

雌雄异株树种东北鼠李营养资源需求性别二态性
收稿日期: 2022-11-18
录用日期: 2023-04-06
网络出版日期: 2023-04-20
基金资助
国家重点研发计划(2022YFD2201004-04)
Gender dimorphism in nutritional resource requirements of dioecious tree species Rhamnus schneideri var. manshurica
Received date: 2022-11-18
Accepted date: 2023-04-06
Online published: 2023-04-20
Supported by
Supported by the National Key R&D Program of China(2022YFD2201004-04)
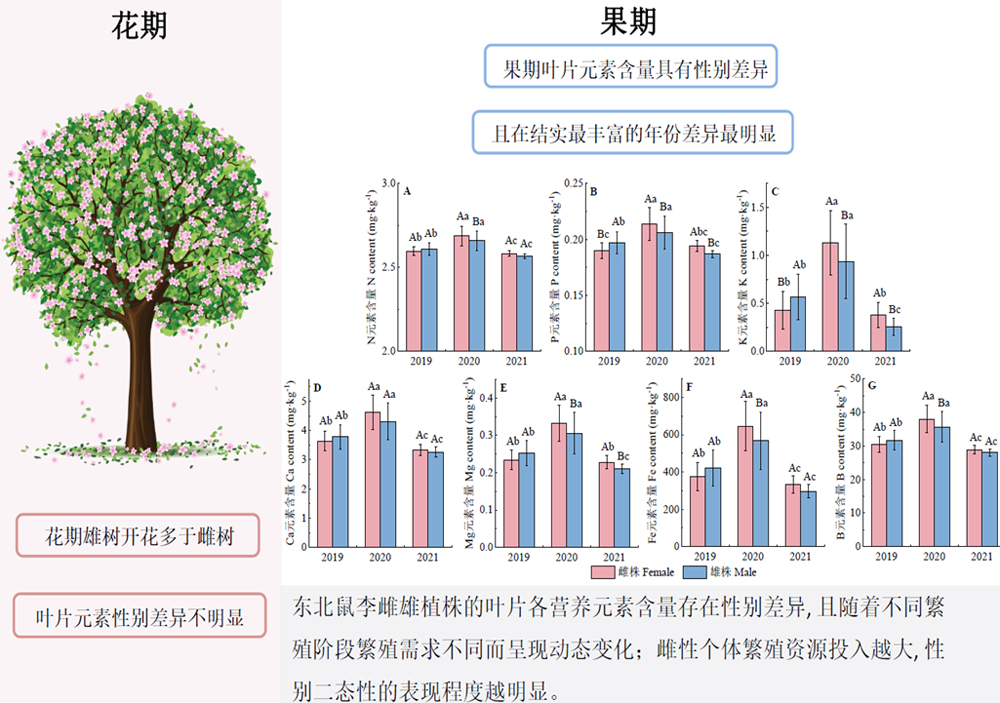
以雌雄异株植物东北鼠李(Rhamnus schneideri var. manshurica)为研究对象, 分析不同繁殖阶段雌雄植株叶片元素含量的性别差异和动态变化, 可为雌雄异株植物性别特异性资源需求及利用策略提供理论参考。该研究在吉林蛟河天然针阔混交林内建立1块23.76 hm2的永久性固定监测样地, 于2019-2021年花期和果期(共5个繁殖阶段)选择样地内一定数量的东北鼠李雌雄植株, 测定每株的叶片营养元素(氮、磷、钾、钙、镁、铁、硼)含量, 比较东北鼠李雌雄植株在不同繁殖阶段叶片各营养元素含量的差异及动态变化; 并计算各个繁殖阶段雌雄植株的花、果生物量, 利用方差分析方法检验雌雄植株各个繁殖阶段的花果生物量差异, 以分析雌雄植株叶片营养元素出现性别差异及动态变化的背后机制。结果表明: (1)东北鼠李雌雄植株的叶片营养元素含量具有显著的性别差异, 但这种性别差异仅在某些繁殖阶段存在。在2019年花期, 雌雄植株的各叶片元素含量均无显著差异, 2021年花期, 雌株叶片的磷含量显著小于雄株。在2019年果期, 雌株叶片的磷、钾含量显著小于雄株; 而在2020年果期, 雌株叶片的氮、磷、钾、钙、镁、铁、硼含量均显著大于雄株; 在2021年果期, 雌株叶片的磷、钾、镁含量均显著大于雄株。(2)无论2019年还是2021年花期, 雄株的花生物量及花数均显著大于雌株。(3)果期雌株果数和果生物量大小排序为: 2020年> 2021年> 2019年。对不同繁殖阶段叶片营养元素含量进行分析, 发现东北鼠李雌雄植株的叶片各营养元素含量存在性别差异, 然而这种性别差异在不同繁殖阶段表现不同。在花期, 雌雄植株叶片元素含量性别差异不明显, 表明雄株并不会因为大量开花造成的高繁殖投入增加叶片元素含量储存; 而在果期, 雌株的叶片营养元素含量受其繁殖活动影响, 在结实较为丰厚的年份增加元素储存, 雌雄植株叶片营养元素含量的性别差异会更为显著。表明东北鼠李雌雄植株营养元素含量的性别二态性表现程度可能受植物个体所处繁殖阶段的影响和限制, 并且雌性个体繁殖资源投入越大, 这种性别二态性的表现就会越明显。
张琦, 叶尔江·拜克吐尔汉, 王娟 . 雌雄异株树种东北鼠李营养资源需求性别二态性[J]. 植物生态学报, 2023 , 47(12) : 1708 -1717 . DOI: 10.17521/cjpe.2022.0470
Aims To provide theoretical references for sex-specific resource requirement and utilization strategies of dioecious plants, we investigated the gender differences and dynamic changes of leaf element contents in male and female plants of Rhamnus schneideri var. manshurica at different stages of reproduction.
Methods Both male and female R. schneideri var. manshurica were selected from a permanent fixed monitoring sample plot of 23.76 hm2 in a natural mixed coniferous and broadleaf forest in Jiaohe, Jilin. Foliar nutrient (nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), ferrum (Fe), and boron (B)) contents of each plant during five reproductive stages from 2019 to 2021 were determined and compared. The flower and fruit biomass of male and female plants were calculated for each reproduction stage. Differences in flower and fruit biomass between male and female plants at each stages of reproduction were tested using ANOVAs. Furthermore, we analyzed the mechanism underlying the occurrence and dynamics of gender differences in foliar nutrient elements of male and female plants.
Important findings (1) Gender differences in foliar nutrient contents of male and female R. schneideri var. manshurica were significant only at certain reproductive stages. During the 2019 flower period, there were no significant differences in foliar elemental contents between male and female plants. During the 2021 flower period, P content in female leaves was significantly lower than that of male plants. During the fruit period, foliar P and K content of female plants was significantly less than that of male plants. In contrast, foliar N, P, K, Ca, Mg, Fe and B contents of female plants were significantly greater than male plants in the 2020 fruit period. During the 2021 fruit period, foliar P, K, and Mg contents in female plants was significantly greater than male plants. (2) Flower biomass and flower number of male plants were significantly greater than female plants during flower periods in both 2019 and 2021. (3) At the fruiting stage, the order of fruit number and fruit biomass of female plants was 2020 fruit period > 2021 fruit period > 2019 fruit period. There were gender differences in foliar nutrient contents, which were expressed differently at different stages of reproduction. During the flower period, there was no significant difference in leaf element content between male and female plants, indicating that male plants do not increase leaf element content stores due to high reproductive inputs during heavy flowering. However, during the fruit period, foliar nutrient contents in female plants was influenced by reproductive activities, and the increase in nutrient stocks in more fruit-bearing years contributed to the more pronounced sex difference in nutrient contents. Our results suggest that the degree of sexually dimorphic expression of nutrient content in male and female R. schneideri var. manshurica may be influenced and limited by the reproductive stages, and that the greater reproductive resource inputs of female, the more pronounced sexually dimorphic expression.

Key words: dioecious plant; nutrient element; dynamic change; reproduction
| [1] | ?gren GI, Wetterstedt J?M, Billberger MFK (2012). Nutrient limitation on terrestrial plant growth—Modeling the interaction between nitrogen and phosphorus. New Phytologist, 194, 953-960. |
| [2] | ?gren J, Danell K, Elmqvist T, Ericson L, Hj?ltén J (1999). Sexual dimorphism and biotic interactions//Geber MA, Dawson TE, Delph LF. Gender and Sexual Dimorphism in Flowering Plants. Springer, Berlin. 217-246. |
| [3] | Aschan G, Pfanz H (2003). Non-foliar photosynthesis—A strategy of additional carbon acquisition. Flora, 198, 81-97. |
| [4] | Ashman TL (1994). A dynamic perspective on the physiological cost of reproduction in plants. The American Naturalist, 144, 300-316. |
| [5] | Bullock SH (1984). Biomass and nutrient allocation in a neotropical dioecious palm. Oecologia, 63, 426-428. |
| [6] | Chen LH, Dong TF, Duan BL (2014). Sex-specific carbon and nitrogen partitioning under N deposition in Populus cathayana. Trees, 28, 793-806. |
| [7] | Chen YL, Zhou BK (1982). Flora of China. Tomus 48(1). Rhamnaceae. Science Press, Beijing. |
| [ 陈艺林, 周邦楷 (2004). 中国植物志. 第四十八卷(第一分册). 鼠李科. 科学出版社, 北京.] | |
| [8] | Cipollini ML, Stiles EW (1991). Costs of reproduction in Nyssa sylvatica: sexual dimorphism in reproductive frequency and nutrient flux. Oecologia, 86, 585-593. |
| [9] | Dawson TE, Geber MA (1999). Sexual dimorphism in physiology and morphology//Geber MA, Dawson TE, Delph LF. Gender and Sexual Dimorphism in Flowering Plants. Springer, Berlin. 175-215. |
| [10] | de Marco A, Vittozzi P, de Santo AV (2023). Elements dynamics, from leaf to stable leaf litter residue and soil, for two functional types of tree planted on volcanic deposits. Plant and Soil, 482, 127-140. |
| [11] | Delph LF (1999). Sexual dimorphism in life history//Geber MA, Dawson TE, Delph LF. Gender and Sexual Dimorphism in Flowering Plants. Springer, Berlin. 149-173. |
| [12] | Ehlers BK, Thompson JD (2004). Temporal variation in sex allocation in hermaphrodites of gynodioecious Thymus vulgaris L. Journal of Ecology, 92, 15-23. |
| [13] | Gao M, Chen YC, Wu LW, Wang YD (2019). Dynamic patterns of sex-specific difference of water and nitrogen use efficiency in Litsea cubeba. Scientia Silvae Sinicae, 55(4), 62-68. |
| [ 高暝, 陈益存, 吴立文, 汪阳东 (2019). 山鸡椒水分及氮素利用效率性别特异性动态. 林业科学, 55(4), 62-68.] | |
| [14] | García MB, Antor RJ (1995). Sex ratio and sexual dimorphism in the dioecious Borderea pyrenaica (Dioscoreaceae). Oecologia, 101, 59-67. |
| [15] | Hemborg ?M (1999). Sexual differences in biomass and nutrient allocation of first-year Silene dioica plants. Oecologia, 118, 453-460. |
| [16] | Huang KS, Pan XF, Ou J, Zeng XY, Liao JM, Liang WH (2021). Variation of nutrient elements content in leaves of different varieties of Illicium verum at flowering and fruit-setting stage and regularity of flowers and fruits dropping. Journal of West China Forestry Science, 50(6), 1-7. |
| [ 黄开顺, 潘晓芳, 欧军, 曾祥艳, 廖健明, 梁文汇 (2021). 不同品种八角开花坐果期叶片营养元素含量变化及落花落果规律. 西部林业科学, 50(6), 1-7.] | |
| [17] | Jiang H, Zhang S, Lei YB, Xu G, Zhang D (2016). Alternative growth and defensive strategies reveal potential and gender specific trade-offs in dioecious plants Salix paraplesia to nutrient availability. Frontiers in Plant Science, 7, 1064. DOI: 10.3389/fpls.2016.01064. |
| [18] | Kachi N, Hirose T (1983). Limiting nutrients for plant growth in coastal sand dune soils. Journal of Ecology, 71, 937-944. |
| [19] | Li H, C Crabbe MJ, Xu F, Wang W, Ma L, Niu R, Gao X, Li X, Zhang P, Ma X, Chen H (2017). Seasonal variations in carbon, nitrogen and phosphorus concentrations and C:N:P stoichiometry in different organs of a Larix principis-rupprechtii Mayr. plantation in the Qinling Mountains, China. PLoS ONE, 12, e0185163. DOI: 10.1371/journal.pone.0185163. |
| [20] | Li Z, Wu N, Liu T, Tang M, Chen H (2020). Gender-related responses of dioecious plant Populus cathayana to AMF, drought and planting pattern. Scientific Reports, 10, 11530. DOI: 10.1038/s41598-020-68112-0. |
| [21] | Liu M, Ma ZG, Wang W, Zhang JL (2018). Dynamic variation and resorption efficiencies of foliar nutrients in Fraxinus pennysylvanica street tree. Tianjin Agricultural Sciences, 24(8), 74-76. |
| [ 刘明, 马作国, 王武, 张金龙 (2018). 洋白蜡行道树叶片养分动态及再吸收特征. 天津农业科学, 24(8), 74-76.] | |
| [22] | Mahajan G, Chauhan BS, Timsina J, Singh PP, Singh K (2012). Crop performance and water- and nitrogen-use efficiencies in dry-seeded rice in response to irrigation and fertilizer amounts in northwest India. Field Crops Research, 134, 59-70. |
| [23] | McDowell SCL, McDowell NG, Marshall JD, Hultine K (2000). Carbon and nitrogen allocation to male and female reproduction in Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca, Pinaceae). American Journal of Botany, 87, 539-546. |
| [24] | Montesinos D, Villar-Salvador P, García-Fayos P, Verdú M (2012). Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytologist, 193, 705-712. |
| [25] | Ning P, Li S, Yu P, Zhang Y, Li CJ (2013). Post-silking accumulation and partitioning of dry matter, nitrogen, phosphorus and potassium in maize varieties differing in leaf longevity. Field Crops Research, 144, 19-27. |
| [26] | Nowak-Dyjeta K, Giertych MJ, Thomas P, Iszku?o G (2017). Males and females of Juniperus communis L. and Taxus baccata L. show different seasonal patterns of nitrogen and carbon content in needles. Acta Physiologiae Plantarum, 39, 191. DOI: 10.1007/s11738-017-2489-3. |
| [27] | Obeso JR (2002). The costs of reproduction in plants. New Phytologist, 155, 321-348. |
| [28] | Pearse IS, Koenig WD, Kelly D (2016). Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytologist, 212, 546-562. |
| [29] | Pers-Kamczyc E, M?derek E, Kamczyc J (2022). Seed quantity or quality?—Reproductive responses of females of two dioecious woody species to long-term fertilisation. International Journal of Molecular Sciences, 23, 3187. DOI: 10.3390/ijms23063187. |
| [30] | Pickup M, Barrett SCH (2012). Reversal of height dimorphism promotes pollen and seed dispersal in a wind-pollinated dioecious plant. Biology Letters, 8, 245-248. |
| [31] | Rabska M, Pers-Kamczyc E, ?ytkowiak R, Adamczyk D, Iszku?o G (2020). Sexual dimorphism in the chemical composition of male and female in the dioecious tree, Juniperus communis L., growing under different nutritional conditions. International Journal of Molecular Sciences, 21, 8094. DOI: 10.3390/ijms21218094. |
| [32] | Rabska M, Warwick NWM, Iszku?o G, Gross CL (2021). Intersexual differences in leaf size and shape in dioecious Adriana tomentosa. Journal of Plant Ecology, 14, 67-83. |
| [33] | Robakowski P, Pers-Kamczyc E, Ratajczak E, Thomas PA, Ye ZP, Rabska M, Iszku?o G (2018). Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Frontiers in Plant Science, 9, 742. DOI: 10.3389/fpls. 2018.00742. |
| [34] | Sánchez Vilas J, Pannell JR (2011). Sexual dimorphism in resource acquisition and deployment: both size and timing matter. Annals of Botany, 107, 119-126. |
| [35] | Sánchez Vilas J, Retuerto R (2017). Sexual dimorphism in water and nitrogen use strategies in Honckenya peploides: timing matters. Journal of Plant Ecology, 10, 702-712. |
| [36] | Song AY, Dong LS, Chen JX, Peng L, Liu JT, Xia JB, Chen YP (2019). Comparation of seasonal dynamics of mineral elements contents in different organs of male and female plants of Fraxinus velutina. Scientia Silvae Sinicae, 55(10), 162-170. |
| [ 宋爱云, 董林水, 陈纪香, 彭玲, 刘京涛, 夏江宝, 陈印平 (2019). 绒毛白蜡雌雄株不同器官矿质元素季节动态的比较. 林业科学, 55(10), 162-170.] | |
| [37] | Song HF, Cai ZY, Liao J, Tang DT, Zhang S (2019). Sexually differential gene expressions in poplar roots in response to nitrogen deficiency. Tree Physiology, 39, 1614-1629. |
| [38] | Teitel Z, Pickup M, Field DL, Barrett SCH (2016). The dynamics of resource allocation and costs of reproduction in a sexually dimorphic, wind-pollinated dioecious plant. Plant Biology, 18, 98-103. |
| [39] | Wang J, Zhang C, Zhao X,von Gadow K (2014). Reproductive allocation of two dioecious Rhamnus species in temperate forests of northeast China. Iforest - Biogeosciences and Forestry, 7, 25-32. |
| [40] | Wang LB, Huang YH, Fan CY, Zhang XN, Wang J (2020). Study on gender differentiation of reproduction cost in Rhamnus schneideri var. manshurica. Journal of Central South University of Forestry & Technology, 40(12), 69-74. |
| [ 王俪玢, 黄云浩, 范春雨, 张新娜, 王娟 (2020). 东北鼠李生殖耗费的性别分异研究. 中南林业科技大学学报, 40(12), 69-74.] | |
| [41] | Xia Z, He Y, Yu L, Lv R, Korpelainen H, Li C (2020). Sex-specific strategies of phosphorus (P) acquisition in Populus cathayana as affected by soil P availability and distribution. New Phytologist, 225, 782-792. |
| [42] | Yu K, Fan QL, Wang Y, Wei JR, Ma Q, Yu D, Li JR (2013). Function of leafy sepals in Paris polyphylla: photosynthate allocation and partitioning to the fruit and rhizome. Functional Plant Biology, 40, 393-399. |
/
| 〈 |
|
〉 |