 Root morphological characteristics | 根系系统 Root system | 方程 Regression model | 拟合优度 R2 | 样本量 n |
|---|---|---|---|---|
| 基径 Basal diameter | 纯林毛白杨 P. tomentosa in pure forest | y = 0.86381 + 99.24471exp(-2.32112x) | 0.484 0 | 940 |
| 混交林毛白杨 P. tomentosa in mixed forest | y = 0.70036 + 157.41951exp(-2.77342x) | 0.414 9 | 1 527 | |
| 混交林刺槐 R. pseudoacacia in mixed forest | y = 0.80671 + 91.76619exp(-2.63586x) | 0.374 7 | 541 | |
| 长度 Length | 纯林毛白杨 P. tomentosa in pure forest | y = 67.63168 + 1098.3759exp(-3.32236x) | 0.014 6 | 940 |
| 混交林毛白杨 P. tomentosa in mixed forest | y = 39.71891 + 1313.84526exp(-1.98679x) | 0.117 2 | 1 527 | |
| 混交林刺槐 R. pseudoacacia in mixed forest | y = 92.10741 + 477664.65953exp(-8.10278x) | 0.079 5 | 541 | |
| 数量 Numbers | 纯林毛白杨 P. tomentosa in pure forest | y = 5.97738 + 396.37817exp(-0.5((x-2.93757)/0.90608)2) | 0.989 5 | 7 |
| 混交林毛白杨 P. tomentosa in mixed forest | y = 22.41349 + 474.80796exp(-0.5((x-3.28655)/1.13184)2) | 0.912 7 | 8 | |
| 混交林刺槐 R. pseudoacacia in mixed forest | y = 5.83356 + 196.21954exp(-0.5((x-2.79311)/1.01443)2) | 0.936 7 | 8 |
Table 4 Results of fitting regression models of Populus tomentosa and Robinia pseudoacacia—regressing root diameter, length, and numbers (y) on root orders (x), respectively
| 根形态指标 Root morphological characteristics | 根系系统 Root system | 方程 Regression model | 拟合优度 R2 | 样本量 n |
|---|---|---|---|---|
| 基径 Basal diameter | 纯林毛白杨 P. tomentosa in pure forest | y = 0.86381 + 99.24471exp(-2.32112x) | 0.484 0 | 940 |
| 混交林毛白杨 P. tomentosa in mixed forest | y = 0.70036 + 157.41951exp(-2.77342x) | 0.414 9 | 1 527 | |
| 混交林刺槐 R. pseudoacacia in mixed forest | y = 0.80671 + 91.76619exp(-2.63586x) | 0.374 7 | 541 | |
| 长度 Length | 纯林毛白杨 P. tomentosa in pure forest | y = 67.63168 + 1098.3759exp(-3.32236x) | 0.014 6 | 940 |
| 混交林毛白杨 P. tomentosa in mixed forest | y = 39.71891 + 1313.84526exp(-1.98679x) | 0.117 2 | 1 527 | |
| 混交林刺槐 R. pseudoacacia in mixed forest | y = 92.10741 + 477664.65953exp(-8.10278x) | 0.079 5 | 541 | |
| 数量 Numbers | 纯林毛白杨 P. tomentosa in pure forest | y = 5.97738 + 396.37817exp(-0.5((x-2.93757)/0.90608)2) | 0.989 5 | 7 |
| 混交林毛白杨 P. tomentosa in mixed forest | y = 22.41349 + 474.80796exp(-0.5((x-3.28655)/1.13184)2) | 0.912 7 | 8 | |
| 混交林刺槐 R. pseudoacacia in mixed forest | y = 5.83356 + 196.21954exp(-0.5((x-2.79311)/1.01443)2) | 0.936 7 | 8 |
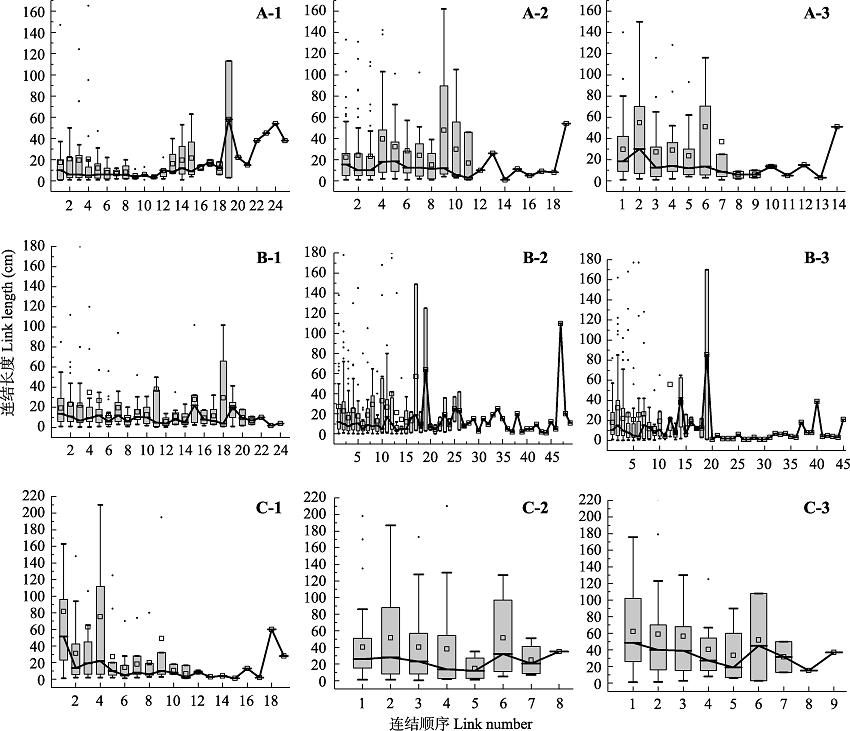
Fig. 3 Link length variations along link sequence from the root base to the root tip in root systems of Populus tomentosa in pure forest (A), P. tomentosa in mixed forest (B), and Robinia pseudoacacia in mixed forest (C). 1-3 refer to root categories from first order to third order. The individual points on the box plots represent outliers, the squares on the box plots represent the average, and the lines connected the box plots.
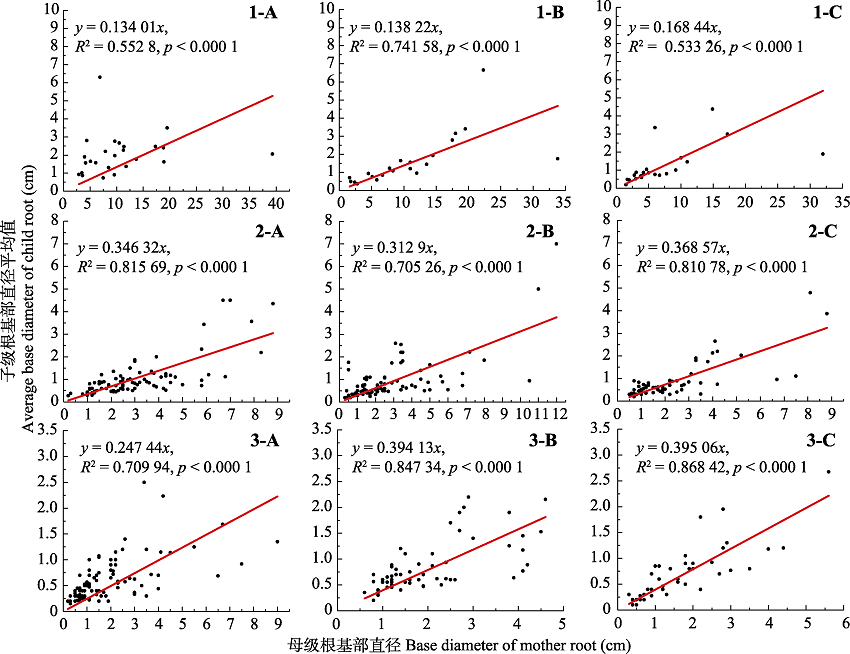
Fig. 4 Basal diameter relationships between the mother roots and the child roots. 1, refer to mother-root categories from first order. 2, refer to mother-root categories from second order. 3, refer to mother-root categories from third order. A, root system of Populus tomentosa in pure forest. B, root system of P. tomentosa in mixed forest. C, root system of Robinia pseudoacacia in mixed forest.
