

Chin J Plant Ecol ›› 2013, Vol. 37 ›› Issue (9): 830-838.DOI: 10.3724/SP.J.1258.2013.00087
• Research Articles • Previous Articles Next Articles
ZHAO Ha-Lin1,*( ), QU Hao1, ZHOU Rui-Lian2, LI Jin1, PAN Cheng-Chen1, WANG Jin2
), QU Hao1, ZHOU Rui-Lian2, LI Jin1, PAN Cheng-Chen1, WANG Jin2
Received:2013-01-29
Accepted:2013-06-28
Online:2013-01-29
Published:2013-09-02
Contact:
ZHAO Ha-Lin
ZHAO Ha-Lin, QU Hao, ZHOU Rui-Lian, LI Jin, PAN Cheng-Chen, WANG Jin. Effects of sand burial on growth in two psammophyte seedlings and differences in their physiological responses[J]. Chin J Plant Ecol, 2013, 37(9): 830-838.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.3724/SP.J.1258.2013.00087
| 沙埋深度 Sand burial depth (cm) | 细沙 Fine sand (%) | 黏粉粒 Clay + silt (%) | 容重 Bulk density (g·cm-3) | 温度 Temperature (℃) | 含水量 Moisture (%) | 硬度 Hardness (kg·cm-2) | 光照强度 Light intensity (lx) | 有机碳 Organic carbon (g·kg-1) | 全氮 Total nitrogen (g·kg-1) | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 72.96a | 0.18a | 1.58a | 33.2a | 4.5a | 0.121a | 67 900a | 0.68a | 0.088a | 8.31a |
| 10 | 70.0a | 1.2a | 1.60a | 21.2b | 16.2b | 0.159b | 80b | 0.73b | 0.102b | 8.45a |
| 20 | 69.1a | 1.6a | 1.56a | 20.8b | 20.7b | 0.187c | 0c | 0.65a | 0.098b | 8.29a |
Table 1 Comparison on soil physical-chemical properties in different sand burial depths
| 沙埋深度 Sand burial depth (cm) | 细沙 Fine sand (%) | 黏粉粒 Clay + silt (%) | 容重 Bulk density (g·cm-3) | 温度 Temperature (℃) | 含水量 Moisture (%) | 硬度 Hardness (kg·cm-2) | 光照强度 Light intensity (lx) | 有机碳 Organic carbon (g·kg-1) | 全氮 Total nitrogen (g·kg-1) | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 72.96a | 0.18a | 1.58a | 33.2a | 4.5a | 0.121a | 67 900a | 0.68a | 0.088a | 8.31a |
| 10 | 70.0a | 1.2a | 1.60a | 21.2b | 16.2b | 0.159b | 80b | 0.73b | 0.102b | 8.45a |
| 20 | 69.1a | 1.6a | 1.56a | 20.8b | 20.7b | 0.187c | 0c | 0.65a | 0.098b | 8.29a |
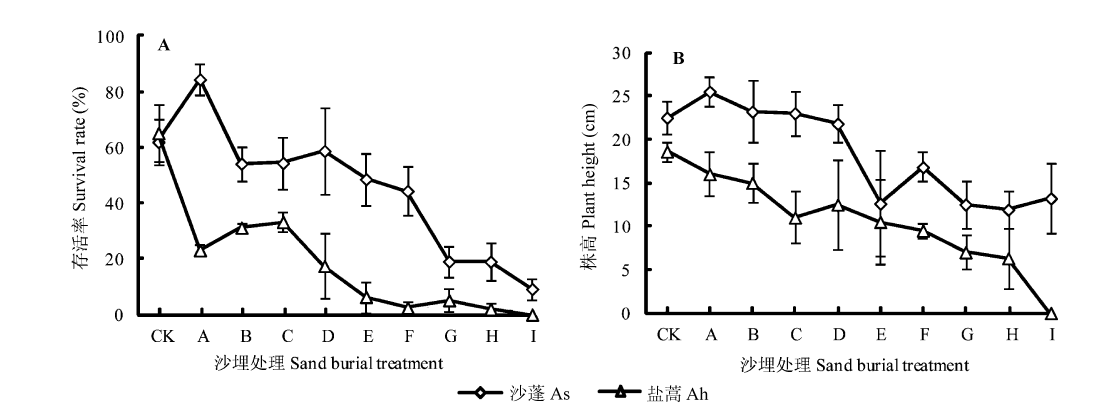
Fig. 1 Survival rate (A) and plant height (B) of the two species in different treatments (mean ± SD). CK, A, B, C, D, E, F, G, H, I, sand burial depth 0% and 25%, 50%, 75% and 100% of seedling height and 2, 4, 6, 8 and 10 cm above seedlings, respectively. Ah, Artemisia halodendron; As, Agriophyllum squarrosum.
