

Chin J Plant Ecol ›› 2013, Vol. 37 ›› Issue (2): 173-182.DOI: 10.3724/SP.J.1258.2013.00018
• Research Articles • Previous Articles Next Articles
LI Xiao-Juan1,2, WANG Qiang2,*( ), NI Sui1,*(
), NI Sui1,*( ), RUAN Xiao2, WANG Yong-Hong2, ZHANG Huan2, Geoff WANG3
), RUAN Xiao2, WANG Yong-Hong2, ZHANG Huan2, Geoff WANG3
Received:2012-11-26
Accepted:2012-12-29
Online:2013-11-26
Published:2013-01-31
Contact:
WANG Qiang,NI Sui
LI Xiao-Juan, WANG Qiang, NI Sui, RUAN Xiao, WANG Yong-Hong, ZHANG Huan, Geoff WANG. Allelopathy comparison between Castanea mollissima and C. dentata[J]. Chin J Plant Ecol, 2013, 37(2): 173-182.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.3724/SP.J.1258.2013.00018
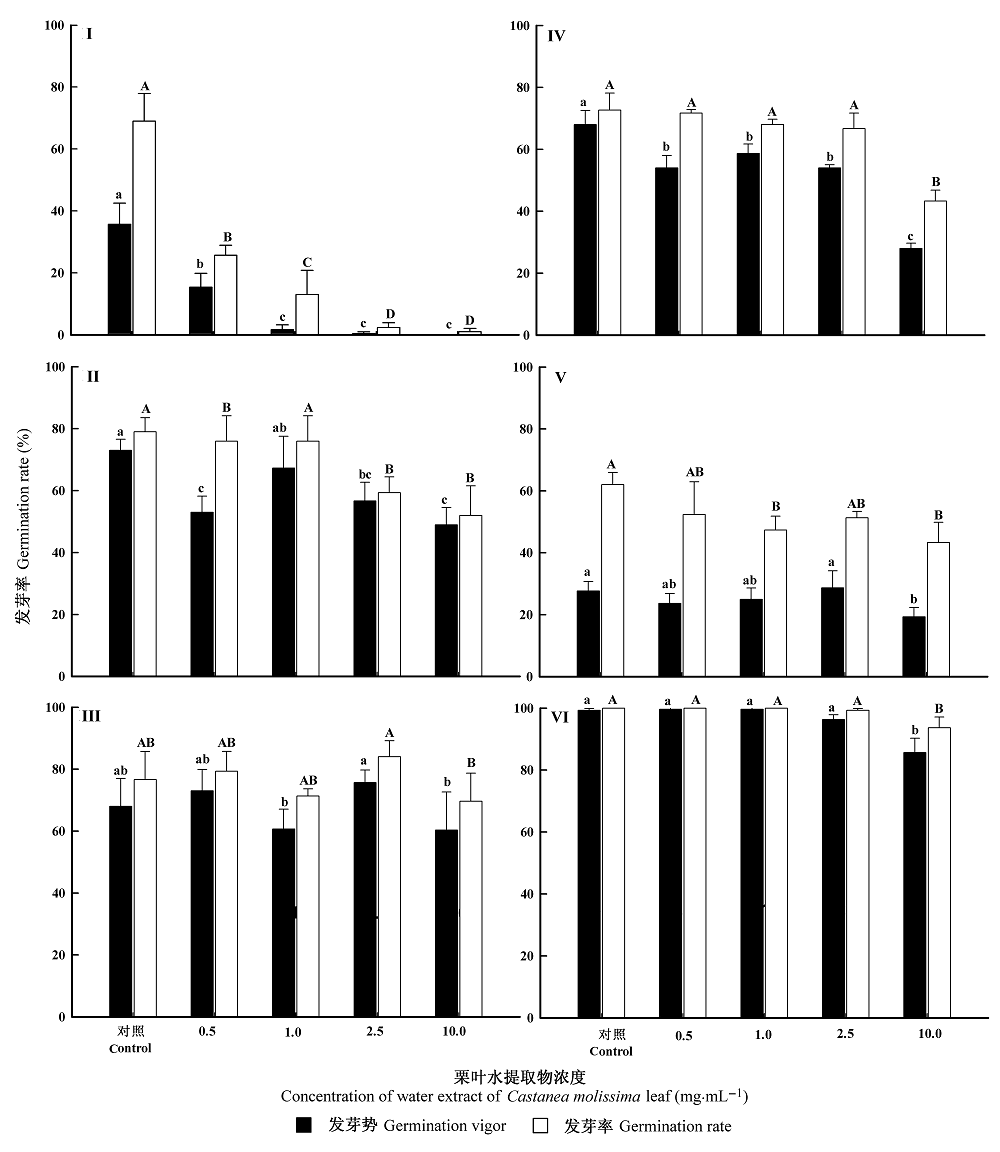
Fig. 1 Effect of water extract of Castanea molissima leaf on germination of test plants (mean ± SD, n = 3). I, Lactuca sativa. II, Raphanus sativus. III, Cucumis sativus. IV, Allium cepa. V, Oryza sativa. VI, Triticum aestivum. Means marked with different letters are significantly different according to least significant difference multiple comparisons (p = 0.05).