| [1] | Bakker MR, Augusto L, Achat DL ( 2006). Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant and Soil, 286, 37-51. |
| [2] | Bécel C, Vercambre G, Pagès L ( 2012). Soil penetration resistance, a suitable soil property to account for variations in root elongation and branching. Plant and Soil, 353, 169-180. |
| [3] | Berntson GM ( 1994). Modelling root architecture: Are there tradeoffs between efficiency and potential of resource acquisition? New Phytologist, 127, 483-493. |
| [4] | Chang WJ, Guo DL ( 2008). Variation in root diameter among 45 common tree species in temperate, subtropical and tropical forests in China. Journal of Plant Ecology (Chinese Version), 32, 1248-1257. |
| [ 常文静, 郭大立 ( 2008). 中国温带、亚热带和热带森林45个常见树种细根直径变异. 植物生态学报, 32, 1248-1257.] | |
| [5] | Danjon F, Khuder H, Stokes A ( 2013). Deep phenotyping of coarse root architecture in R. pseudoacacia reveals that tree root system plasticity is confined within its architectural model. PLOS ONE, 8, e83548. DOI: 10.1371/journal.pone.0083548. |
| [6] | Danjon F, Reubens B ( 2008). Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant and Soil, 303, 1-34. |
| [7] | Danjon F, Barker DH, Drexhage M, Stokes A ( 2008). Using three-dimensional plant root architecture in models of shallow-slope stability. Annals of Botany, 101, 1281-1293. |
| [8] | Danjon F, Fourcaud T, Bert D ( 2005). Root architecture and wind-firmness of mature Pinus pinaster. New Phytologist, 168, 387-400. |
| [9] | Dawson TE, Pate JS ( 1996). Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia, 107, 13-20. |
| [10] | De Deurwaerder H, Herve-Fernandez P, Stahl C, Burban B, Petronelli P, Hoffman B, Bonal D, Boeckx P, Verbeeck H ( 2018). Liana and tree below-ground water competition— Evidence for water resource partitioning during the dry season. Tree Physiology, 38, 1071-1083. |
| [11] | Di N, Liu Y, Mead DJ, Xie YQ, Jia LM, Xi BY ( 2018). Root-system characteristics of plantation-grown Populus tomentosa adapted to seasonal fluctuation in the groundwater table. Trees, 32, 137-149. |
| [12] | Fan Y, Miguez-Macho G, Jobbagy EG, Jackson RB, Otero- Casal C ( 2017). Hydrologic regulation of plant rooting depth. Proceedings of the National Academy of Sciences of the United States of America, 114, 10572-10577. |
| [13] | Fitter AH ( 1986). The topology and geometry of plant root systems: Influence of watering rate on root system topology in trifolium pretense. Annals of Botany, 58, 91-101. |
| [14] | Fitter AH ( 1987). An architectural approach to the comparative ecology of plant root systems. New Phytologist, 106, 61-77. |
| [15] | Fitter AH, Stickland TR ( 1991). Architectural analysis of plant root systems: 2. Influence of nutrient supply on architecture in contrasting plant species. New Phytologist, 118, 383-389. |
| [16] | Gärtner H, Wagner B, Heinrich I, Denier C ( 2009). 3D-laser scanning: A new method to analyze coarse tree root systems. Forest Snow and Landscape Research, 82, 95-106. |
| [17] | Guo DL, Xia MX, Wei X, Chang WJ, Liu Y, Wang ZQ ( 2008). Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist, 180, 673-683. |
| [18] | Kalliokoski T, Sievänen R, Nygren P ( 2010). Tree roots as self-similar branching structures: Axis differentiation and segment tapering in coarse roots of three boreal forest tree species. Trees, 24, 219-236. |
| [19] | Lecompte F, Pages L, Ozier-Lafontaine H ( 2005). Patterns of variability in the diameter of lateral roots in the banana root system. New Phytologist, 167, 841-850. |
| [20] | Lynch J ( 1995). Root architecture and plant productivity. Plant physiology, 109, 7-13. |
| [21] | Nagel KA, Kastenholz B, Jahnke S, Van Dusschoten D, Aach T, Muhlich M, Truhn D, Scharr H, Terjung S, Walter A, Schurr U ( 2009). Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology, 36, 947-959. |
| [22] | Oppelt AL, Kurth W, Godbold DL ( 2001). Topology, scaling relations and Leonardo’s rule in root systems from African tree species. Tree Physiology, 21, 117-128. |
| [23] | Osawa A, Zyryanova OA, Matsuura Y, Kajimoto T, Wein RW ( 2010). Permafrost Ecosystems: Siberian Larch Forests. Springer, Dordrecht, the Netherlands. |
| [24] | Ozier-Lafontaine H, Sillon JF ( 1999). Fractal analysis of the root architecture of Gliricidia sepium for the spatial prediction of root branching, size and mass: Model development and evaluation in agroforestry. Plant and Soil, 209, 167-179. |
| [25] | Pagès L, Doussan C, Vercambre G ( 2000). An introduction on below-ground environment and resource acquisition, with special reference on trees. Simulation models should include plant structure and function. Annals of Forest Science, 57, 513-520. |
| [26] | Pagès L, Xie J, Serra V ( 2013). Potential and actual root growth variations in root systems: Modeling them with a two-step stochastic approach. Plant and Soil, 373, 723-735. |
| [27] | Pagès L ( 2014). Branching patterns of root systems: Quantitative analysis of the diversity among dicotyledonous species. Annals of Botany, 114, 591-598. |
| [28] | Pate JS, Jeschke WD, Aylward MJ ( 1995). Hydraulic architecture and xylem structure of the dimorphic root systems of South-West Australian species of Proteaceae. Journal of Experimental Botany, 46, 907-915. |
| [29] | Pregitzer KS, Kubiske ME, Yu CK, Hendrick RL ( 1997). Relationships among root branch order, carbon, and nitrogen in four temperate species. Oecologia, 111, 302-308. |
| [30] | Pregitzer KS, Deforest JL, Burton AJ, Allen MF, Hendrick RRL ( 2002). Fine root architecture of nine North American trees. Ecological Monographs, 72, 293-309. |
| [31] | Read DJ ( 1991). Mycorrhizas in ecosystems. Experientia, 47, 376-391. |
| [32] | Rose DA ( 1983). The description of the growth of root systems. Plant and Soil, 75, 405-415. |
| [33] | Salahuddin, Rewald B, Razaq M, Lixue Y, Li J, Khan F, Jie Z ( 2018). Root order-based traits of Manchurian walnut & larch and their plasticity under interspecific competition. Scientific Reports, 8, 9815. DOI: 10.1038/s41598-018-27832-0. |
| [34] | Schenk HJ, Jackson RB ( 2002 a). The global biogeography of roots. Ecological Monographs, 72, 311-328. |
| [35] | Schenk HJ, Jackson RB ( 2002 b). Rooting depths, lateral spreads, and below-ground/above-ground allometries of plants in water-limited ecosystems. Journal of Ecology, 90, 480-494. |
| [36] | Schenk HJ, Jackson RB ( 2005). Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma, 126, 129-140. |
| [37] | Shafroth PB, Stromberg JC, Patten DT ( 2000). Woody riparian vegetation response to different alluvial water table regimes. Western North American Naturalist, 60, 66-76. |
| [38] | Smit AL, Bengough AG, Engels C, Noordwijk M, Pellerin S, Geijn SC ( 2000). Root Methods. Springer, Berlin. |
| [39] | Strahler AN ( 1957). Quantitative analysis of watershed geomorphology. Eos Transactions American Geophysical Union, 38, 913-920. |
| [40] | Tardieu F, Pellerin S ( 1991). Influence of soil temperature during root appearance on the trajectory of nodal roots of field grown maize. Plant and Soil, 131, 207-214. |
| [41] | Valdes-Rodriguez OA, Sanchez-Sanchez O, Perez-Vazquez A, Caplan JS, Danjon F (2013). Jatropha curcas L. root structure and growth in diverse soils. The Scientific World Journal, 2013,827295. DOI: 10.1155/2013/827295. |
| [42] | Vercambre G, Pages L, Doussan C, Habib R ( 2003). Architectural analysis and synthesis of the plum tree root system in an orchard using a quantitative modelling approach. Plant and Soil, 251, 1-11. |
| [43] | Vennetier M, Zanetti C, Meriaux P, Mary B ( 2015). Tree root architecture: New insights from a comprehensive study on dikes. Plant and Soil, 387, 81-101. |
| [44] | Wagner B, Gärtner H, Santini S, Ingensand H ( 2011). Cross- sectional interpolation of annual rings within a 3D root model. Dendrochronologia, 29, 201-210. |
| [45] | Wu Q ( 2016). Analyzing and Modeling of Root Architecture of Individual Maize Plants Based on High-accuracy Field Data and Its Applications. PhD dissertation, China Agricultural University, Beijing. |
| [ 吴茜 ( 2016). 基于精确数据的大田玉米植株根系结构分析、建模及其应用. 博士学位论文, 中国农业大学, 北京.] | |
| [46] | Wu Q, Pagès L, Wu J ( 2016). Relationships between root diameter, root length and root branching along lateral roots in adult, field-grown maize. Annals of Botany, 117, 379. |
| [47] | Xu GQ, Li Y ( 2008). Rooting depth and leaf hydraulic conductance in the xeric tree Haloxyolon ammodendron growing at sites of contrasting soil texture. Functional Plant Biology, 35, 1234-1242. |
| [48] | Yan XL, Liao H, Ge ZY, Luo XW ( 2000). Root architectural characteristics and phosphorus acquisition efficiency in plants. Chinese Bulletin of Botany, 17, 511-519. |
| [ 严小龙, 廖红, 戈振扬, 罗锡文 ( 2000). 植物根构型特性与磷吸收效率. 植物学通报, 17, 511-519.] | |
| [49] | Yang M, Défossez P, Danjon F, Fourcaud T ( 2018). Analyzing key factors of roots and soil contributing to tree anchorage of Pinus species. Trees, 32, 703-712. |
| [50] | Yu P, Hochholdinger F, Li C ( 2015). Root-type-specific plasticity in response to localized high nitrate supply in maize (Zea mays). Annals of Botany, 116, 751-762. |
| [51] | Zhao YY, Lu ZH, Xia JB, Liu JT ( 2015). Root architecture and adaptive strategy of 3 shrubs in Shell Bay in Yellow River Delta. Acta Ecologica Sinica , 35, 1688-1695. |
| [ 赵艳云, 陆兆华, 夏江宝, 刘京涛 ( 2015). 黄河三角洲贝壳堤岛3种优势灌木的根系构型. 生态学报, 35, 1688-1695.] |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn