

植物生态学报 ›› 2019, Vol. 43 ›› Issue (8): 718-728.DOI: 10.17521/cjpe.2019.0036
• 研究论文 • 上一篇
崔利1,2,3,郭峰1,2,3,张佳蕾1,2,3,杨莎1,2,3,王建国1,2,3,孟静静1,2,3,耿耘1,2,3,李新国1,2,3,*( ),万书波2,3,4,*(
),万书波2,3,4,*( )
)
收稿日期:2019-02-21
修回日期:2019-08-05
出版日期:2019-08-20
发布日期:2020-01-03
通讯作者:
李新国 ORCID:0000-0003-3277-9808,万书波
基金资助:
CUI Li1,2,3,GUO Feng1,2,3,ZHANG Jia-Lei1,2,3,YANG Sha1,2,3,WANG Jian-Guo1,2,3,MENG Jing-Jing1,2,3,GENG Yun1,2,3,LI Xin-Guo1,2,3,*( ),WAN Shu-Bo2,3,4,*(
),WAN Shu-Bo2,3,4,*( )
)
Received:2019-02-21
Revised:2019-08-05
Online:2019-08-20
Published:2020-01-03
Contact:
LI Xin-Guo ORCID:0000-0003-3277-9808,WAN Shu-Bo
Supported by:摘要:
花生(Arachis hypogaea)长期连作导致土壤环境恶化, 严重影响产量和品质。丛枝菌根真菌(AMF)作为有益真菌能够与80%的陆生植物根系形成共生关系, 这种共生体能够改善植物根系微环境, 提高植物对营养物质的吸收和对逆境胁迫的抗性。为了探究AMF对花生连作土壤微环境的影响, 该研究通过对花生连作土壤接种和未接种摩西斗管囊霉(Funneliformis mosseae)试验, 在花生不同生长期检测根际土壤的酶活性、土壤矿物质含量、土壤微生物群落结构和多度的变化情况, 以及对连作花生产量和品质的影响。研究结果表明: 1)摩西斗管囊霉能够显著提高花生根际土壤蔗糖酶、脲酶、碱性磷酸酶和硝酸还原酶的活性; 2)摩西斗管囊霉显著增加花生连作土壤中全氮、全磷、全钾、速效磷和速效钾的含量; 3)摩西斗管囊霉显著降低土壤中有害真菌曲霉菌属(Aspergillus)的多度, 减少镰刀菌属(Fusarium)和赤霉菌属(Gibberella)的多度, 但是没有达到显著水平, 显著增加有益细菌放线菌Gaiella属的多度; 4)摩西斗管囊霉显著提高连作花生的产量, 增加籽仁中蛋白质、油酸和亚油酸的含量。因此, 摩西斗管囊霉能够改善连作花生根际土壤微生态环境, 增强连作土壤对致病菌的抵抗能力, 从而缓解连作障碍对花生根系的危害。
崔利, 郭峰, 张佳蕾, 杨莎, 王建国, 孟静静, 耿耘, 李新国, 万书波. 摩西斗管囊霉改善连作花生根际土壤的微环境. 植物生态学报, 2019, 43(8): 718-728. DOI: 10.17521/cjpe.2019.0036
CUI Li, GUO Feng, ZHANG Jia-Lei, YANG Sha, WANG Jian-Guo, MENG Jing-Jing, GENG Yun, LI Xin-Guo, WAN Shu-Bo. Improvement of continuous microbial environment in peanut rhizosphere soil by Funneliformis mosseae. Chinese Journal of Plant Ecology, 2019, 43(8): 718-728. DOI: 10.17521/cjpe.2019.0036
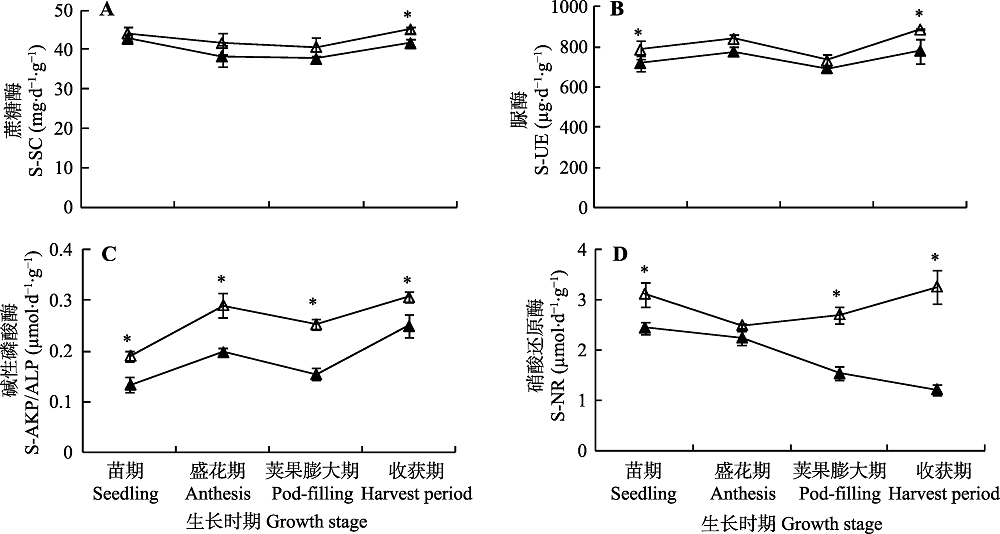
图1 接种摩西斗管囊霉对连作花生不同生长时期的根际土壤蔗糖酶(A)、脲酶(B)、碱性磷酸酶(C)和硝酸还原酶(D)活力的影响(平均值±标准误差)。*表示不同处理之间差异显著(p < 0.05)。▲, 未接种摩西斗管囊霉; △, 接种摩西斗管囊霉。
Fig. 1 Effects of inoculation with Funneliformis mosseae on enzyme activities of solid-sucrase (S-SC)(A), solid-urease (S-UE)(B), solid alkaline-phosphatase (S-AKP/ALP)(C) and solid-nitrate reductase (S-NR)(D) in rhizosphere soil of continuing cropping peanut at different growth stages (mean ± SE). * indicates significant difference between the two treatments (p < 0.05). ▲, without F. mosseae inoculation; △, F. mosseae inoculation.
| 处理 Treatment | 全氮 Total N (g·kg-1) | 全磷 Total P (g·kg-1) | 全钾 Total K (mg·g-1) | |||
|---|---|---|---|---|---|---|
| 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | |
| -AMF | 1.23 ± 0.06 | 0.74 ± 0.02 | 0.59 ± 0.06 | 0.37 ± 0.04 | 6.67 ± 0.22 | 3.57 ± 0.67 |
| +AMF | 1.44 ± 0.06* | 0.82 ± 0.03* | 0.70 ± 0.03* | 0.47 ± 0.07* | 7.41 ± 0.57 | 5.39 ± 0.73* |
表1 接种和未接种摩西斗管囊霉的连作花生根际土壤全氮、全磷和全钾含量比较(平均值±标准误差, n = 3)
Table 1 Comparison of soil contents of total nitrogen, total phosphorus and total potassium between the treatments with and without Funneliformis mosseae inoculation under continuing cropping of peanuts (mean ± SE, n = 3)
| 处理 Treatment | 全氮 Total N (g·kg-1) | 全磷 Total P (g·kg-1) | 全钾 Total K (mg·g-1) | |||
|---|---|---|---|---|---|---|
| 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | |
| -AMF | 1.23 ± 0.06 | 0.74 ± 0.02 | 0.59 ± 0.06 | 0.37 ± 0.04 | 6.67 ± 0.22 | 3.57 ± 0.67 |
| +AMF | 1.44 ± 0.06* | 0.82 ± 0.03* | 0.70 ± 0.03* | 0.47 ± 0.07* | 7.41 ± 0.57 | 5.39 ± 0.73* |
| 处理 Treatment | 碱解氮 Alkaline N (mg·kg-1) | 速效磷 Effective P (g·kg-1) | 速效钾 Effective K (mg·kg-1) | |||
|---|---|---|---|---|---|---|
| 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | |
| -AMF | 73.39 ± 3.66 | 75.77 ± 3.66 | 0.18 ± 0.01 | 0.15 ± 0.01 | 15.40 ± 0.56 | 11.03 ± 0.64 |
| +AMF | 74.86 ± 5.54 | 81.67 ± 7.00 | 0.21 ± 0.02* | 0.18 ± 0.00* | 16.17 ± 0.47 | 13.20 ± 0.66* |
表2 接种摩西斗管囊霉对连作花生根际土壤碱解氮、速效磷和速效钾含量的影响(平均值±标准误差, n = 3)
Table 2 Effects of Funneliformis mosseae inoculation on the soil contents of alkaline nitrogen, available phosphorus and available potassium (mean ± SE, n = 3)
| 处理 Treatment | 碱解氮 Alkaline N (mg·kg-1) | 速效磷 Effective P (g·kg-1) | 速效钾 Effective K (mg·kg-1) | |||
|---|---|---|---|---|---|---|
| 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | 盛花期 Anthesis | 收获期 Harvest period | |
| -AMF | 73.39 ± 3.66 | 75.77 ± 3.66 | 0.18 ± 0.01 | 0.15 ± 0.01 | 15.40 ± 0.56 | 11.03 ± 0.64 |
| +AMF | 74.86 ± 5.54 | 81.67 ± 7.00 | 0.21 ± 0.02* | 0.18 ± 0.00* | 16.17 ± 0.47 | 13.20 ± 0.66* |
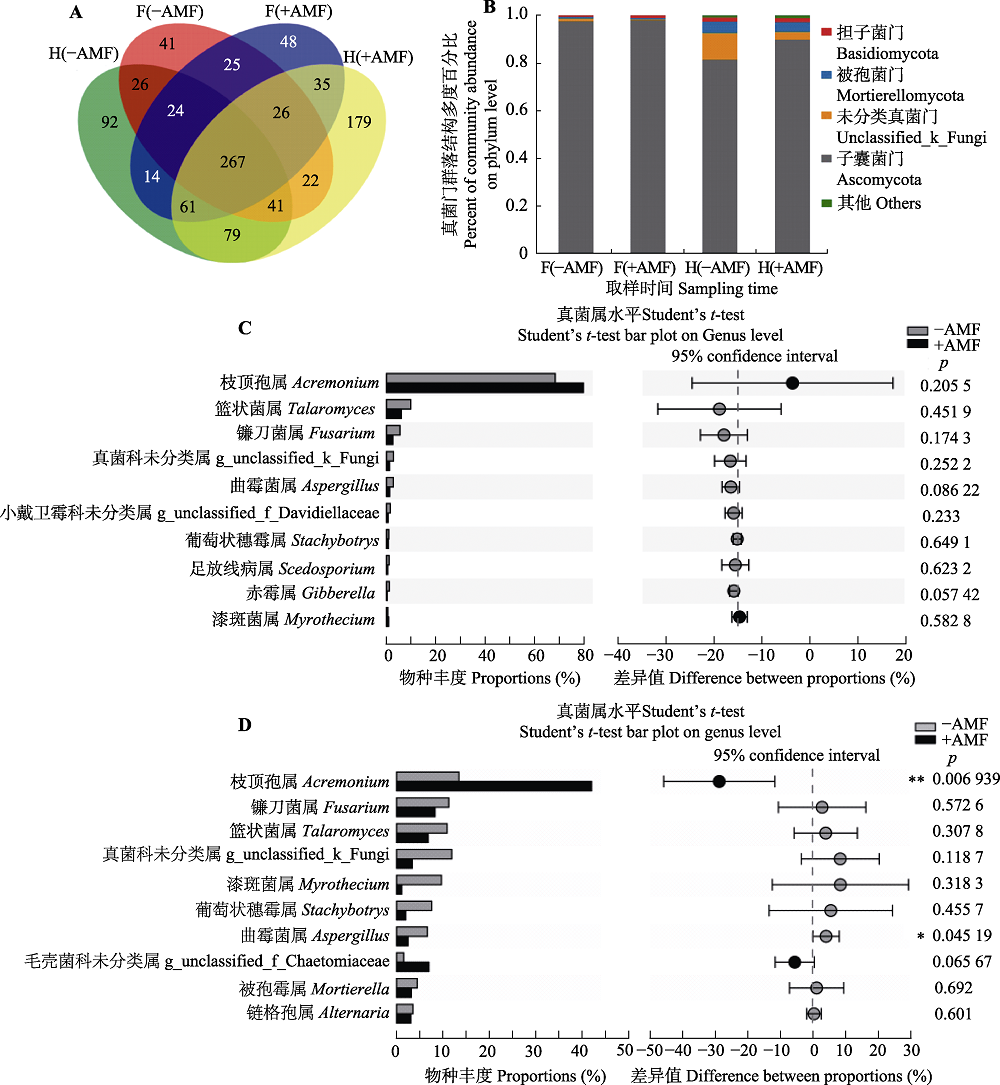
图2 接种和未接种摩西斗管囊霉对花生连作土壤真菌群落结构及多度的影响。A, 不同时期不同处理间真菌分类操作单元丰度的韦恩图。B, 接种摩西斗管囊霉改变花生连作土壤真菌门的多度。C, 接种摩西斗管囊霉对盛花期花生根际土壤真菌属影响不显著(平均值±标准误差, n = 3)。D, 接种摩西斗管囊霉对收获期花生根际土壤真菌属影响显著(平均值±标准误差, n = 3)。-AMF, 未接种摩西斗管囊霉; +AMF, 接种摩西斗管囊霉。F(-AMF), 未接种摩西斗管囊霉的盛花期花生根际土壤; F(+AMF), 接种摩西斗管囊霉的盛花期花生根际土壤; H(-AMF), 未接种摩西斗管囊霉的收获期花生根际土壤; H(+AMF), 接种摩西斗管囊霉的收获期花生根际土壤。*, p < 0.05; **, p < 0.01。
Fig. 2 Effects of with and without Funneliformis mosseae inoculation on the structure and abundance of soil fungi community under continuing cropping of peanut. A, The Venn figure shows the number of fungal operational taxonomic units in different treatments. B, Abundance of soil fungi in continuing cropping of peanut was changed by F. mosseae. C, Abundance of fungal genera were not significantly different between with and without F. mosseae inoculation in the flowering period of continuing cropping peanuts (mean ± SE, n = 3). D, Abundance of some fungal genera were significantly different between with and without F. mosseae inoculation at harvest period (mean ± SE, n = 3). -AMF, without F. mosseae inoculation; +AMF, F. mosseae inoculation. F(-AMF), peanut rhizosphere soil without F. mosseae inoculation during the flowering period; F(+AMF), rhizosphere soil of peanut with F. mosseae inoculation; H(-AMF), peanut rhizosphere soil without F. mosseae inoculation during the harvest period; H(+AMF), peanut rhizosphere soil with F. mosseae inoculation during the harvest period. *, p < 0.05; **, p < 0.01.
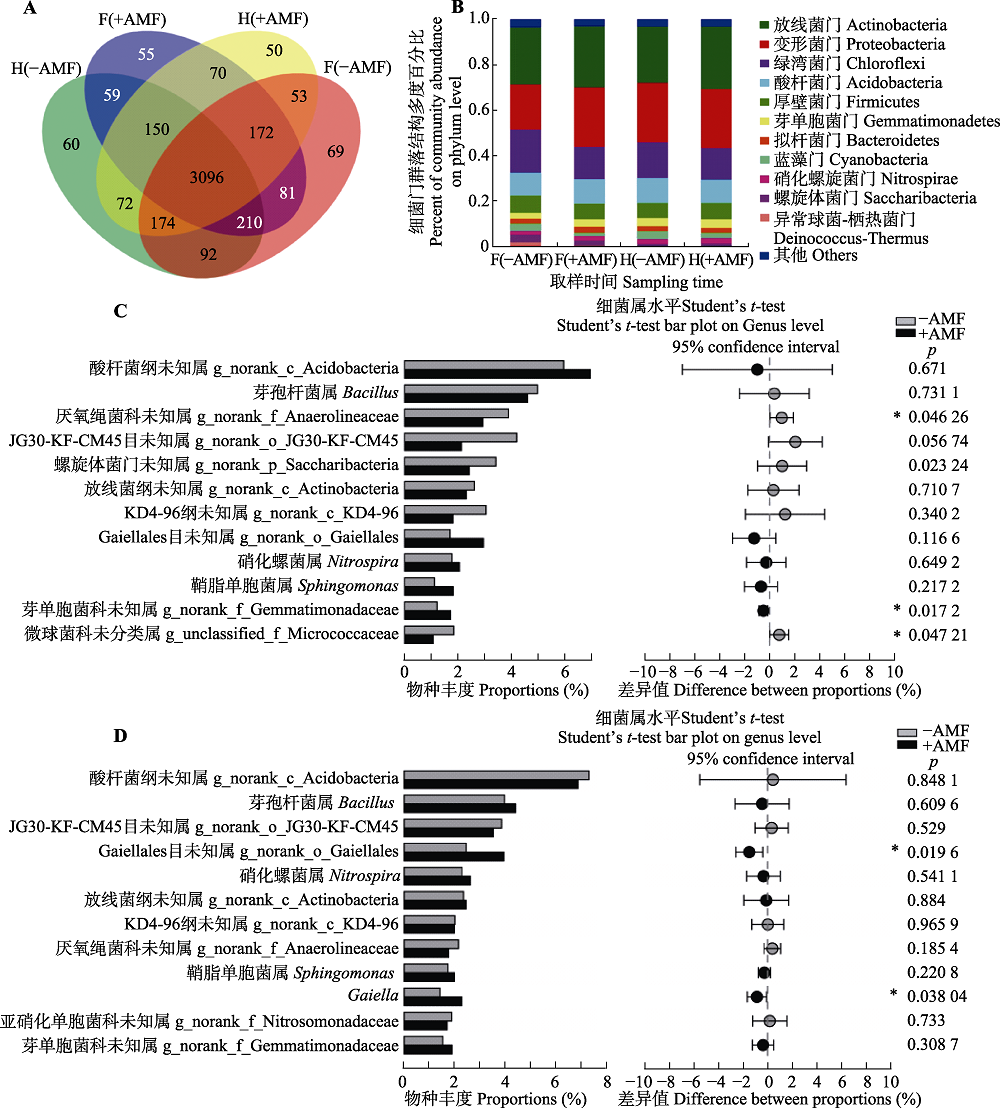
图3 接种摩西斗管囊霉对花生连作土壤细菌群落结构及丰度的影响。A, 不同时期不同处理间细菌操作分类单元丰度的韦恩图。B, 接种摩西斗管囊霉改变细菌门多度。C, 接种摩西斗管囊霉显著改变盛花期花生根际土壤细菌属的多度(平均值±标准误差, n = 3)。D, 接种摩西斗管囊霉显著改变收获期花生根际土壤细菌属的多度(平均值±标准误差, n = 3)。-AMF, 未接种摩西斗管囊霉; +AMF, 接种摩西斗管囊霉。F(-AMF), 未接种摩西斗管囊霉的盛花期花生根际土壤; F (+AMF), 接种摩西斗管囊霉的盛花期花生根际土壤; H(-AMF), 未接种摩西斗管囊霉的收获期花生根际土壤; H(+AMF), 接种摩西斗管囊霉的收获期花生根际土壤。*, p < 0.05。
Fig. 3 Effects of Funneliformis mosseae on the structure and abundance of soil bacterial community in rhizosphere soil of peanut under continuing cropping. A, The Venn figure shows the number of bacterial operational taxonomic units in the two treatments. B, Abundance of soil bacteria in continuous cropping of peanut changed in the F. mosseae inoculation tratement C, Abundance of bacterial genera were significantly different between with and without F. mosseae inoculation in the flowering period of continuing cropping peanuts (mean ± SE, n = 3). D, Abundance of bacterial genera were significantly different between with and without F. mosseae inoculation in the harvesting period of continuous cropping peanuts (mean ± SE, n = 3). -AMF, without F. mosseae inoculation; +AMF, F. mosseae inoculation. F(-AMF), peanut rhizosphere soil without F. mosseae inoculation during the flowering period; F(+AMF), rhizosphere soil of peanut with F. mosseae inoculation; H(-AMF), peanut rhizosphere soil without F. mosseae inoculation during the harvest period; H(+AMF), peanut rhizosphere soil with F. mosseae inoculation during the harvest period. *, p < 0.05.
| 处理 Treatment | 单株结果数 Pod number per plant | 单株果质量 Pod mass per plant (g) | 饱果率 Full fruit rate (%) | 蛋白质 Protein (%) | 总氨基酸 Total amino acid (%) | 油酸 Oleic (%) | 亚油酸 Linoleic (%) |
|---|---|---|---|---|---|---|---|
| -AMF | 34.67 ± 2.08 | 41.85 ± 2.87 | 60.82 ± 0.02 | 18.34 ± 0.17 | 18.29 ± 1.68 | 52.46 ± 1.16 | 24.12 ± 1.37 |
| +AMF | 39.33 ± 0.58* | 52.05 ± 0.79* | 70.33 ± 0.04* | 21,89 ± 0.22* | 21.30 ± 0.97* | 57.38 ± 1.32* | 27.20 ± 1.19* |
表3 接种摩西斗管囊霉对连作花生产量和品质的影响
Table 3 Effects of Funneliformis mosseae inoculation on the yield and quality of continuous cropping peanut
| 处理 Treatment | 单株结果数 Pod number per plant | 单株果质量 Pod mass per plant (g) | 饱果率 Full fruit rate (%) | 蛋白质 Protein (%) | 总氨基酸 Total amino acid (%) | 油酸 Oleic (%) | 亚油酸 Linoleic (%) |
|---|---|---|---|---|---|---|---|
| -AMF | 34.67 ± 2.08 | 41.85 ± 2.87 | 60.82 ± 0.02 | 18.34 ± 0.17 | 18.29 ± 1.68 | 52.46 ± 1.16 | 24.12 ± 1.37 |
| +AMF | 39.33 ± 0.58* | 52.05 ± 0.79* | 70.33 ± 0.04* | 21,89 ± 0.22* | 21.30 ± 0.97* | 57.38 ± 1.32* | 27.20 ± 1.19* |
| [1] | Adolfsson L, Nziengui H, Abreu IN, Šimura J, Beebo A, Herdean A, Aboalizadeh J, Široká J, Moritz T, Novák O, Ljung K, Schoefs B, Spetea C (2017). Enhanced secondary- and hormone metabolism in leaves of arbuscular mcorrhizal Medicago truncatula. Plant Physiology, 175, 392-411. |
| [2] | Azcón-Aguilar C, Barea JM (1997). Arbuscular mycorrhizas and biological control of soil-borne plant pathogens—An overview of the mechanisms involved. Mycorrhiza, 6, 457-464. |
| [3] | Bagyaraj DJ, Manjunath A, Patil RB (1979). Interaction between a vesicular-arbuscular mycorrhiza and rhizobium and their effects on soybean in the field. New Phytologist, 82, 141-145. |
| [4] | Bao SD (1999). Soil Agrochemical Analysis. 3rd edn. China Agricultural Press, Beijing. 42-56, 71-80, 100-108. |
| [ 鲍士旦 (1999). 土壤农化分析. 第三版. 中国农业出版社, 北京. 42-56, 71-80, 100-108.] | |
| [5] | Chen MN, Li X, Yang QL, Chi XY, Pan LJ, Chen N, Yang Z, Wang T, Wang M, Yu SL (2012). Soil eukaryotic microorganism succession as affected by continuous cropping of peanut-pathogenic and beneficial fungi were selected. PLOS ONE, 7, e40659. DOI: 10.1371/journal.pone.0040659. |
| [6] | Chen MN, Li X, Yang QL, Chi XY, Pan LJ, Chen N, Yang Z, Wang T, Wang M, Yu SL (2014). Dynamic succession of soil bacterial community during continuous cropping of peanut ( Arachis hypogaea L.). PLOS ONE, 9, e101355. DOI: 10.1371/journal.pone.0101355. |
| [7] | Cui L, Guo F, Zhang JL, Yang S, Meng JJ, Geng Y, Wang Q, Li XG, Wan SB (2019). Arbuscular mycorrhizal fungi combined with exogenous calcium improves the growth of peanut ( Arachis hypogaea L.) seedlings under continuous cropping. Journal of Integrative Agriculture, 18, 407-416. |
| [8] | Dehne H, Schonbeck F, Baltruschat H (1978). The influence of endotrophic mycorrhiza on plant disease. 3. Chitinase-activity and ornithine-cylce. Journal of Plant Disease Protection, 85, 666-678. |
| [9] | Desjardins AE (2003). Gibberella from A (venaceae) to Z (eae). Annual Review of Phytopathology, 41, 177-198. |
| [10] | Feng HS, Wan SB, Zuo XQ, Cheng B (1999). Changes of main microbial groups in soil and rhizosphere of peanut continuous cropping and their correlation with yield. Peanut Science and Technology, (Suppl. 1), 277-283. |
| [ 封海胜, 万书波, 左学青, 成波 (1999). 花生连作土壤及根际主要微生物类群的变化及与产量的相关. 花生科技, (增刊1), 277-283.] | |
| [11] | Feng Y (2017). Effects of arbuscular mycorrhizal fungi (AMF) on soil microbes and nutrients in rhizosphere of Salvia miltiorrhiza. China Agriculture Information, (10), 57-58. |
| [ 封晔 (2017). 丛枝菌根真菌(AMF)对丹参根际土壤微生物及养分的影响. 中国农业信息, (10), 57-58.] | |
| [12] | Fu XF, Zhang GP, Zhang XW, Ren JH (2016). Effects of PSB and AMF on growth, microorganisms and soil enzyme activities in the rhizosphere of Taxus chinensis var. mairei seedlings. Acta Botanica Boreali-Occidentalia Sinica, 36, 353-360. |
| [ 付晓峰, 张桂萍, 张小伟, 任嘉红 (2016). 溶磷细菌和丛枝菌根真菌接种对南方红豆杉生长及根际微生物和土壤酶活性的影响. 西北植物学报, 36, 353-360.] | |
| [13] | Gao P, Li F, Guo YE, Duan TY (2017). Advances in AM fungi and rhizobium to control plant fungal diseases. Acta Agrestia Sinica, 25, 236-242. |
| [ 高萍, 李芳, 郭艳娥, 段廷玉 (2017). 丛枝菌根真菌和根瘤菌防控植物真菌病害的研究进展. 草地学报, 25, 236-242.] | |
| [14] | Garcia K, Chasman D, Roy S, Ané JM (2017). Physiological responses and gene co-expression network of mycorrhizal roots under K+ deprivation . Plant Physiology, 173, 1811-1823. |
| [15] | Guan SY (1986). Soil Enzyme and Its Research Method. China Agricultural Press, Beijing. 274-276, 294-297, 309-313, 332-333. |
| [ 关松荫 (1986). 土壤酶及其研究方法. 中国农业出版社, 北京. 274-276, 294-297, 309-313, 332-333.] | |
| [16] | Han B, Guo SR, He CX, Yan Y, Yu XC (2012). Effects of arbuscular mycorrhiza fungi on the plant growth, fruit yield and fruit quality of cucumber under salt stress. Chinese Journal of Applied Ecology, 23, 154-158. |
| [ 韩冰, 郭世荣, 贺超兴, 闫妍, 于贤昌 (2012). 丛枝菌根真菌对盐胁迫下黄瓜植株生长果实产量和品质的影响. 应用生态学报, 23, 154-158. ] | |
| [17] | He ZQ, Li HX, Tang HR (2010). Effect of arbuscular mycorrhizal fungi on cucumber rhizoctonia rot. Journal of Sichuan Agricultural University, 28, 200-204. |
| [ 贺忠群, 李焕秀, 汤浩茹 (2010). 丛枝菌根真菌对黄瓜立枯病的影响. 四川农业大学学报, 28, 200-204.] | |
| [18] | Huang YQ, Han XR, Yang JF, Liu N, Liu XH (2011). Studies on the changes of soil microbial communities under peanuts continuous cropping. Chinese Journal of Soil Science, 42, 552-556. |
| [ 黄玉茜, 韩晓日, 杨劲峰, 刘宁, 刘小虎 (2011). 花生连作土壤微生物区系变化研究. 土壤通报, 42, 552-556.] | |
| [19] | Jakobsen I, Gazey C, Abbott LK (2001). Phosphate transport by communities of arbuscular mycorrhizal fungi in intact soil cores. New Phytologist, 149, 95-103. |
| [20] | Kunoh H (2002). Endophytic actinomycetes: Attractive biocontrol agents. Journal of General Plant Pathology, 68, 249-252. |
| [21] | LeBlanc N, Kinkel L, Kistler HC (2017). Plant diversity and plant identity influence Fusarium communities in soil. Mycologia, 109, 128-139. |
| [22] | Lehmann A, Rillig MC (2015). Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—A meta-analysis. Soil Biology & Biochemistry, 81, 147-158. |
| [23] | Lei H, Wang JZ, Tan Y, Zeng M (2019). Effects of reduced fertilization and inoculation with AMF on growth and soil enzyme activities of potted citrus. South China Fruits, 48(2), 11-17. |
| [ 雷卉, 王久照, 谭嫣, 曾明 (2019). 减量施肥和接种AMF对盆栽柑橘生长及土壤酶活性的影响. 中国南方果树, 48(2), 11-17.] | |
| [24] | Li H, Chen XW, Wong MH (2016). Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chemosphere, 145, 224-230. |
| [25] | Li XG, Ding CF, Zhang TL, Wang XX (2014). Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biology & Biochemistry, 72, 11-18. |
| [26] | Li XG, Zhang TL, Wang XX (2015). Advances in mechanism of peanut continuous cropping obstacle. Soils, 47, 266-271. |
| [ 李孝刚, 张桃林, 王兴祥 (2015). 花生连作土壤障碍机制研究进展. 土壤, 47, 266-271.] | |
| [27] | Li Z, Jiang LG, Tang RH, Guo WF (2018). Effects of long-term continuous peanut cropping on dry matter weight of different peanut varieties, soil nutrient contents and enzyme activities. Soils, 50, 491-497. |
| [ 李忠, 江立庚, 唐荣华, 郭文峰 (2018). 连作对花生土壤酶活性、养分含量和植株产量的影响. 土壤, 50, 491-497.] | |
| [28] | Liu XL, Xi XY, Shen H, Liu B, Guo T (2014). Influences of arbuscular mycorrhizal (AM) fungi inoculation on resistance of tobacco to bacterial wilt. Tobacco Science & Technology, 47(5), 94-98. |
| [ 刘先良, 习向银, 申鸿, 刘斌, 郭涛 (2014). 接种丛枝菌根真菌对烟草青枯病抗性的影响. 烟草科技, 47(5), 94-98.] | |
| [29] | Luo WQ, Li J, Ma XN, Niu H, Hou SW, Wu FY (2019). Effect of arbuscular mycorrhizal fungi on uptake of selenate, selenite, and selenomethionine by roots of winter wheat. Plant and Soil, 438, 71-83. |
| [30] | Marschner P, Crowley D, Lieberei R (2001). Arbuscular mycorrhizal infection changes the bacterial 16S rDNA community composition in the rhizosphere of maize. Mycorrhiza, 11, 297-302. |
| [31] | Ning CH, Li WB, Liu RJ (2019). Research advances in plant symbiotic Actinomyces. Chinese Journal of Ecology, 38, 256-266. |
| [ 宁楚涵, 李文彬, 刘润进 (2019). 植物共生放线菌研究进展. 生态学杂志, 38, 256-266.] | |
| [32] | Ozgonen H, Akgul DS, Erkili A (2010). The effects of arbuscular mycorrhizal fungi on yield and stem rot caused by Sclerotium rolfsii Sacc. in peanut. African Journal of Agricultural Research, 5(2), 128-132. |
| [33] | Pozo MJ, López-Ráez JA, Azcón-Aguilar C, García-Garrido JM (2015). Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytologist, 205, 1431-1436. |
| [34] | Qu MH, Yu YC, Li S, Zhang JC (2019). Advances in research on activation of mineral nutrients by arbuscular mycorrhizal fungi. Journal of Zhejiang A&F University, 36, 394-405. |
| [ 屈明华, 俞元春, 李生, 张金池 (2019). 丛枝菌根真菌对矿质养分活化作用研究进展. 浙江农林大学学报, 36, 394-405.] | |
| [35] | Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti M, Agnolucci M, De Pascale S, Bonini P, Colla G (2015). Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Scientia Horticulturae, 196, 91-108. |
| [36] | Shen ML, Zhao C, Liao P, Li J, Cheng XF, Li CC, Zhang Q, Li YB, Zhang LL, Zhao K (2018). The isolation and identification of endophytic actinobacteria from Glycyrrhiza glabra in the Tarim basin and their stress resistance and ability to promote plant growth. Pratacultural Science, 35, 1624-1633. |
| [ 申枚灵, 赵翀, 廖萍, 李静, 程雪芬, 李成成, 张琴, 李艳宾, 张利莉, 赵珂 (2018). 塔里木盆地光果甘草内生放线菌的分离鉴定及抗逆、促生特性. 草业科学, 35, 1624-1633.] | |
| [37] | Smith SE, Manjarrez M, Stonor R, McNeill A, Smith FA (2015). Indigenous arbuscular mycorrhizal (AM) fungi contribute to wheat phosphate uptake in a semi-arid field environment, shown by tracking with radioactive phosphorus. Applied Soil Ecology, 96, 68-74. |
| [38] | Sun RL, Zhao BQ, Zhu LS, Xu J, Zhang FD (2008). Dynamic changes of soil enzyme activities in long-term fertilization soil. Ecology and Environment, 17, 2059-2063. |
| [ 孙瑞莲, 赵秉强, 朱鲁生, 徐晶, 张夫道 (2008). 长期定位施肥田土壤酶活性的动态变化特征. 生态环境, 17, 2059-2063.] | |
| [39] | Sun XS, Feng HS, Wan SB, Zuo XQ (2001). Changes of main microbial strains and enzymes activities in peanut continuous cropping soil and their interactions. Acta Agronomica Sinica, 27, 617-621. |
| [ 孙秀山, 封海胜, 万书波, 左学青 (2001). 连作花生田主要微生物类群与土壤酶活性变化及交互作用. 作物学报, 27, 617-621.] | |
| [40] | Sun XX, He CX, Li YS, Yu XC (2017). Effects of arbuscular mycorrhizal fungi on microbial community and function in the rhizosphere soil of cucumber plants. Mycosystema, 36, 892-903. |
| [ 孙秀秀, 贺超兴, 李衍素, 于贤昌 (2017). AM真菌对黄瓜根围土壤微生物群落功能的影响. 菌物学报, 36, 892-903.] | |
| [41] | Teng Y, Ren WJ, Li ZG, Wang XB, Liu WX, Luo YM (2015). Advance in mechanism of peanut continuous cropping obstacle. Soils, 47, 259-265. |
| [ 滕应, 任文杰, 李振高, 王小兵, 刘五星, 骆永明 (2015). 花生连作障碍发生机理研究进展. 土壤, 47, 259-265.] | |
| [42] | Wan SB, Wang CB, Lu JL, Li GM, Lun WZ, Wu ZF, Cheng B (2007). Study on the growth characteristics of continuous cropping peanut. Shandong Agricultural Sciences,(2), 32-36. |
| [ 万书波, 王才斌, 卢俊玲, 李光敏, 伦伟志, 吴正峰, 成波 (2007). 连作花生的生育特性研究. 山东农业科学, (2), 32-36.] | |
| [43] | Wang J, Bi YL, Zhang YX, Hong TC, Qiu L, Chen SL (2014). Effects of arbuscular mycorrhiza on soil microorganisms and enzyme activities in disturbed coal mine areas. Journal of Southern Agriculture, 45, 1417-1423. |
| [ 王瑾, 毕银丽, 张延旭, 洪天才, 裘浪, 陈书琳 (2014). 接种丛枝菌根对矿区扰动土壤微生物群落及酶活性的影响. 南方农业学报, 45, 1417-1423.] | |
| [44] | Yao XD, Li XG, Ding CF, Han ZM, Wang XX (2019). Microzone distribution characteristics of soil microbial community with peanut cropping system, monocropping or rotation. Acta Pedologica Sinica, 56, 975-985. |
| [ 姚小东, 李孝刚, 丁昌峰, 韩正敏, 王兴祥 (2019). 连作和轮作模式下花生土壤微生物群落不同微域分布特征. 土壤学报, 56, 975-985.] | |
| [45] | Zhang CS (2013). Study on the Distribution, Aflatoxin Production and Genetic Diversity of Aspergillus flavus in Soils of Peanut Fields in Four Agroecological Zones of China. PhD dissertation, Chinese Academy of Agricultural Science, Beijing. |
| [ 张初署 (2013). 中国四个生态区花生土壤中黄曲霉菌分布、产毒特征及遗传多样性研究. 博士学位论文, 中国农业科学院, 北京.] | |
| [46] | Zhou BL, Zheng JD, Bi XH, Cai LL, Guo WW (2015). Effects of mycorrhizal fungi on eggplant verticillium wilt and eggplant growth. Chinese Journal of Ecology, 34, 1026-1030. |
| [ 周宝利, 郑继东, 毕晓华, 蔡莲莲, 郭伟伟 (2015). 丛枝菌根真菌对茄子黄萎病的防治效果和茄子植株生长的影响. 生态学杂志, 34, 1026-1030.] |
| [1] | 龙吉兰 蒋铮 刘定琴 缪宇轩 周灵燕 冯颖 裴佳宁 刘瑞强 周旭辉 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 陈保冬 付伟 伍松林 朱永官. 菌根真菌在陆地生态系统碳循环中的作用[J]. 植物生态学报, 2024, 48(1): 0-0. |
| [3] | 李柳, 刘庆华, 尹春英. 植物硒生物强化及微生物在其中的应用潜力[J]. 植物生态学报, 2023, 47(6): 756-769. |
| [4] | 何斐, 李川, Faisal SHAH, 卢谢敏, 王莹, 王梦, 阮佳, 魏梦琳, 马星光, 王卓, 姜浩. 丛枝菌根菌丝桥介导刺槐-魔芋间碳转运和磷吸收[J]. 植物生态学报, 2023, 47(6): 782-791. |
| [5] | 罗娜娜, 盛茂银, 王霖娇, 石庆龙, 何宇. 长期植被恢复对中国西南喀斯特石漠化土壤活性有机碳组分含量和酶活性的影响[J]. 植物生态学报, 2023, 47(6): 867-881. |
| [6] | 杨佳绒, 戴冬, 陈俊芳, 吴宪, 刘啸林, 刘宇. 丛枝菌根真菌多样性对植物群落构建和稀有种维持的研究进展[J]. 植物生态学报, 2023, 47(6): 745-755. |
| [7] | 张琦, 冯可, 常智慧, 何双辉, 徐维启. 灌丛化对林草交错带植物和土壤微生物的影响[J]. 植物生态学报, 2023, 47(6): 770-781. |
| [8] | 郑炀, 孙学广, 熊洋阳, 袁贵云, 丁贵杰. 叶际微生物对马尾松凋落针叶分解的影响[J]. 植物生态学报, 2023, 47(5): 687-698. |
| [9] | 赵小祥, 朱彬彬, 田秋香, 林巧玲, 陈龙, 刘峰. 叶片凋落物分解的主场优势研究进展[J]. 植物生态学报, 2023, 47(5): 597-607. |
| [10] | 张雅琪, 庞丹波, 陈林, 曹萌豪, 何文强, 李学斌. 荒漠草原土壤氨氧化细菌群落结构对氮添加和枯落物输入的响应[J]. 植物生态学报, 2023, 47(5): 699-712. |
| [11] | 冯可, 刘冬梅, 张琦, 安菁, 何双辉. 旅游干扰对松山油松林土壤微生物多样性及群落结构的影响[J]. 植物生态学报, 2023, 47(4): 584-596. |
| [12] | 何敏, 许秋月, 夏允, 杨柳明, 范跃新, 杨玉盛. 植物磷获取机制及其对全球变化的响应[J]. 植物生态学报, 2023, 47(3): 291-305. |
| [13] | 白雪, 李玉靖, 景秀清, 赵晓东, 畅莎莎, 荆韬羽, 刘晋汝, 赵鹏宇. 谷子及其根际土壤微生物群落对铬胁迫的响应机制[J]. 植物生态学报, 2023, 47(3): 418-433. |
| [14] | 张尧, 陈岚, 王洁莹, 李益, 王俊, 郭垚鑫, 任成杰, 白红英, 孙昊田, 赵发珠. 太白山不同海拔森林根际土壤微生物碳利用效率差异性及其影响因素[J]. 植物生态学报, 2023, 47(2): 275-288. |
| [15] | 陈林康 赵平 王顶 向蕊 龙光强. 玉米马铃薯秸秆混合腐解的非加性效应研究[J]. 植物生态学报, 2023, 47(12): 1728-1738. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19