Chinese Journal of Plant Ecology >
Effects of Artemisia ordosica on the nitrogen uptake rate and proportion of associated herbaceous plants in the Mau Us Sandy Land
- MIAO Chun ,
- LIU Liang ,
- ZHU Guan-Nan ,
- BAI Yu-Xuan ,
- SHE Wei-Wei ,
- QIN Shu-Gao ,
- GUO Yan-Pei ,
- ZHANG Yu-Qing
- 1Yanchi Research Station, School of Soil and Water Conservation, Beijing Forestry University, Beijing 100083, China
2State Key Laboratory of Efficient Production of Forest Resource, Beijing Forestry University, Beijing 100083, China
3Key Laboratory of State Forestry and Grassland Administration on Soil and Water Conservation, Beijing Forestry University, Beijing 100083, China
Received date: 2024-03-27
Accepted date: 2024-09-28
Online published: 2024-09-29
Supported by
National Natural Science Foundation of China(U22A20504);National Natural Science Foundation of China(32071844)
Abstract
Aims Nitrogen is a key nutrient element for maintaining species diversity in plant communities. In nitrogen-limited desert ecosystems, the impact of interspecific interactions within sand-fixed plant communities on nitrogen uptake rates and distribution among different functional groups of plants remains unclear.
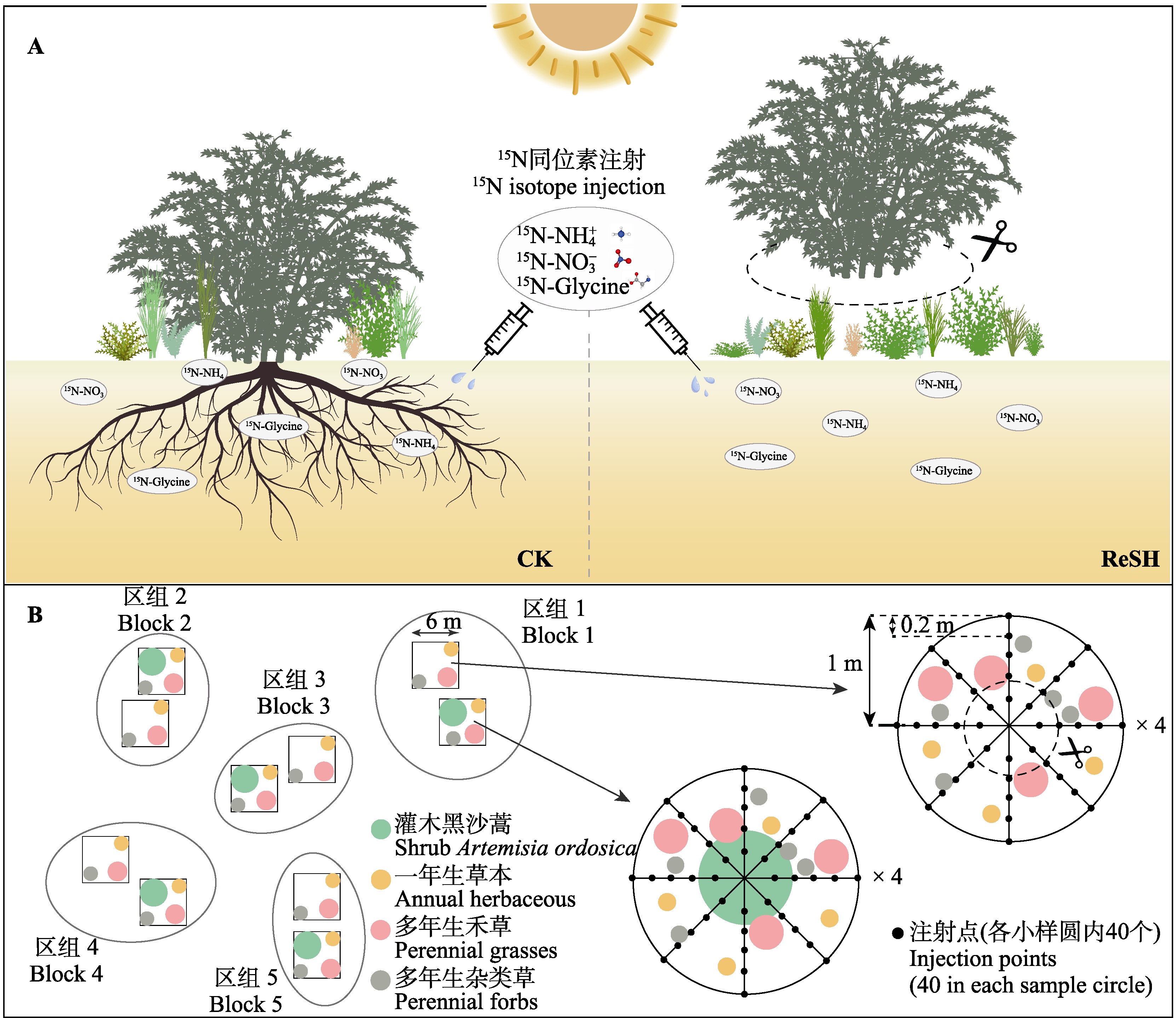
Methods A field experiment was conducted in the Mau Us Sandy Land, northern China, with two treatments: removal of Artemisia ordosica and a control that retained A. ordosica. Nitrogen uptake rate and proportion of nitrate nitrogen, ammonium nitrogen, and glycine by three associated functional groups of herbaceous plants (annual herbaceous, perennial grasses, and perennial forbs) and the overall herbaceous community, were measured using 15N isotope labeling. Environmental factors, such as soil ammonium nitrogen, nitrate nitrogen, total nitrogen, soluble organic nitrogen content, soil moisture, and plot light transmittance were also determined. Additionally, the relationships between nitrogen uptake rate, proportion and environmental factors for each functional group and herbaceous community were analyzed.
Important findings Inorganic nitrogen and micromolecular organic nitrogen were effective nitrogen sources for herbaceous plants in A. ordosica community. The preference of herbaceous plants for different forms of nitrogen followed the order: nitrate nitrogen, ammonium nitrogen, and micromolecule organic nitrogen. After the removal of A. ordosica, the nitrogen uptake rate of annual herbaceous, perennial grasses, and the overall herbaceous community increased by 48.32%, 129.77%, and 55.53%, respectively, with perennial grasses showing a 10.65% increase in the proportion of nitrate nitrogen uptake. Artemisia ordosica affected the nitrogen uptake absorption mode of herbaceous plants by altering the microenvironment under the plants, particularly by reducing plot light transmittance and soil nitrate nitrogen content. Nitrogen uptake rate and proportion among different functional groups of herbaceous plants were influenced by various environmental factors, among which perennial grasses demonstrating flexible nitrogen source plasticity and higher nitrogen use efficiency. The study showed that the differentiated nitrogen acquisition strategies of herbaceous plants may be a crucial mechanism underlying that herbaceous plants in A. ordosica community coped with interspecific nitrogen competition and resource scarcity, thereby enhancing community stability and resilience. Perennial grasses, with flexible nitrogen source utilization and efficient absorption capabilities, may gradually become the dominant functional group as community succession. These findings may enhance the understanding of nutrient competition and species coexistence mechanisms in the typical sand-fixed community, which would provide scientific evidence for vegetation restoration and management in desertification areas.
Cite this article
MIAO Chun , LIU Liang , ZHU Guan-Nan , BAI Yu-Xuan , SHE Wei-Wei , QIN Shu-Gao , GUO Yan-Pei , ZHANG Yu-Qing . Effects of Artemisia ordosica on the nitrogen uptake rate and proportion of associated herbaceous plants in the Mau Us Sandy Land[J]. Chinese Journal of Plant Ecology, 2025 , 49(3) : 446 -459 . DOI: 10.17521/cjpe.2024.0086
References
| [1] | Andersen KM, Mayor JR, Turner BL (2017). Plasticity in nitrogen uptake among plant species with contrasting nutrient acquisition strategies in a tropical forest. Ecology, 98, 1388-1398. |
| [2] | Andersen KM, Turner BL (2013). Preferences or plasticity in nitrogen acquisition by understorey palms in a tropical montane forest. Journal of Ecology, 101, 819-825. |
| [3] | Ashton IW, Miller AE, Bowman WD, Suding KN (2010). Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology, 91, 3252-3260. |
| [4] | Bell CW, Asao S, Calderon F, Wolk B, Wallenstein MD (2015). Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biology & Biochemistry, 85, 170-182. |
| [5] | Bremner JM, Sparks DL (1996). Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America, Madison, USA. |
| [6] | Bruno JF, Stachowicz JJ, Bertness MD (2003). Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution, 18, 119-125. |
| [7] | Callaway RM (2007). Positive Interactions and Interdependence in Plant Communities. Springer, Dordrecht, The Netherlands. |
| [8] | Du EZ, Terrer C, Pellegrini AFA, Ahlstr?m A, van Lissa CJ, Zhao X, Xia N, Wu XH, Jackson RB (2020). Global patterns of terrestrial nitrogen and phosphorus limitation. Nature Geoscience, 13, 221-226. |
| [9] | Fan JW, Wang K, Harris W, Zhong HP, Hu ZM, Han B, Zhang WY, Wang JB (2009). Allocation of vegetation biomass across a climate-related gradient in the grasslands of Inner Mongolia. Journal of Arid Environments, 73, 521-528. |
| [10] | Fernando AL, Boléo S, Barbosa B, Costa J, Duarte MP, Monti A (2015). Perennial grass production opportunities on marginal Mediterranean land. BioEnergy Research, 8, 1523-1537. |
| [11] | Fowler N (1986). The role of competition in plant communities in arid and semiarid regions. Annual Review of Ecology and Systematics, 17, 89-110. |
| [12] | Fraterrigo JM, Strickland MS, Keiser AD, Bradford MA (2011). Nitrogen uptake and preference in a forest understory following invasion by an exotic grass. Oecologia, 167, 781-791. |
| [13] | Friedman J (2020). The evolution of annual and perennial plant life histories: ecological correlates and genetic mechanisms. Annual Review of Ecology, Evolution, and Systematics, 51, 461-481. |
| [14] | Gao L, Cui XY, Hill PW, Guo YF (2020). Uptake of various nitrogen forms by co-existing plant species in temperate and cold-temperate forests in northeast China. Applied Soil Ecology, 147, 103398. DOI: 10.1016/j.apsoil.2019.103398. |
| [15] | Gebauer RLE, Ehleringer JR (2000). Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology, 81, 1415-1424. |
| [16] | Gherardi LA, Sala OE, Yahdjian L (2013). Preference for different inorganic nitrogen forms among plant functional types and species of the Patagonian steppe. Oecologia, 173, 1075-1081. |
| [17] | Hachiya T, Sakakibara H (2017). Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. Journal of Experimental Botany, 68, 2501-2512. |
| [18] | Hart SP (2023). How does facilitation influence the outcome of species interactions? Journal of Ecology, 111, 2094-2104. |
| [19] | Hesse E, Pannell JR (2011). Sexual dimorphism in a dioecious population of the wind-pollinated herb Mercurialis annua: the interactive effects of resource availability and competition. Annals of Botany, 107, 1039-1045. |
| [20] | Hietz P, Turner BL, Wanek W, Richter A, Nock CA, Wright SJ (2011). Long-term change in the nitrogen cycle of tropical forests. Science, 334, 664-666. |
| [21] | Hong JT, Ma XX, Zhang XK, Wang XD (2017). Nitrogen uptake pattern of herbaceous plants: coping strategies in altered neighbor species. Biology and Fertility of Soils, 53, 729-735. |
| [22] | Hu XF, Li WT, Liu QH, Yin CY (2022). Interactions between species change the uptake of ammonium and nitrate in Abies faxoniana and Picea asperata. Tree Physiology, 42, 1396-1410. |
| [23] | Huberty AF, Denno RF (2006). Consequences of nitrogen and phosphorus limitation for the performance of two planthoppers with divergent life-history strategies. Oecologia, 149, 444-455. |
| [24] | Inselsbacher E, N?sholm T (2012). The below-ground perspective of forest plants: soil provides mainly organic nitrogen for plants and mycorrhizal fungi. New Phytologist, 195, 329-334. |
| [25] | Jin VL, Evans RD (2010). Elevated CO2 increases plant uptake of organic and inorganic N in the desert shrub Larrea tridentata. Oecologia, 163, 257-266. |
| [26] | Jin YH, Zhang YJ, Xu JW, Tao Y, He HS, Guo M, Wang AL, Liu YX, Niu LP (2018). Comparative assessment of tundra vegetation changes between north and southwest slopes of Changbai Mountains, China, in response to global warming. Chinese Geographical Science, 28, 665-679. |
| [27] | Jones DL, Healey JR, Willett VB, Farrar JF, Hodge A (2005). Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil Biology & Biochemistry, 37, 413-423. |
| [28] | Kempers AJ, Zweers A (1986). Ammonium determination in soil extracts by the salicylate method. Communications in Soil Science and Plant Analysis, 17, 715-723. |
| [29] | Kielland K (1994). Amino acid absorption by Arctic plants: implications for plant nutrition and nitrogen cycling. Ecology, 75, 2373-2383. |
| [30] | Lai CM, Peng F, Sun JB, Zhou J, Li CY, Xu XL, Chen XJ, You QG, Sun HY, Sun J, Xue X, Lambers H (2023). Niche differentiation and higher uptake of available nitrogen maintained the productivity of alpine meadow at early degradation. Biology and Fertility of Soils, 59, 35-49. |
| [31] | Lehman CL, Tilman D (2000). Biodiversity, stability, and productivity in competitive communities. The American Naturalist, 156, 534-552. |
| [32] | Lim SL, Voon CP, Guan XQ, Yang Y, Gardestr?m P, Lim BL (2020). In planta study of photosynthesis and photorespiration using NADPH and NADH/NAD+ fluorescent protein sensors. Nature Communications, 11, 3238. DOI:10.1038/s41467-020-17056-0. |
| [33] | Lind EM, Borer E, Seabloom E, Adler P, Bakker JD, Blumenthal DM, Crawley M, Davies K, Firn J, Gruner DS, Stanley Harpole W, Hautier Y, Hillebrand H, Knops J, Melbourne B, et al.(2013). Life-history constraints in grassland plant species: a growth-defence trade-off is the norm. Ecology Letters, 16, 513-521. |
| [34] | Lipson D, N?sholm T (2001). The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia, 128, 305-316. |
| [35] | Liu J, Peng B, Xia ZW, Sun JF, Gao DC, Dai WW, Jiang P, Bai E (2017). Different fates of deposited and in a temperate forest in Northeast China: a 15N tracer study. Global Change Biology, 23, 2441-2449. |
| [36] | Liu M, Adl S, Cui XY, Tian YQ, Xu XL, Kuzyakov Y (2020). In situ methods of plant-microbial interactions for nitrogen in rhizosphere. Rhizosphere, 13, 100186. DOI: 10.1016/j.rhisph.2020.100186. |
| [37] | Liu QY, Xu MJ, Yuan Y, Wang HM (2021). Interspecific competition for inorganic nitrogen between canopy trees and underlayer-planted young trees in subtropical pine plantations. Forest Ecology and Management, 494, 119331. DOI: 10.1016/j.foreco.2021.119331. |
| [38] | Liu XY, Koba K, Koyama LA, Hobbie SE, Weiss MS, Inagaki Y, Shaver GR, Giblin AE, Hobara S, Nadelhoffer KJ, Sommerkorn M, Rastetter EB, Kling GW, Laundre JA, Yano Y, et al.(2018). Nitrate is an important nitrogen source for Arctic tundra plants. Proceedings of the National Academy of Sciences of the United States of America, 115, 3398-3403. |
| [39] | Ma QX, Xu M, Liu MJ, Cao XC, Hill PW, Chadwick DR, Wu LH, Jones DL (2022). Organic and inorganic sulfur and nitrogen uptake by co-existing grassland plant species competing with soil microorganisms. Soil Biology & Biochemistry, 168, 108627. DOI: 10.1016/j.soilbio.2022.108627. |
| [40] | Maestre FT, Eldridge DJ, Soliveres S, Kéfi S, Delgado- Baquerizo M, Bowker MA, García-Palacios P, Gaitán J, Gallardo A, Lázaro R, Berdugo M (2016). Structure and functioning of dryland ecosystems in a changing world. Annual Review of Ecology, Evolution, and Systematics, 47, 215-237. |
| [41] | Mayor JR, Wright SJ, Schuur EAG, Brooks ME, Turner BL (2014). Stable nitrogen isotope patterns of trees and soils altered by long-term nitrogen and phosphorus addition to a lowland tropical rainforest. Biogeochemistry, 119, 293-306. |
| [42] | McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G (2002). Resource-based niches provide a basis for plant species diversity and dominance in Arctic tundra. Nature, 415, 68-71. |
| [43] | Miao C, Bai YX, Zhang YQ, She WW, Liu L, Qiao YG, Qin SG (2022). Interspecific interactions alter plant functional strategies in a revegetated shrub-dominated community in the Mu Us Desert, China. Annals of Botany, 130, 149-158. |
| [44] | Miller AE, Bowman WD, Suding KN (2007). Plant uptake of inorganic and organic nitrogen: neighbor identity matters. Ecology, 88, 1832-1840. |
| [45] | Pastore AI, Barabás G, Bimler MD, Mayfield MM, Miller TE (2021). The evolution of niche overlap and competitive differences. Nature Ecology & Evolution, 5, 330-337. |
| [46] | Pierce NA, Archer SR, Bestelmeyer BT, James DK (2019). Grass-shrub competition in arid lands: an overlooked driver in grassland-shrubland state transition? Ecosystems, 22, 619-628. |
| [47] | Qiao JJ, Zuo XA, Yue P, Wang SK, Hu Y, Guo XX, Li XY, Lv P, Guo AX, Sun SS (2023). High nitrogen addition induces functional trait divergence of plant community in a temperate desert steppe. Plant and Soil, 487, 133-156. |
| [48] | Qiao YG, Bai YX, Zhang YQ, She WW, Lai ZR, Qin SG (2019). Arbuscular mycorrhizal fungi shape the adaptive strategy of plants by mediating nutrient acquisition in a shrub-dominated community in the Mu Us Desert. Plant and Soil, 443, 549-564. |
| [49] | Quan Z, Liu X, Liu D (2022). Research progress on soil soluble organic nitrogen. Chinese Journal of Applied Ecology, 33, 277-288. |
| [全智, 刘轩, 刘东 (2022), 土壤可溶性有机氮研究进展. 应用生态学报, 33, 277-288.] | |
| [50] | Raab TK, Lipson DA, Monson RK (1999). Soil amino acid utilization among species of the Cyperaceae: plant and soil processes. Ecology, 80, 2408-2419. |
| [51] | Rahman S, Mehta S, Husen A (2024). Use of amino acids in plant growth, photosynthetic assimilation, and nutrient availability//Husen A. Biostimulants in Plant Protection and Performance. Elsevier, Amsterdam, The Netherlands. 117-127. |
| [52] | Ramond JB, Jordaan K, Díez B, Heinzelmann SM, Cowan DA (2022). Microbial biogeochemical cycling of nitrogen in arid ecosystems. Microbiology and Molecular Biology Reviews, 86, e0010921. DOI: 10.1128/mmbr.00109-21. |
| [53] | Rejmánková E (2005). Nutrient resorption in wetland macrophytes: comparison across several regions of different nutrient status. New Phytologist, 167, 471-482. |
| [54] | Sah RN (1994). Nitrate-nitrogen determination—A critical review. Communications in Soil Science and Plant Analysis, 25, 2841-2869. |
| [55] | Schimann H, Ponton S, H?ttenschwiler S, Ferry B, Lensi R, Domenach AM, Roggy JC (2008). Differing nitrogen use strategies of two tropical rainforest late successional tree species in French Guiana: evidence from 15N natural abundance and microbial activities. Soil Biology & Biochemistry, 40, 487-494. |
| [56] | She WW, Bai YX, Zhang YQ, Qin SG, Feng W, Lai ZR, Qiao YG, Liu L, Zhang WJ, Miao C (2021). Nitrogen-enhanced herbaceous competition threatens woody species persistence in a desert ecosystem. Plant and Soil, 460, 333-345. |
| [57] | She WW, Bai YX, Zhang YQ, Qin SG, Jia X, Feng W, Lai ZR, Fu J, Qiao YG (2020). Nitrogen enrichment suppresses revegetated shrub growth under increased precipitation via herb-induced topsoil water limitation in a desert ecosystem in Northern China. Plant and Soil, 446, 97-110. |
| [58] | Simon J (2023). Relevance of organic vs. inorganic nitrogen in intra- and interspecific competition of seven central European tree species. Trees, 37, 1583-1591. |
| [59] | Skuji?? J (1981). Nitrogen cycling in arid ecosystems. Ecological Bulletins, 33, 477-491. |
| [60] | Steidinger BS, Turner BL, Corrales A, Dalling JW (2015). Variability in potential to exploit different soil organic phosphorus compounds among tropical montane tree species. Functional Ecology, 29, 121-130. |
| [61] | Templer PH, Dawson TE (2004). Nitrogen uptake by four tree species of the Catskill Mountains, New York:implications for forest N dynamics. Plant and Soil, 262, 251-261. |
| [62] | von Felten S, Niklaus PA, Scherer-Lorenzen M, Hector A, Buchmann N (2012). Do grassland plant communities profit from N partitioning by soil depth? Ecology, 93, 2386-2396. |
| [63] | Wang RX, Tian YQ, Ouyang SN, Xu XL, Xu FZ, Zhang Y (2016). Nitrogen acquisition strategies used by Leymus chinensis and Stipa grandis in temperate steppes. Biology and Fertility of Soils, 52, 951-961. |
| [64] | Wang WY, Ma YG, Xu J, Wang HC, Zhu JF, Zhou HK (2012). The uptake diversity of soil nitrogen nutrients by main plant species in Kobresia humilis alpine meadow on the Qinghai-Tibet Plateau. Science China: Earth Sciences, 55, 1688-1695. |
| [王文颖, 马永贵, 徐进, 王慧春, 朱锦福, 周华坤 (2012). 高寒矮嵩草(Kobresia humilis)草甸植物吸收土壤氮的多元化途径研究. 中国科学: 地球科学, 55, 1688-1695.] | |
| [65] | White JR, Reddy KR (2009). Biogeochemical dynamics I: nitrogen cycling in wetlands. The Wetlands Handbook, 2, 213-227. |
| [66] | Xu XL, Ouyang H, Cao GM, Richter A, Wanek W, Kuzyakov Y (2011a). Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant and Soil, 341, 495-504. |
| [67] | Xu XL, Ouyang H, Kuzyakov Y, Richter A, Wanek W (2006). Significance of organic nitrogen acquisition for dominant plant species in an alpine meadow on the Tibet Plateau, China. Plant and Soil, 285, 221-231. |
| [68] | Xu XL, Ouyang H, Richter A, Wanek W, Cao GM, Kuzyakov Y (2011b). Spatio-temporal variations determine plant-microbe competition for inorganic nitrogen in an alpine meadow. Journal of Ecology, 99, 563-571. |
| [69] | Yahdjian L, Gherardi L, Sala OE (2014). Grasses have larger response than shrubs to increased nitrogen availability: a fertilization experiment in the Patagonian steppe. Journal of Arid Environments, 102, 17-20. |
| [70] | Zak DR, Grigal DF, Gleeson S, Tilman D (1990). Carbon and nitrogen cycling during old-field succession: constraints on plant and microbial biomass. Biogeochemistry, 11, 111-129. |
| [71] | Zhang XJ, Liang XS, Ma W, Wang ZW (2021). Temporal variation and resorption of nutrients in plant culms and leaves in Hulun Buir grassland. Chinese Journal of Plant Ecology, 45, 738-748. |
| [张效境, 梁潇洒, 马望, 王正文 (2021). 呼伦贝尔草地植物茎秆和叶片中养分的时间动态与回收. 植物生态学报, 45, 738-748.] | |
| [72] | Zhang XS (1994). Principles and optimal models for development of Maowusu sandy grassland. Acta Phytoecologia et Geobotanica Sinica, 18, 1-16. |
| [张新时 (1994). 毛乌素沙地的生态背景及其草地建设的原则与优化模式. 植物生态学报, 18, 1-16.] | |
| [73] | Zhuang W, Wang M, Xiao Y, Zhou X, Wu N (2022). Differential uptake of nitrogen forms by two herbs in the Gurbantunggut desert, Central Asia. Plant Biology, 24, 758-765. |