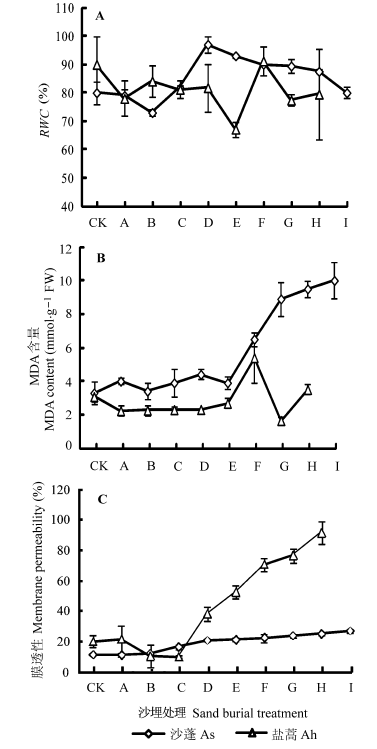
Fig. 2 Relative water content (RWC) (A), malonaldehyde (MDA) content (B) and membrane permeability (C) of leaves (mean ± SD). Sand burial treatment see Ah,Artemisia halodendron; As,Agriophyllum squarrosum.
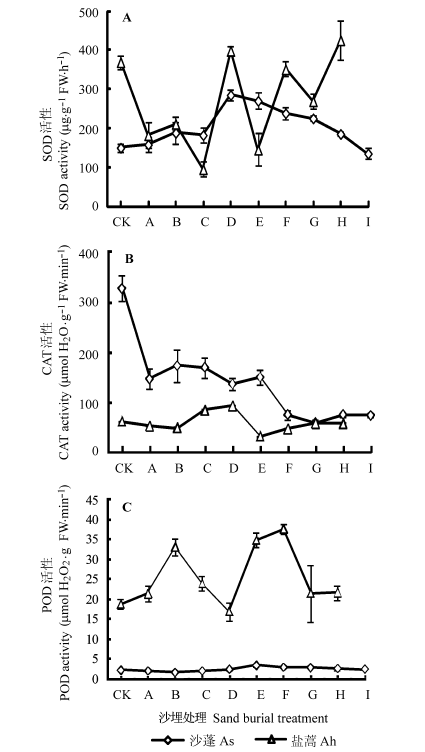
Fig. 3 Activities of superoxide dismutase (SOD) (A), catalase (CAT) (B) and peroxidase (POD) (C) (mean ± SD). Sand burial treatment see Ah,Artemisia halodendron; As,Agrio- phyllum squarrosum.
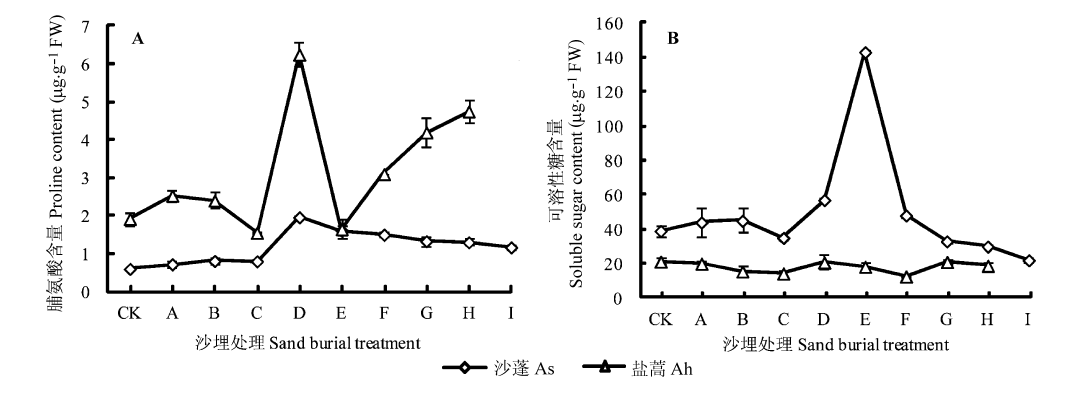
Fig. 4 Variation of proline (A) and soluble sugar (B) content in different sand burial treatments (mean ± SD). Sand burial treatment see Ah,Artemisia halodendron; As,Agriophyllum squarrosum.
| 项目 Item | 物种 Species | 存活率 Survival rate | 株高 Plant height | 脯氨酸含量 Proline content | 可溶性糖 含量 Soluble sugar content | SOD活性 SOD activity | CAT活性 CAT activity | POD活性 POD activity | MDA含量 MDA content | PF |
|---|---|---|---|---|---|---|---|---|---|---|
| 株高 Plant height | 沙蓬 As | 0.861** | 1.000 | |||||||
| 盐蒿 Ah | 0.844** | 1.000 | ||||||||
| MDA含量 MDA content | 沙蓬 As | -0.897** | -0.777** | 0.285 | -0.388 | 0.171 | -0.748* | 0.372 | 1.000 | |
| 盐蒿 Ah | -0.221 | -0.173 | -0.024 | -0.548 | 0.445 | -0.289 | 0.504 | 1.000 | ||
| PF | 沙蓬 As | -0.862** | -0.880** | 0.687* | 0.011 | 0.282 | -0.781* | 0.674* | 0.842** | 1.000 |
| 盐蒿 Ah | -0.771* | -0.825** | 0.512 | 0.085 | 0.520 | -0.284 | 0.106 | 0.400 | 1.000 | |
| FWC | 沙蓬 As | -0.175 | -0.457 | 0.902** | 0.457 | 0.841** | -0.365 | 0.762* | 0.175 | 0.568 |
| 盐蒿 Ah | 0.443 | 0.335 | 0.051 | -0.294 | 0.491 | 0.277 | -0.036 | 0.526 | -0.170 |
Table 2 Correlation analysis among plant growth properties and physiological indexes
| 项目 Item | 物种 Species | 存活率 Survival rate | 株高 Plant height | 脯氨酸含量 Proline content | 可溶性糖 含量 Soluble sugar content | SOD活性 SOD activity | CAT活性 CAT activity | POD活性 POD activity | MDA含量 MDA content | PF |
|---|---|---|---|---|---|---|---|---|---|---|
| 株高 Plant height | 沙蓬 As | 0.861** | 1.000 | |||||||
| 盐蒿 Ah | 0.844** | 1.000 | ||||||||
| MDA含量 MDA content | 沙蓬 As | -0.897** | -0.777** | 0.285 | -0.388 | 0.171 | -0.748* | 0.372 | 1.000 | |
| 盐蒿 Ah | -0.221 | -0.173 | -0.024 | -0.548 | 0.445 | -0.289 | 0.504 | 1.000 | ||
| PF | 沙蓬 As | -0.862** | -0.880** | 0.687* | 0.011 | 0.282 | -0.781* | 0.674* | 0.842** | 1.000 |
| 盐蒿 Ah | -0.771* | -0.825** | 0.512 | 0.085 | 0.520 | -0.284 | 0.106 | 0.400 | 1.000 | |
| FWC | 沙蓬 As | -0.175 | -0.457 | 0.902** | 0.457 | 0.841** | -0.365 | 0.762* | 0.175 | 0.568 |
| 盐蒿 Ah | 0.443 | 0.335 | 0.051 | -0.294 | 0.491 | 0.277 | -0.036 | 0.526 | -0.170 |
| [1] | Bao SD (2000). Soil and Agricultural Chemistry Analysis. China Agricultural Press, Beijing. (in Chinese) |
| [鲍士旦 (2000). 土壤农业化学分析. 中国农业出版社, 北京.] | |
| [2] | Benvenuti S, Macchia M, Miele S (2001). Light, temperature and burial depth effects on Rumex obtusifolius seed germination and emergence. Weed Research, 41, 177-186. |
| [3] | Harris D, Davy AJ (1988). Carbon and nutrient allocation in Elymus farctus seedlings after burial with sand. Annals of Botany, 61, 147-157. |
| [4] |
Izanloo A, Condon AG, Langridge P, Tester M, Schnurbusch T (2008). Different mechanisms of adaptation to cyclic water stress in two south Australian bread wheat cultivars. Journal of Experimental Botany, 59, 3327-3346.
URL PMID |
| [5] |
Jouili H, El Ferjani E (2003). Changes in antioxidant and lignifying enzyme activities in sunflower roots ( Helianthus annuus L.) stressed with copper excess. Comptes Rendus Biologies, 326, 639-644.
DOI URL PMID |
| [6] | Li HJ, Wang P, Baoyin DLGE, Tian PF (2012). Response of seed germination and seedling emergence of Pugionium gaerth to different sand burial depth. Journal of Inner Mongolia Agricultural University, 33(3),39-44. (in Chinese with English abstract) |
| [李海静, 王萍, 宝音德力格尔, 田鹏飞 (2012). 沙埋深度对沙芥属植物种子萌发和出苗的影响. 内蒙古农业大学学报, 33(3),39-44.] | |
| [7] | Li QY, Fang HY (2008). Effects of sand burial depth on seedling emergence and growth of Reaumuria soongorica. Bulletin of Soil and Water Conservation, 28, 30-33. (in Chinese with English abstract) |
| [李秋艳, 方海燕 (2008). 沙埋对红砂幼苗出土和生长的影响. 水土保持通报, 28, 30-33.] | |
| [8] | Li WT, Zhang C, Wang F, Zheng MQ, Zheng TR, Zhang F (2010). Effects of sand burial and water supply on seedlings growth of two dominant psammophytes in Mu Us sandland. Acta Ecologica Sinica, 30, 1192-1199. (in Chinese with English abstract) |
| [李文婷, 张超, 王飞, 郑明清, 郑元润, 张峰 (2010). 沙埋与供水对毛乌素沙地两种重要沙生植物幼苗生长的影响. 生态学报, 30, 1192-1199.] | |
| [9] | Liu B, Liu ZM, Guan DX (2008). Seedling growth variation in response to sand burial in four Artemisia species from different habitats in the semi-arid dune field. Trees, 22, 41-47. |
| [10] | Liu SE, Feng ZW, Zhao DC (1959). On some matters of principle in China plant division. Acta Botanica Sinica, 8, 87-105. (in Chinese with English abstract) |
| [刘慎谔, 冯宗炜, 赵大昌 (1959). 关于中国植物区划的若干原则问题. 植物学报, 8, 87-105.] | |
| [11] | Luisa MM, Maun MA (1999). Responses of dune mosses to experimental burial by sand under natural and greenhouse conditions. Plant Ecology, 145, 209-219. |
| [12] | Martínez ML, Moreno-Casasola P (1996). Effects of burial by sand on seedling growth and survival in six tropical sand dune species from the Gulf of Mexico. Journal of Coastal Research, 12, 406-419. |
| [13] | Maun MA (1996). The effects of burial by sand on survival and growth of Calamovilfa longifolia. Écoscience, 3, 93-100. |
| [14] | Maun MA, Lapierre J (1984). The effects of burial by sand on Ammophila breviligulata. Journal of Ecology, 72, 827-839. |
| [15] | Mi ZY, Zhou DD, Wu YD (2005). Influence of wind erosion and sand bury on the morphological characteristics of Salix psammophila. Inner Mongolia Forestry Science & Technology, (1),9-13. (in Chinese with English abstract) |
| [米志英, 周丹丹, 吴亚东 (2005). 风蚀沙埋对沙柳形态特征的影响. 内蒙古林业科技, (1),9-13.] | |
| [16] | Pagter M, Bragato C, Brix H (2005). Tolerance and physiological responses of Phragmites australis to water deficit. Aquatic Botany, 81, 285-299. |
| [17] | Qayyum A, Razzaq A, Ahmad M, Jenks MA (2011). Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat ( Triticum aestivum L.) genotypes. African Journal of Biotechnology, 10, 14038-14045. |
| [18] |
Shi L, Zhang ZJ, Zhang CY (2004). Effects of sand burial on survival, growth, gas exchange and biomass allocation of Ulmus pumila seedlings in the Hunshandak Sandland, China. Annals of Botany, 94, 553-560.
URL PMID |
| [19] | Sykes MT, Wilson JB (1990). An experimental investigation into the response of New Zealand sand dune species to different depths of burial by sand. Acta Botanica Neerlandica, 39, 171-181. |
| [20] | Wang J, Zhou RL, Zhao HL, Zhao YH, Hou YP (2012). Growth and physiological adaptation of Messerschmidia sibirica to sand burial on coastal sandy. Acta Ecologica Sinica, 32, 4291-4299. (in Chinese with English abstract) |
| [王进, 周瑞莲, 赵哈林, 赵彦宏, 侯玉萍 (2012). 海滨沙地砂引草对沙埋的生长和生理适应对策. 生态学报, 32, 4291-4299.] | |
| [21] |
Yang HL, Cao ZP, Dong M, Ye YZ, Huang ZY (2007). Effects of sand burying on caryopsis germination and seedling growth of Bromus inermis Leyss. Chinese Journal of Applied Ecology, 18, 2438-2443. (in Chinese with English abstract)
URL PMID |
|
[杨慧玲, 曹志平, 董鸣, 叶永忠, 黄振英 (2007). 沙埋对无芒雀麦种子萌发和幼苗生长的影响. 应用生态学报, 18, 2438-2443.]
PMID |
|
| [22] | Zhang ZL, Zhai WJ (2003). Experimental Guide of Plant Physiology. Higher Education Press, Beijing. (in Chinese) |
| [张志良, 瞿伟菁 (2003). 植物生理学实验指导. 高等教育出版社, 北京.] | |
| [23] | Zhao HL (2012). Desert Ecology. Science Press, Beijing. (in Chinese) |
| [赵哈林 (2012). 沙漠生态学. 科学出版社, 北京.] | |
| [24] | Zhao HL, He YH, Yue GY (2010). Effects of wind blow and sand burial on the seedling growth and photosynthetic and transpiration rates of desert plants. Chinese Journal of Ecology, 29, 413-419. (in Chinese with English abstract) |
| [赵哈林, 何玉惠, 岳广阳 (2010). 风吹、沙埋对沙地植物幼苗生长和光合蒸腾特性的影响. 生态学杂志, 29, 413-419.] | |
| [25] | Zhao HL, Zhao XY, Zhang TH (2004). Plants Adaption Strategies and Vegetation Stability in the Desertification Process. China Ocean Press, Beijing. (in Chinese) |
| [赵哈林, 赵学勇, 张铜会 (2004). 沙漠化过程中植物的适应对策及植被稳定性机理. 海洋出版社, 北京.] | |
| [26] | Zhou RL (2001). The physiological mechanism of plant succession in Kerqin Sandy Land. Arid Zone Research, 18(3),13-19. (in Chinese with English abstract) |
| [周瑞连 (2001). 科尔沁沙地植物演替的生理机制. 干旱区研究, 18(3),13-19.] | |
| [27] | Zhou RL, Wang HO (1999). Correlation between resistance to dehydration and lipid peroxidation of desert plants under atmosphere dehydration and high temperature stresses. Journal of Desert Research, 19, 60-64. (in Chinese with English abstract) |
| [周瑞莲, 王海鸥 (1999). 在干旱、高温胁迫中沙生植物抗脱水性与膜脂过氧化关系的研究. 中国沙漠, 19, 60-64.] |
| [1] | WU Qi-Mei,ZHOU Qi-Xing. Eco-physiological responses of Polytrichum commune to soil contamination by polychlorinated biphenyls [J]. Chin J Plan Ecolo, 2015, 39(3): 275-282. |
| [2] | LÜ Jin-Hui,REN Lei,LI Yan-Feng,WANG Xuan,ZHAO Xia-Lu,ZHANG Chun-Lai. Responses to salt stress among different genotypes of tea Chrysanthemum [J]. Chin J Plant Ecol, 2013, 37(7): 656-664. |
| [3] | WANG Hai-Cui, HU Lin-Lin, LI Min, CHEN Wei-Feng, WANG Ying, ZHOU Jia-Jia. Growth effects and accumulations of polycyclic aromatic hydrocarbons (PAHs) in rape [J]. Chin J Plant Ecol, 2013, 37(12): 1123-1131. |
| [4] | XU Hao, LI Yan, XIE Jing-Xia, CHENG Lei, ZHAO Yan, LIU Ran. Influence of solar radiation and groundwater table on carbon balance of phreatophytic desert shrub Tamarix [J]. Chin J Plant Ecol, 2010, 34(4): 375-386. |
| [5] | LI Yang, HUANG Jian-Hui. PHOTOSYNTHETIC PHYSIOLOGICAL RESPONSES OF GLYCYRRHIZA URALENSISUNDER DIFFERENT WATER AND NUTRIENT SUPPLIES IN KUBUQI DESERT, CHINA [J]. Chin J Plant Ecol, 2009, 33(6): 1112-1124. |
| [6] | LIU Bin-Yang, LIU Wei-Qiu, LEI Chun-Yi, ZHANG Yi-Shun. PHYSIOLOGICAL RESPONSES OF THREE BRYOPHYTE SPECIES OF SOUTH CHINA TO SIMULATED NITROGEN DEPOSITION [J]. Chin J Plant Ecol, 2009, 33(1): 141-149. |
| [7] | LIU Peng, YANG YS, XU Gen-Di, GUO Shui-Liang, WANG Min. PHYSIOLOGICAL RESPONSE OF FOUR SOUTHERN HERBACEOUS PLANTS TO ALUMINIUM STRESS [J]. Chin J Plant Ecol, 2005, 29(4): 644-651. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn