Chinese Journal of Plant Ecology >
Seasonal dynamics of soil microbial biomass carbon, nitrogen and phosphorus stoichiometry across global forest ecosystems
- 1 State Key Laboratory of Urban and Regional Ecology, Research Centre for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
2 College of Life Sciences, Capital Normal University, Beijing 100048, China
Received date: 2019-04-04
Revised date: 2019-06-12
Online published: 2019-09-30
Supported by
Supported by the National Natural Science Foundation of China(31870458);the Key Research Program of Frontier Sciences, Chinese Academy of Sciences(QYZDB-SSW-DQC019)
Abstract
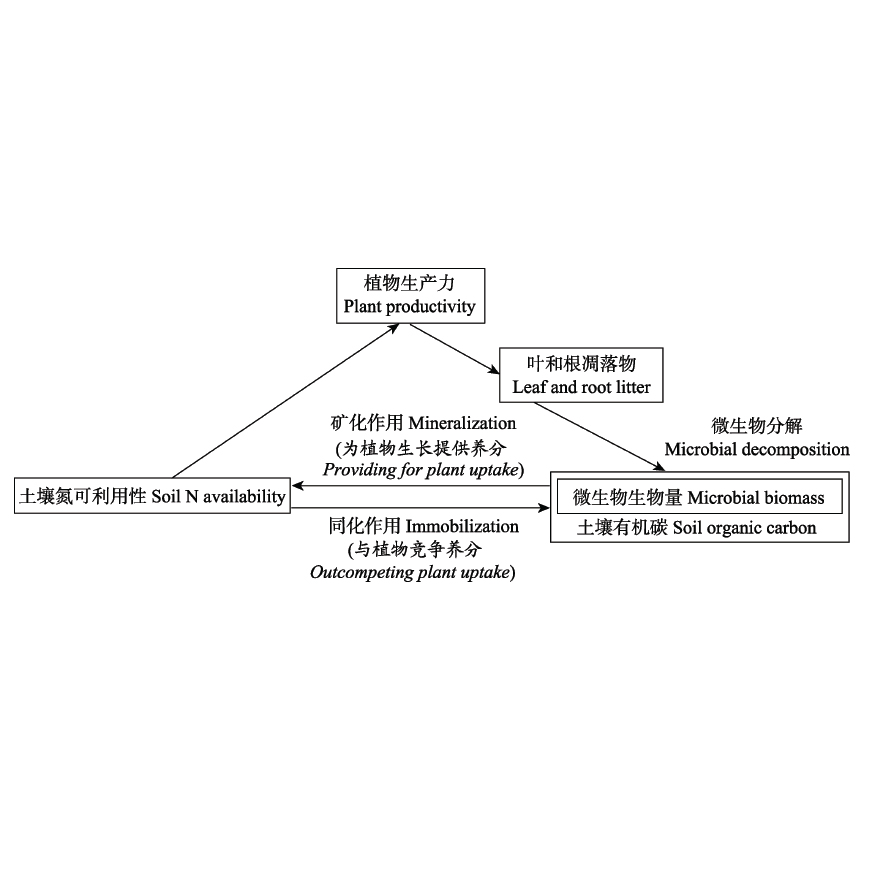
Aims Soil microorganisms in forest ecosystems play vital roles in regulating above- and belowground ecosystem processes and functions such as soil nutrient cycling, litter decomposition, net ecosystem productivity, and ecosystem succession. We aim to investigate broad-scale seasonal patterns of soil microbial biomass carbon (C), nitrogen (N) and phosphorus (P) stoichiometry. Methods By synthesizing 164 samples of soil microbial biomass C, N and P content derived from the published literature, we investigated global seasonal patterns of soil microbial C, N, P content and their ratios across three vegetation types of global forests. Important findings Soil microbial biomass C, N and P content in temperate and subtropical forests were lower in summer and higher in winter. Soil microbial biomass C, N and P content in tropical forests were lower than those in temperate and subtropical forests in four seasons. Soil microbial biomass C and N content in tropical forests were relatively the lowest in autumn, and soil microbial biomass P content was relatively constant in all seasons. The soil microbial biomass C:N of temperate forest was significantly higher than that of other two forest types in spring, and that of tropical forest was significantly higher than that of other two forest types in autumn. Soil microbial biomass N:P and C:P in temperate forests remained relatively constant in four seasons, while those in tropical forests were higher than those in other three seasons in summer. The soil microbial biomass C content, N content, N:P and C:P of broad-leaved trees were significantly higher than those of conifers in four seasons, while the soil microbial biomass P content of conifers was significantly higher than that of broad-leaved trees in four seasons. There was no significant difference in soil microbial biomass C:N between broad-leaved and coniferous trees in both spring and winter, but the soil microbial biomass C:N of coniferous trees was significantly higher than that of broad-leaved trees in summer and autumn. For the change of soil microbial biomass, season is not but forest type is the main significant factor, suggesting that the seasonal fluctuation of soil microbial biomass changes with the inherent periodic change of trees. Asynchronous nutrient uptake by plants and soil microorganisms is a trade-off mechanism between nutrient retention and ecological function maintenance.
Cite this article
LI Pin, Muledeer TUERHANBAI, TIAN Di, FENG Zhao-Zhong . Seasonal dynamics of soil microbial biomass carbon, nitrogen and phosphorus stoichiometry across global forest ecosystems[J]. Chinese Journal of Plant Ecology, 2019 , 43(6) : 532 -542 . DOI: 10.17521/cjpe.2019.0075
References
| [1] | Barbhuiya AR, Arunachalam A, Pandey HN, Arunachalam K, Khan ML, Nath PC (2004). Dynamics of soil microbial biomass C, N and P in disturbed and undisturbed stands of a tropical wet-evergreen forest. European Journal of Soil Biology, 40, 113-121. |
| [2] | Bonan GB (2014). Connecting mathematical ecosystems, real-world ecosystems, and climate science. New Phytologist, 202, 731-733. |
| [3] | Brookes PC, Ocio JA, Wu J (1990). The soil microbial biomass: Its measurement, properties and role in nitrogen carbon dynamics following substrate incorporation. Soil Microorganisms, 35, 39-51. |
| [4] | Brooks PD, Williams MW, Schmidt SK (1996). Microbial activity under alpine snowpacks, Niwot Ridge, Colorado. Biogeochemistry, 32, 93-113. |
| [5] | Brooks PD, Williams MW, Schmidt SK (1998). Inorganic N and microbial biomass dynamics before and during spring snowmelt. Biogeochemistry, 43, 1-15. |
| [6] | Cleveland CC, Liptzin D (2007). C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry, 85, 235-252. |
| [7] | Deng M, Liu L, Jiang L, Liu W, Wang X, Li S, Yang S, Wang B (2018). Ecosystem scale trade-off in nitrogen acquisition pathways. Nature Ecology & Evolution, 2, 1724-1734. |
| [8] | Devi NB, Yadava PS (2006). Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-east India. Applied Soil Ecology, 31, 220-227. |
| [9] | Edwards KA, McCulloch J, Peter Kershaw G, Jefferies RL (2006). Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biology & Biochemistry, 38, 2843-2851. |
| [10] | Fang CM, Smith P, Moncrieff JB, Smith JU (2005). Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature, 433, 57-59. |
| [11] | Fauci MF, Dick RP (1994). Soil microbial dynamics: Short- and long-term effects of inorganic and organic nitrogen. Soil Science Society of America Journal, 58, 801-806. |
| [12] | Feng WT, Zou XM, Schaefer D (2009). Above- and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biology & Biochemistry, 41, 978-983. |
| [13] | Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009). Global patterns in belowground communities. Ecology Letters, 12, 1238-1249. |
| [14] | Groffmann PM, Zak DR, Christensen S, Mosier A, Tiedje JM (1993). Early spring nitrogen dynamics in a temperate forest landscape. Ecology, 74, 1579-1585. |
| [15] | Harte J, Kinzig AP (1993). Mutualism and competition between plants and decomposers: Implications for nutrient allocation in ecosystems. The American Naturalist, 141, 829-846. |
| [16] | He ZL, Wu J, O’Donnel AG, Syers JK (1997). Seasonal responses in microbial biomass carbon, phosphorus and sulphur in soils under pasture. Biology and Fertility of Soils, 24, 421-428. |
| [17] | Kaiser C, Franklin O, Dieckmann U, Richter A (2014). Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecology Letters, 17, 680-690. |
| [18] | Kaiser C, Fuchslueger L, Koranda M, Gorfer M, Stange CF, Kitzler B, Rasche F, Strauss J, Sessitsch A, Zechmeister-?Boltenstern S, Richter A (2011). Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology, 92, 1036-1051. |
| [19] | Kaye JP, Hart SC (1997). Competition for nitrogen between plants and soil microorganisms. Trends in Ecology & Evolution, 12, 139-143. |
| [20] | Koranda M, Kaiser C, Fuchslueger L, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2013). Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biology & Biochemistry, 60, 95-104. |
| [21] | Landgraf D, Klose S (2002). Mobile and readily available C and N fractions and their relationship to microbial biomass and selected enzyme activities in a sandy soil under different management systems. Journal of Plant Nutrition and Soil Science, 165, 9-16. |
| [22] | Li P, Yang YH, Han WX, Fang JY (2014). Global patterns of soil microbial nitrogen and phosphorus stoichiometry in forest ecosystems. Global Ecology and Biogeography, 23, 979-987. |
| [23] | Lipson DA, Schadt CW, Schmidt SK (2002). Changes in soil microbial community structure and function in an alpine dry meadow following spring snow melt. Microbial Ecology, 43, 307-314. |
| [24] | Lipson DA, Schmidt SK, Monson RK (1999). Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology, 80, 1623-1631. |
| [25] | Lipson DA, Schmidt SK, Monson RK (2000). Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biology & Biochemistry, 32, 441-448. |
| [26] | Liu S, Wang CK (2010). Spatio-temporal patterns of soil microbial biomass carbon and nitrogen in five temperate forest ecosystems. Acta Ecologica Sinica, 30, 3135-3143. |
| [26] | [ 刘爽, 王传宽 (2010). 五种温带森林土壤微生物生物量碳氮的时空格局. 生态学报, 30, 3135-3143.] |
| [27] | López-Mondéjar R, Vo?í?ková J, Větrovsky T, Baldrian P (2015). The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biology & Biochemistry, 87, 43-50. |
| [28] | Medlyn BE, Zaehle S, De Kauwe MG, Walker AP, Dietze MC, Hanson PJ, Hickler T, Jain AK, Luo Y, Parton W, Prentice IC, Thornton PE, Wang S, Wang YP, Weng E, Iversen CM, McCarthy HR, Warren JM, Oren R, Norby RJ (2015). Using ecosystem experiments to improve vegetation models. Nature Climate Change, 5, 528-534. |
| [29] | Muller RN, Herbert Bormann F (1976). Role of Erythronium americanum Ker. in energy flow and nutrient dynamics of a northern hardwood forest ecosystem. Science, 193, 1126-1128. |
| [30] | Neill C, Gignoux J (2006). Soil organic matter decomposition driven by microbial growth: A simple model for a complex network of interactions. Soil Biology & Biochemistry, 38, 803-811. |
| [31] | Nemergut DR, Costello EK, Meyer AF, Pescador MY, Weintraub MN, Schmidt SK (2005). Structure and function of alpine and arctic soil microbial communities. Research in Microbiology, 156, 775-784. |
| [32] | Schimel JP, Weintraub MN (2003). The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biology & Biochemistry, 35, 549-563. |
| [33] | Schmidt SK, Lipson DA (2004). Microbial growth under the snow: Implications for nutrient and allelochemical availability in temperate soils. Plant and Soil, 259, 1-7. |
| [34] | Scott-Denton LE, Rosenstiel TN, Monson RK (2006). Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Global Change Biology, 12, 205-216. |
| [35] | Singh JS, Raghubanshi AS, Singh RS, Srivastava SC (1989). Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature, 338, 499-500. |
| [36] | Terrer C, Vicca S, Stocker BD, Hungate BA, Phillips RP, Reich PB, Finzi AC, Prentice IC (2018). Ecosystem responses to elevated CO2 governed by plant-soil interactions and the cost of nitrogen acquisition. New Phytologist, 217, 507-522. |
| [37] | Vance ED, Brookes PC, Jenkinson DS (1987). An extraction method for measuring soil microbial biomass C. Soil Biology & Biochemistry, 19, 703-707. |
| [38] | Wang GB, Ruan HH, Tang YF, Luan YL, Chen YQ, Tao ZF (2008). Seasonal fluctuation of soil microbial biomass carbon in secondary oak forest and Pinus taeda plantation in north subtropical area of China. Chinese Journal of Applied Ecology, 19, 37-42. |
| [38] | [ 王国兵, 阮宏华, 唐燕飞, 栾以玲, 陈月琴, 陶忠芳 (2008). 北亚热带次生栎林与火炬松人工林土壤微生物生物量碳的季节动态. 应用生态学报, 19, 37-42.] |
| [39] | Wang XQ, Han Y, Wang CK (2017). Soil microbial biomass and its seasonality in deciduous broadleaved forests with different stand ages in the Mao’ershan region, Northeast China. Chinese Journal of Plant Ecology, 41, 597-609. |
| [39] | [ 王薪琪, 韩轶, 王传宽 (2017). 帽儿山不同林龄落叶阔叶林土壤微生物生物量及其季节动态. 植物生态学报, 41, 597-609.] |
| [40] | Warren M, Zou XM (2003). Seasonal nitrogen retention in temperate hardwood forests: The “vernal dam” hypothesis and case studies. Acta Phytoecologica Sinica, 27, 11-15. |
| [40] | [ Warren M, 邹晓明 (2003) 温带阔叶林中氮的保留机制: “春坝”假设及研究实例. 植物生态学报, 27, 11-15.] |
| [41] | Yang K, Zhu JJ, Zhang JX, Yan QL (2009). Seasonal dynamics of soil microbial biomass C and N in two larch plantation forests with different ages in Northeastern China. Acta Ecologica Sinica, 29, 5500-5507. |
| [41] | [ 杨凯, 朱教君, 张金鑫, 闫巧玲 (2009). 不同林龄落叶松人工林土壤微生物生物量碳氮的季节变化. 生态学报, 29, 5500-5507.] |
| [42] | Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL (1993). Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant and Soil, 151, 105-117. |
| [43] | Zak DR, Pregitzer KS, King JS, Holmes WE (2000). Elevated atmospheric CO2 fine roots and the response of soil microorganisms: A review and hypothesis. New Phytologist, 147, 201-222. |