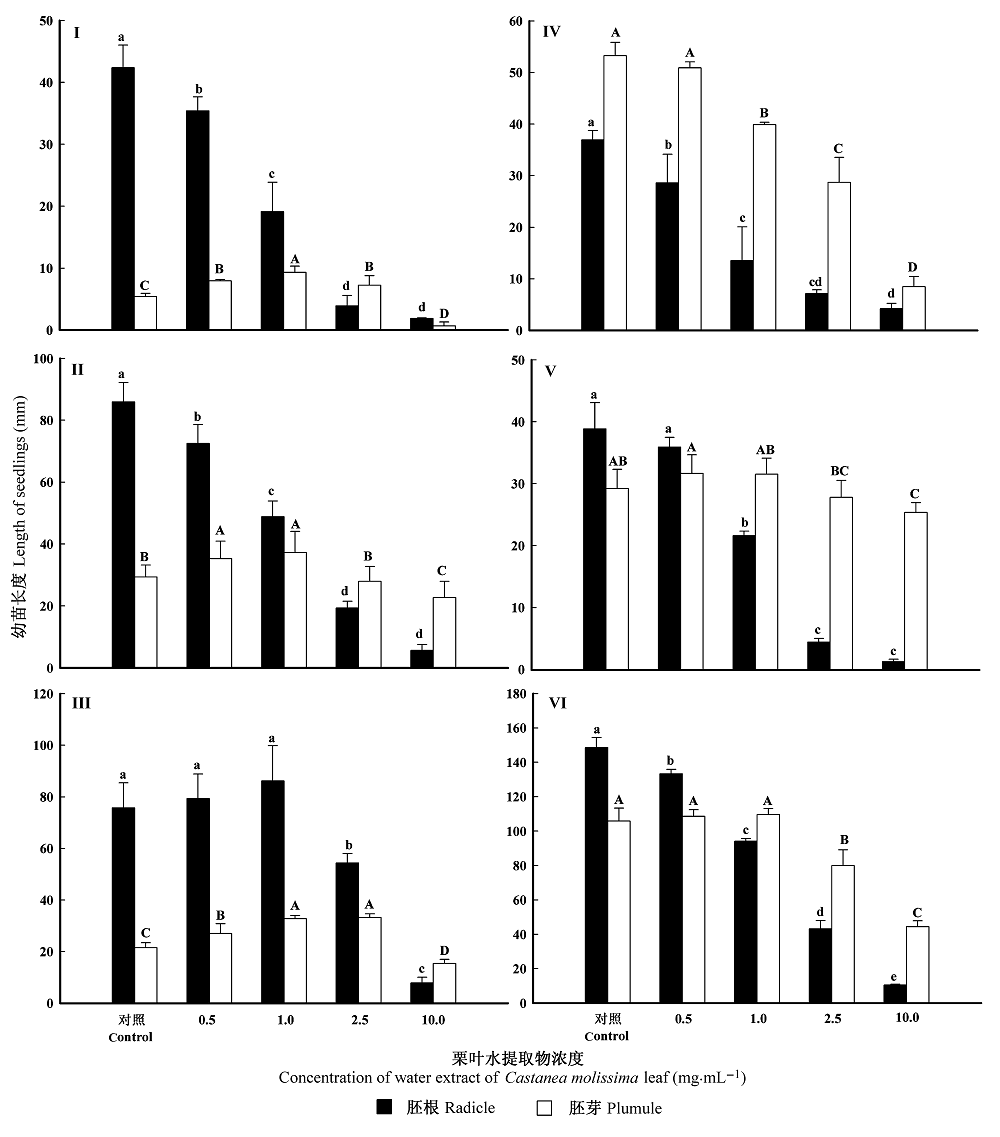
Fig. 2 Effect of water extract of Castanea molissima leaf on radicle and plumule length of test plants (mean ± SD, n = 3). I, Lactuca sativa. II, Raphanus sativus. III, Cucumis sativus. IV, Allium cepa. V, Oryza sativa. VI, Triticum aestivum. Means marked with different letters are significantly different according to least significant difference multiple comparisons ( p = 0.05).
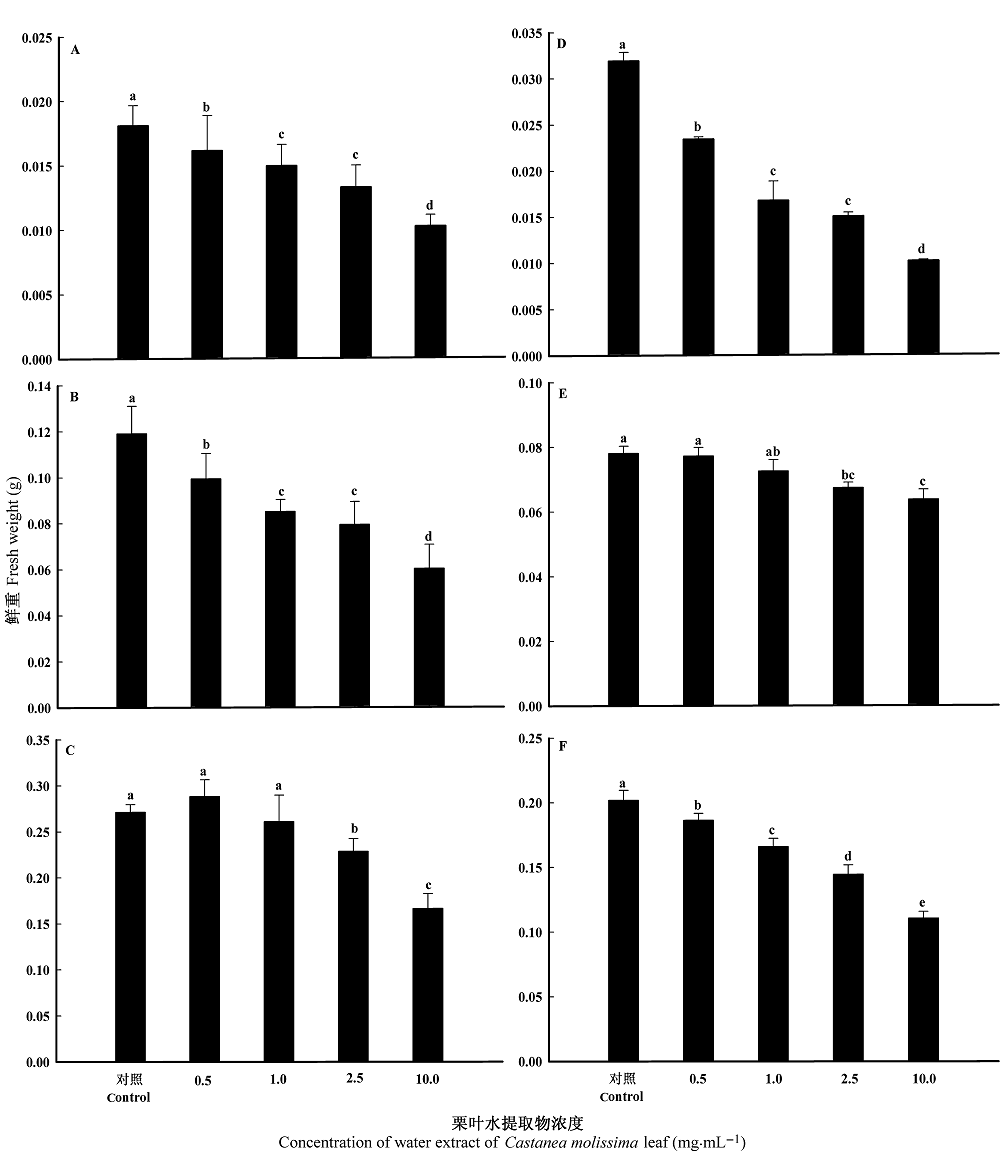
Fig. 3 Effect of water extract of Castanea molissima leaf on the fresh weight of test plants (mean ± SD, n = 3). A, Lactuca sativa. B, Raphanus sativus. C, Cucumis sativus. D, Allium cepa. E, Oryza sativa. F, Triticum aestivum. Means marked with different letters are significantly different according to least significant difference multiple comparisons (p = 0.05).
| 组分中水:乙醇 Ratio of water to ethanol in component | 干重 Dry weight (g) | |
|---|---|---|
| 美国板栗 Castanea dentata | 栗 Castanea molissima | |
| 10:0 | 3.355 ± 0.121 | 3.331 ± 0.152 |
| 9:1 | 1.145 ± 0.056 | 0.334 ± 0.020 |
| 8:2 | 0.873 ± 0.022 | 0.780 ± 0.028 |
| 7:3 | 0.813 ± 0.030 | 0.925 ± 0.030* |
| 6:4 | 0.364 ± 0.012 | 0.422 ± 0.024* |
| 5:5 | 0.101 ± 0.005 | 0.109 ± 0.008 |
| 4:6 | 0.043 ± 0.009 | 0.090 ± 0.012 |
| 3:7 | 0.056 ± 0.018 | 0.137 ± 0.040 |
| 2:8 | 0.051 ± 0.012 | 0.051 ± 0.033 |
| 1:9 | 0.054 ± 0.028 | 0.060 ± 0.045 |
| 0:10 | 0.043 ± 0.022 | 0.081 ± 0.055 |
| 总重量 Total weight | 6.025 ± 0.059A | 6.021 ± 0.034a |
| 总上样量 Total sample weight | 6.089 ± 0.062A | 6.052 ± 0.042a |
Table 1 Quantitative analysis of isolated components of Castanea dentata and C. molissima (mean ± SD, n = 3)
| 组分中水:乙醇 Ratio of water to ethanol in component | 干重 Dry weight (g) | |
|---|---|---|
| 美国板栗 Castanea dentata | 栗 Castanea molissima | |
| 10:0 | 3.355 ± 0.121 | 3.331 ± 0.152 |
| 9:1 | 1.145 ± 0.056 | 0.334 ± 0.020 |
| 8:2 | 0.873 ± 0.022 | 0.780 ± 0.028 |
| 7:3 | 0.813 ± 0.030 | 0.925 ± 0.030* |
| 6:4 | 0.364 ± 0.012 | 0.422 ± 0.024* |
| 5:5 | 0.101 ± 0.005 | 0.109 ± 0.008 |
| 4:6 | 0.043 ± 0.009 | 0.090 ± 0.012 |
| 3:7 | 0.056 ± 0.018 | 0.137 ± 0.040 |
| 2:8 | 0.051 ± 0.012 | 0.051 ± 0.033 |
| 1:9 | 0.054 ± 0.028 | 0.060 ± 0.045 |
| 0:10 | 0.043 ± 0.022 | 0.081 ± 0.055 |
| 总重量 Total weight | 6.025 ± 0.059A | 6.021 ± 0.034a |
| 总上样量 Total sample weight | 6.089 ± 0.062A | 6.052 ± 0.042a |
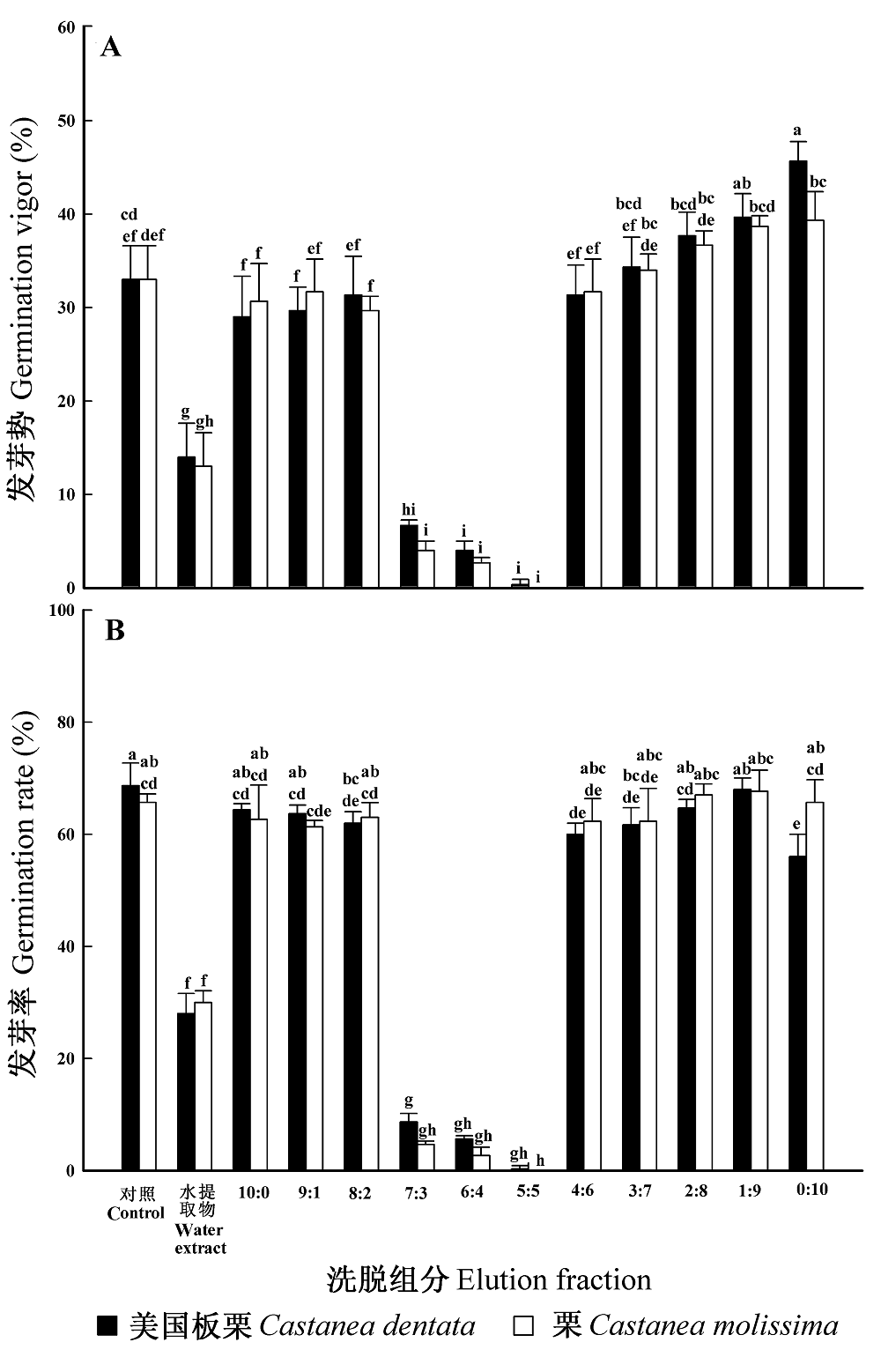
Fig. 4 Effect of isolated components of Castanea dentata and C. molissima on seed germination of lettuce (mean ± SD, n = 3). A, Germination vigor. B, Germination rate. Means marked with different letters are significantly different according to least significant difference multiple comparisons (p = 0.05)
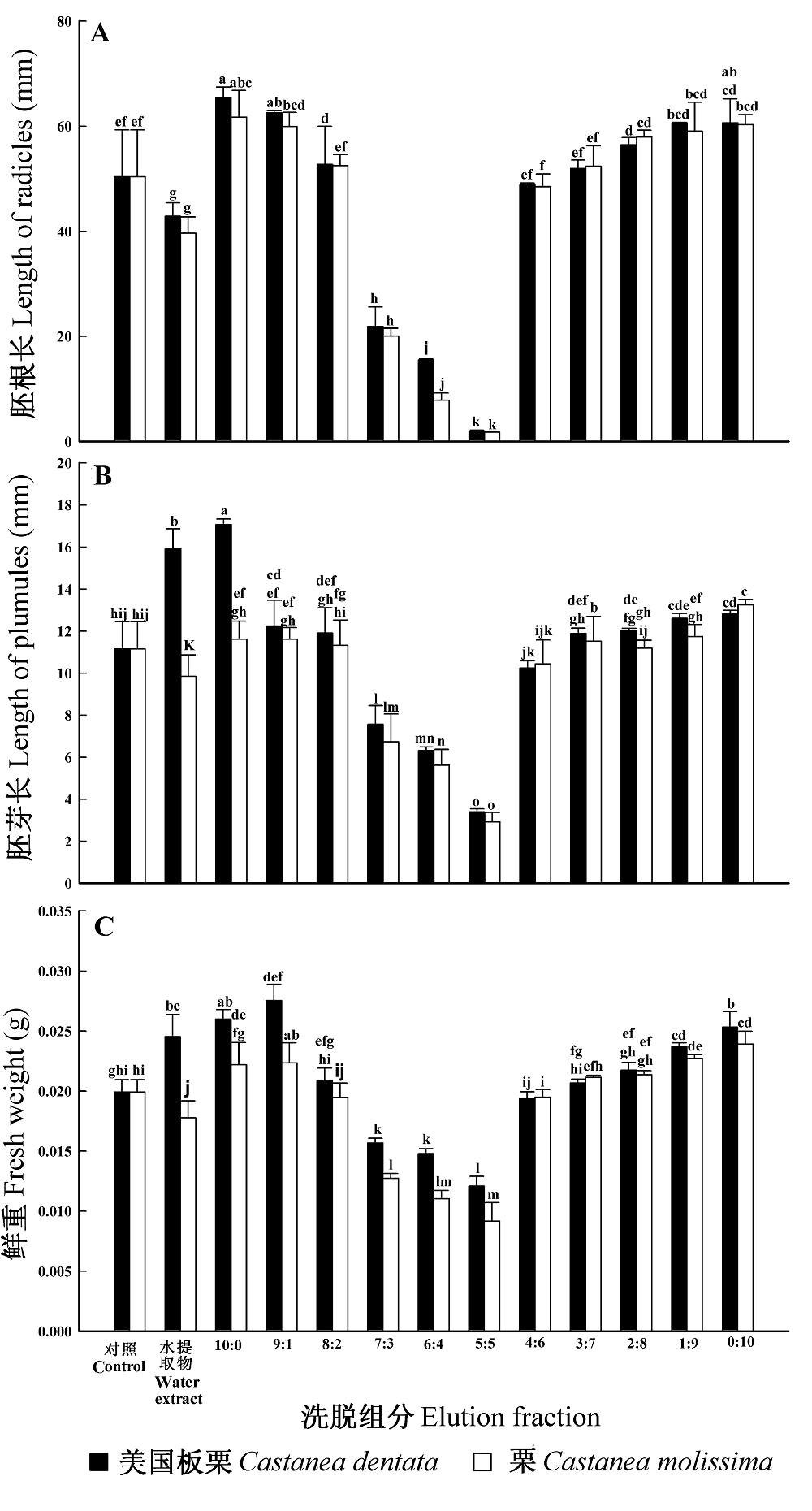
Fig. 5 Effect of isolated components of Castanea dentata and C. molissima on lettuce seedlings (mean ± SD, n = 3). A, Radicle. B, Plumule. C, Fresh weight. Means marked with different letters are significantly different according to least significant difference multiple comparisons (p = 0.05)
| [1] | Anderson PJ (1914). The morphology and life history of the chestnut blight fungus. Cornell University, Harrisburg. 44. |
| [2] | Anderson TW (1974). The chestnut pollen decline as a time horizon in lake sediments in eastern North America. Canadian Journal of Earth Science, 11, 678-685. |
| [3] | Baldwin T (2003). Finally, proof of weapons of mass destruction. Science Signaling, 203, 42. |
| [4] | Barakat A, Staton M, Cheng CH, Park J, Yassin NBM, Ficklin S, Yeh CC, Hebard F, Baier K, Powell W, Schuster SC, Wheeler N, Abbott A, Carlson JE, Sederoff R (2012). Chestnut resistance to the blight disease: insights from transcriptome analysis. BioMed Central Plant Biology, 12, 38. |
| [5] | Barreira JC, Casal S, Ferreira IC, Oliveira MB, Pereira JA (2009). Nutritional, fatty acid and triacylglycerol profiles of Castanea sativa Mill. cultivars: a compositional and chemometric approach. Journal of Agricultural and Food Chemistry, 57, 2836-2842. |
| [6] | Beattie RK, Diller JD (1954). Fifty years of chestnut blight in America. Journal of Forestry, 52, 323-329. |
| [7] | Bennett AJ, Bending GD, Chandler D, Hilton S, Mills P (2012). Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotations. Biological Reviews, 87, 52-71. |
| [8] | Blanco JA (2007). The representation of allelopathy in ecosystem-level forest models. Ecological Modelling, 209, 65-77. |
| [9] | Bounous G (2005). The chestnut: a multipurpose resource for the new millennium. Acta Horticulturae, 693, 33-138. |
| [10] | Callaway RM, Ridenour WM (2004). Novel weapons: invasive success and the evolution of increased competitive ability. The Ecological Society of America, 2, 436-443. |
| [11] | Chou CH, Leu LL (1992). Allelopathic substances and interactions of Delonix regia (Boj) Raf. Journal of Chemical Ecology, 18, 2285-2303. |
| [12] | de Albuquerque MB, Dos Santos RC, Lima LM, Melo PD, Nogueira RJMC, da Cämara CAG, de Rezende Ramos AD (2011). Allelopathy, an alternative tool to improve cropping systems. Agronomy for Sustainable Development, 31, 379-395. |
| [13] | Delcourt PA, Delcourt HR (1998). The influence of prehistoric human set fires on oak-chestnut forests in the southern Appalachians. Castanea, 63, 337-345. |
| [14] | Djurdjević L, Mitrović M, Gajić G, Jarić S, Kostić O, Oberan L, Pavlović P (2011). An allelopathic investigation of the domination of the introduced invasive Conyza canadensis L. Flora, 206, 921-927. |
| [15] | Elliott KJ, Swank WT (2008). Long-term changes in forest composition and diversity following early logging (1919-1923) and the decline of American chestnut (Castanea dentata). Plant Ecology, 197, 155-172. |
| [16] | Exum EM (1992). Tree in a coma. American Forests, 98, 20-25, 59. |
| [17] | Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KHM (2011). The role of allelopathy in agricultural pest management. Pest Management Science, 67, 493-506. |
| [18] | Foster DR, Clayden S, Orwig DA, Hall B, Barry S (2002). Oak, chestnut and fire: climatic and cultural controls of long-term forest dynamics in New England, USA. Journal of Biogeography, 29, 1359-1379. |
| [19] | Freinkel S (2007). American Chestnut: the Life, Death, and Rebirth of a Perfect Tree. University of California Press, Los Angeles. 75. |
| [20] | Gounga ME, Xu SY, Wang Z, Yang WG (2008). Effect of whey protein isolate-pullulan edible coatings on the quality and shelf life of freshly roasted and freeze-dried Chinese chestnut. Food Engineering and Physical Properties, 73, 155-161. |
| [21] | Hebard FV (2006). The backcross breeding program of the American chestnut foundation. Journal of the American Chestnut Foundation, 19, 55-78. |
| [22] | Husaain F, Ilahi I, Malik SA, Dasti AA, Ahmad B (2011). Allelopathic effects of rain leachates and root exudates of Cenchrus ciliaris L. and Bothriochloa pertusa (L.) A. camus. Pakistan Journal of Botany, 43, 341-350. |
| [23] | Leather GR, Einhelling FA (1986). Bioassays in the study of allelopathy. In: Putnam AR, Tang CS eds. The Science of Allelopathy. John Wiley, Sons, New York. 133-145. |
| [24] |
Li ZH, Wang Q, Ruan X, Pan CD, Jiang DA (2010). Phenolics and plant allelopathy. Molecules, 15, 8933-8952.
DOI URL |
| [25] | Lord W (2005). Wildlife food: the pre-blight chestnut and the post-blight acorn. Journal of the American Chestnut Foundation, 6, 29-32. |
| [26] |
Lutts RH (2004). Manna from god: the American chestnut trade in southwestern Virginia. Environmental History, 9, 497-525.
DOI URL |
| [27] |
McCormick JF, Platt RB (1980). Recovery of an Appalachian forest following the chestnut blight or catherine keever― you were right! American Midland Naturalist, 104, 264-273.
DOI URL |
| [28] | Mitrović M, Jarić S, Djurdjević L, Karadzić B, Gajić G, Kostić O, Oberan LJ, Pavlović D, Pavlović M, Pavlović P (2012). Allelopathic and environmental implications of plant phenolic compounds. Allelopathy Journal, 29, 177-197. |
| [29] |
Nowacka J, O1eszek W (1994). Determination of alfalfa (Medicago sativa) saponins by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry, 42, 727-730.
DOI URL |
| [30] | Pan CD, Wang Q, Ruan X, Li ZH (2009). Biological activity and quantification of potential auto-toxins from the leaves of Picea schrenkiana. Chinese Journal of Plant Ecology, 33, 186-196. (in Chinese with English abstract) |
| [ 潘存德, 王强, 阮晓, 李兆慧 (2009). 天山云杉针叶水提取物自毒效应及自毒物质的分离鉴定. 植物生态学报, 33, 186-196.] | |
| [31] |
Pellissier F, Souto XC (1999). Allelopathy in northern temperate and boreal semi-natural woodland. Critical Reviews in Plant Sciences, 18, 637-652.
DOI URL |
| [32] | Pereira-Lorenzo S, Ramos-Cabrer AM (2004). Chestnut, an ancient crop with future. Production Practices and Quality Assessment of Food Crops, 1, 105-161. |
| [33] |
Seal AN, Pratley JE, Haig T, An M (2004). Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. Journal of Chemical Ecology, 30, 1647-1662.
DOI URL |
| [34] |
Sodaeizadeh H, Rafieiolhossaini M, Havlík J, van Damme P (2009). Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Regulation, 59, 227-236.
DOI URL |
| [35] |
Vandermast DB, van Lear DH, Clinton BD (2002). American chestnut as an allelopath in the southern Appalachians. Forest Ecology and Management, 165, 173-181.
DOI URL |
| [36] | Zeng RS (1999). Review on bioassay methods for allelopathy research. Chinese Journal of Applied Ecology, 10, 123-126. (in Chinese with English abstract) |
| [ 曾任森 (1999). 化感作用研究中的生物测定方法综述. 应用生态学报, 10, 123-126.] | |
| [37] |
Zhang JH, Mao ZQ, Wang LQ, Shu HR (2007). Bioassay and identification of root exudates of three fruit tree species. Journal of Integrative Plant Biology, 49, 257-261.
DOI URL |
| [38] |
Zhang M, Chen HX, Zhang Y (2011). Physicochemical, thermal, and pasting properties of Chinese chestnut (Castanea mollissima Bl.) starches as affected by different drying methods. Starch, 63, 260-267.
DOI URL |
| [1] | CHEN Bao-Ming, WEI Hui-Jie, CHEN Wei-Bin, ZHU Zheng-Cai, YUAN Ya-Ru, ZHANG Yong-Long, LAN Zhi-Gang. Effects of plant invasion on soil nitrogen transformation processes and its associated microbes [J]. Chin J Plant Ecol, 2018, 42(11): 1071-1081. |
| [2] | LI Xiao-Feng, XU Xiao, WANG Bi-Xia, HUANG You-You, WANG Zhi-Feng, LI Jun-Yu. Effects of forest litter layer on regeneration of Populus cathayana natural population in Xiaowutai Mountains in China [J]. Chin J Plant Ecol, 2012, 36(2): 109-116. |
| [3] | SHEN Jian-Hong, ZENG Bo, LEI Shu-Tong, SU Xiao-Lei, HUANG Wen-Jun. Seed submergence tolerance of four annual species growing in the water-level-fluctuation zone of Three Gorges Reservoir, China, and effects of long-term submergence on their seed germination [J]. Chin J Plant Ecol, 2011, 35(3): 237-246. |
| [4] | WANG Chuan-Hua, LI Jun-Qing, CHEN Fang-Qing, YANG Ying. Factors affecting seedling regeneration of Liquidambar formosana in the L. formosana forests in hilly regions of Southeast Hubei, China [J]. Chin J Plant Ecol, 2011, 35(2): 187-194. |
| [5] | WANG Hua-Tian, YANG Yang, WANG Yan-Ping, JIANG Yue-Zhong, WANG Zong-Qin. Effects of exogenous phenolic acids on nitrate absorption and utilization of hydroponic cuttings of Populus × euramericana ‘Neva’ [J]. Chin J Plant Ecol, 2011, 35(2): 214-222. |
| [6] | WANG Ming-Dao, CHEN Hong-Ge, LIU Xin-Yu, GAO Yu-Qian, WU Kun, JIA Xin-Cheng. ISOLATION AND IDENTIFICATION OF ALLELOCHEMICALS FROM REHMANNIA GLUTINOSA THAT AFFECT SESAMUM INDICUM [J]. Chin J Plant Ecol, 2009, 33(6): 1191-1198. |
| [7] | YU Xing-Jun, YU Dan, MA Ke-Ping. RELATIONSHIPS BETWEEN ALLELOPATHY AND INVASIVENESS BY EUPATORIUM ADENOPHORUM AT DIFFERENT SITES [J]. Chin J Plan Ecolo, 2004, 28(6): 773-780. |
| [8] | ZHEN Wen-Chao, CAO Ke-Qiang, DAI Li, ZHANG Xue-Ying. SIMULATION OF AUTOTOXICITY OF STRAWBERRY ROOT EXUDATES UNDER CONTINUOUS CROPPING [J]. Chin J Plan Ecolo, 2004, 28(6): 828-832. |
| [9] | CHEN Long-Chi, LIAO Li-Ping, WANG Si-Long. Effect of Vanillin on Nutritient Absorbency of China fir Seedlings [J]. Chin J Plan Ecolo, 2003, 27(1): 41-46. |
| [10] | SONG Qi-Shi, FU Yun, TANG Jian-Wei, FENG Zhi-Li, YANG Chong-Ren. Allelopathic Potential of Eupatorium adeno phorum [J]. Chin J Plan Ecolo, 2000, 24(3): 362-365. |
| [11] | YE Ju-Xin, HE Chi-Quan, CHEN Shao-Feng. Allelopathic Effect of Acorus tatarinowii on Algae Growth [J]. Chin J Plan Ecolo, 1999, 23(4): 379-384. |
| [12] | YU Feng-LAN, MA Mao-Hua, KONG Ling-Shao. Study on Allelopathic Effects of Essential Oil from Artemisia ordosica [J]. Chin J Plan Ecolo, 1999, 23(4): 345-350. |
| [13] | Zhu Xinru, Wang Dali. Potential Effect of Extracts of Roots of Malus pumila and Populus canadensis on Wheat Growth [J]. Chin J Plan Ecolo, 1997, 21(3): 226-233. |
| [14] | Wang Da-li, Zhu Xin-ru. Allelopathic Research of Ambrosia trifida [J]. Chin J Plan Ecolo, 1996, 20(4): 330-337. |
| [15] | Wang Xiang-rong, Song Yong-chang. Studies on the Allelopathy of the Maceration Extracts of Woodwardi japonica and Hicriopteris glauca [J]. Chin J Plan Ecolo, 1993, 17(2): 143-154. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn