美洲黑杨幼苗生长和生理生态指标对干旱-复水响应的性别差异
- 浙江农林大学林业与生物技术学院, 杭州 311300
收稿日期: 2022-04-29
录用日期: 2022-09-28
网络出版日期: 2022-09-28
基金资助
浙江省自然科学基金(LY19C160005)
Sexual divergence of Populus deltoides seedlings growth and ecophysiological response to drought and rewatering
- College of Forestry and Biotechnology, Zhejiang A&F University, Hangzhou 311300, China
Received date: 2022-04-29
Accepted date: 2022-09-28
Online published: 2022-09-28
Supported by
Natural Science Foundation of Zhejiang Province(LY19C160005)
摘要
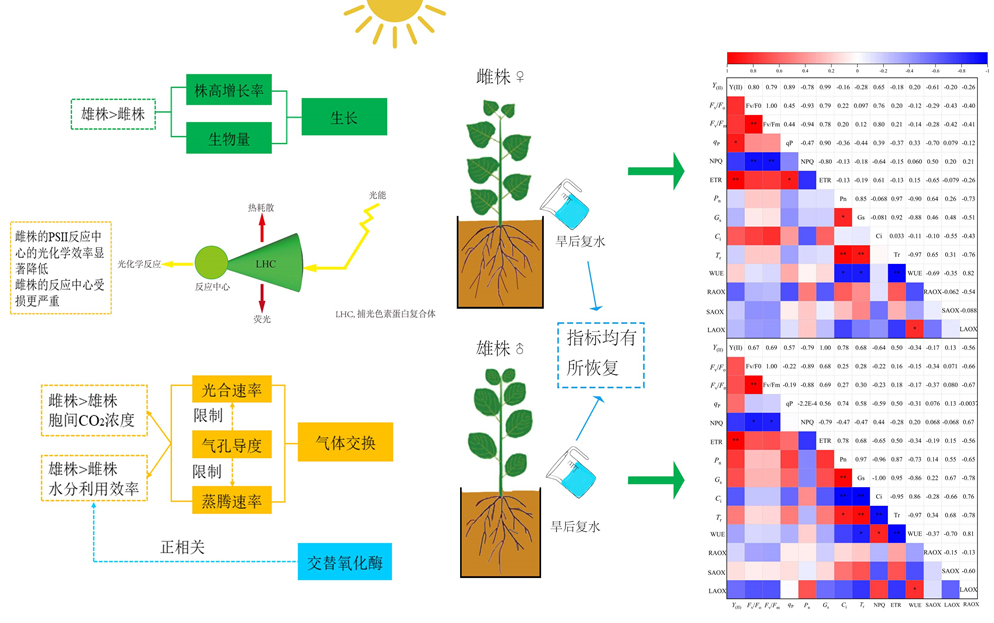
研究雌雄异株植物在干旱-复水过程中的生长表现和生理机制变化有助于了解不同性别植物对环境的适应以及抗逆能力差异, 可为全球气候变化背景下造林树种的选择提供理论依据。该研究以美洲黑杨(Populus deltoides)为研究对象, 采用盆栽控水实验, 通过测定干旱-复水条件下幼苗的生长、叶片水分参数、光合参数等指标, 分析雌雄植株对不同水分处理的生理响应差异。结果显示: 干旱对雌雄植株生长均产生不利影响, 植株株高和地径生长减缓, 叶片的相对含水量、水势、净光合速率、气孔导度、蒸腾速率、光合电子产量、光化学猝灭系数以及电子传递速率均显著降低。干旱胁迫下, 雄株的生长情况优于雌株, 表现为雄株有更高的株高生长速率、地下生物量分配和水分利用效率。干旱胁迫下, 雌株最大光化学效率与光系统II (PSII)的潜在活性显著降低, 胞间CO2浓度显著增加; 雄株PSII受损伤较小, 但根和叶的线粒体交替氧化酶活性显著升高。复水30天后, 植株的各指标均有不同程度的恢复, 但雌雄株的株高、地径增长率和净光合速率等显著低于未经过干旱胁迫的对照组, 且不存在性别差异。干旱胁迫下, 美洲黑杨雌雄株幼苗的生长均受到不同程度的抑制, 雌株较雄株受影响更大。雄株通过降低叶片相对含水量、光合速率和叶绿素荧光参数, 增加交替氧化酶活性等一系列生理响应来提高植株干旱适应性。因此, 雄株比雌株具有更有效的保护机制, 有利于复水后各生理活动的恢复。
本文引用格式
施梦娇, 李斌, 伊力塔, 刘美华 . 美洲黑杨幼苗生长和生理生态指标对干旱-复水响应的性别差异[J]. 植物生态学报, 2023 , 47(8) : 1159 -1170 . DOI: 10.17521/cjpe.2022.0173
Abstract
Aims Global climate change has aggravated the effects of drought which is one of the major factors restricting the sustainable development of agriculture and forestry. It is important to study the growth performance and the changes of physiological mechanism of dioecious plants during drought and rewatering process, which could help to understand the difference of adaptability and stress tolerance to the unfavorable environment in dioecious plants. And this paper also provides a theoretical basis for the selection of tree species for afforestation in the context of global climate change.
Methods Male and female cuttings of Populus deltoides were planted in the pots in a greenhouse, and were treated by drought stress and rewatering. The growth, leaf water parameters, and photosynthetic parameters were measured to analyze the physiological adaptability and stress tolerance of males and females under drought-rewatering conditions.
Important findings Drought stress showed negative effects on plant growth by reducing the growth of plant height and basal diameter, with decreased relative water content, water potential, net photosynthetic rate, stomatal conductance, transpiration rate, photosynthetic electron yield, photochemical quenching coefficient and electron transfer rate of leaves of males and females. There were no significant sexual differences in all parameters between males and females under sufficient water supply. Under drought stress, the growth of male plants was better than that of females, with higher growth rate of plant height and more root biomass accumulation in males. Drought resulted in the decrease of the maximum photochemical efficiency and the potential activity of photosystem II (PSII), and the increase of the intercellular CO2 concentration of females. PSII of male plants was less damaged under drought conditions, and the photosynthetic reaction center still maintained a high light-harvesting efficiency. Meanwhile, alternating oxidase (AOX) activity was significantly increased in roots and leaves of male seedlings, which could alleviate the effect of photoinhibition. All indexes recovered after 30 days of rewatering. However, the growth rate of plant height and ground diameter, and net photosynthetic rate of males and females under drought stress were significantly lower than those of the control group without drought stress. The results showed that the growth of male and female seedlings of P. deltoides was inhibited by drought stress, and the females were more likely affected by water deficit. Water stress induced a series of adaptive physiological effects in males, including decreased leaf relative water content, decreased photosynthetic and chlorophyll fluorescence parameters, and increased activity of alternating oxidase. Therefore, males had a more effective protective mechanism than females, which was also conducive to the recovery of various functions after rewatering.

Key words: drought; rewatering; dioecious; growth physiology; Populus deltoides
参考文献
| [1] | Ali S, Hayat K, Iqbal A, Xie LN (2020). Implications of abscisic acid in the drought stress tolerance of plants. Agronomy, 10, 1323. DOI: 10.3390/agronomy10091323. |
| [2] | Bartoli CG, Gomez F, Gergoff G, Guiamét JJ, Puntarulo S (2005). Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. Journal of Experimental Botany, 56, 1269-1276. |
| [3] | Baurain D, Dinant M, Coosemans N, Matagne RF (2003). Regulation of the alternative oxidase Aox1 gene in Chlamydomonas reinhardtii. Role of the nitrogen source on the expression of a reporter gene under the control of the Aox1 promoter. Plant Physiology, 131, 1418-1430. |
| [4] | Chastain DR, Snider JL, Collins GD, Perry CD, Whitaker J, Byrd SA (2014). Water deficit in field-grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. Journal of Plant Physiology, 171, 1576-1585. |
| [5] | Chen J, Dong TF, Duan BL, Korpelainen H, Niinemets ü, Li CY (2015). Sexual competition and N supply interactively affect the dimorphism and competiveness of opposite sexes in Populus cathayana. Plant, Cell & Environment, 38, 1285-1298. |
| [6] | Chen J, Duan BL, Wang ML, Korpelainen H, Li CY (2014). Intra- and inter-sexual competition of Populus cathayana under different watering regimes. Functional Ecology, 28, 124-136. |
| [7] | Chen LH (2011). Differences of Physiological and Ecological Responses of Male and Female Plants of Populus yunnanensis to Environmental Stress. PhD dissertation, University of Chinese Academy of Sciences, Beijing. |
| [7] | [陈良华 (2011). 滇杨(Populus yunnanensis)雌雄植株对环境胁迫的生理生态响应差异. 博士学位论文, 中国科学院大学, 北京.] |
| [8] | Dahal K, Vanlerberghe GC (2017). Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytologist, 213, 560-571. |
| [9] | Duan QY, Tian Y, E XW, Qin GZ, Zhang JY (2020). Sexual differences in growth and physiological properties of southern-type poplar clones in response to continuous drought and re-watering. Chinese Journal of Ecology, 39, 2140-2150. |
| [9] | [段启英, 田野, 鄂晓伟, 秦广震, 张贾宇 (2020). 南方型黑杨生长和生理特性对持续干旱和复水响应的性别差异. 生态学杂志, 39, 2140-2150.] |
| [10] | Efeo?lu B, Ekmek?i Y, ?i?ek N (2009). Physiological responses of three maize cultivars to drought stress and recovery. South African Journal of Botany, 75, 34-42. |
| [11] | Fang YJ, Xiong LZ (2015). General mechanisms of drought response and their application in drought resistance improvement in plants. Cellular and Molecular Life Sciences, 72, 673-689. |
| [12] | Finnegan PM, Umbach AL, Wilce JA (2003). Prokaryotic origins for the mitochondrial alternative oxidase and plastid terminal oxidase nuclear genes. FEBS Letters, 555, 425-430. |
| [13] | Fu YY (2018). Stomatal and non-stomatal limitation of photosynthesis in plant leaves under water stress. Science and Technology & Innovation, (8), 57-58. |
| [13] | [符玉英 (2018). 水分胁迫下植物叶片光合的气孔和非气孔限制. 科技与创新, (8), 57-58.] |
| [14] | Galvez DA, Landh?usser SM, Tyree MT (2011). Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiology, 31, 250-257. |
| [15] | Gao TH, Shang JZ, Song LT, Wang WF (2021). Responses of leaf photosynthetic and anatomical characteristics in Populus simonii cuttings to drought and re-watering. Science of Soil and Water Conservation, 19(6), 18-26. |
| [15] | [高钿惠, 尚佳州, 宋立婷, 王卫锋 (2021). 小叶杨叶片光合特性与解剖结构对干旱及复水的响应. 中国水土保持科学, 19(6), 18-26.] |
| [16] | Hultine KR, Grady KC, Wood TE, Shuster SM, Stella JC, Whitham TG (2016). Climate change perils for dioecious plant species. Nature Plants, 2, 16109. DOI: 10.1038/nplants.2016.109. |
| [17] | Jing X, Wang JD, Wang JH, Huang WJ, Liu LY (2008). Classifying forest vegetation using sub-region classification based on multi-temporal remote sensing images. Remote Sensing Technology and Application, 23, 394-397. |
| [17] | [竞霞, 王锦地, 王纪华, 黄文江, 刘良云 (2008). 基于分区和多时相遥感数据的山区植被分类研究. 遥感技术与应用, 23, 394-397.] |
| [18] | Lei YB, Yin CY, Li CY (2006). Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiologia Plantarum, 127, 182-191. |
| [19] | Leigh A, Nicotra AB (2003). Sexual dimorphism in reproductive allocation and water use efficiency in Maireana pyramidata (Chenopodiaceae), a dioecious, semi-arid shrub. Australian Journal of Botany, 51, 509-514. |
| [20] | Li CY, Yin CY, Liu SR (2004). Different responses of two contrasting Populus davidiana populations to exogenous abscisic acid application. Environmental and Experimental Botany, 51, 237-246. |
| [21] | Li JW, Wang JX, Zhang ML, Ji ZB, Xue S (2009). Effect of drought and rewater on leaf water potential of Robinia pseudoacacia. Journal of Northwest Forestry University, 24(3), 33-36. |
| [21] | [李继文, 王进鑫, 张慕黎, 吉增宝, 薛设 (2009). 干旱及复水对刺槐叶水势的影响. 西北林学院学报, 24(3), 33-36.] |
| [22] | Li L, Li XY, Lin LS, Wang YJ, Xue W (2011). Photosystem II characteristics of nine Gramineae species in southern Taklamakan Desert. Chinese Journal of Applied Ecology, 22, 2599-2603. |
| [22] | [李磊, 李向义, 林丽莎, 王迎菊, 薛伟 (2011). 塔克拉玛干沙漠南缘9种禾本科牧草光系统II特性. 应用生态学报, 22, 2599-2603.] |
| [23] | Liao T, Wang Y, Xu CP, Li Y, Kang XY (2018). Adaptive photosynthetic and physiological responses to drought and rewatering in triploid Populus populations. Photosynthetica, 56, 578-590. |
| [24] | Limousin JM, Bickford CP, Dickman LT, Pangle RE, Hudson PJ, Boutz AL, Gehres N, Osuna JL, Pockman WT, McDowell NG (2013). Regulation and acclimation of leaf gas exchange in a pi?on-juniper woodland exposed to three different precipitation regimes. Plant, Cell & Environment, 36, 1812-1825. |
| [25] | Liu CG, Wang YJ, Pan KW, Wang QW, Liang J, Jin YQ, Tariq A (2017). The synergistic responses of different photoprotective pathways in dwarf bamboo (Fargesia rufa) to drought and subsequent rewatering. Frontiers in Plant Science, 8, 489. DOI: 10.3389/fpls.2017.00489. |
| [26] | Liu KQ, Liu WH, Jia ZF, Ma X, Liang GL (2020). Effects of water stress on organ growth and water use efficiency of Avena sativa ‘Qingyan No. 1’. Acta Agrestia Sinica, 28, 1552-1562. |
| [26] | [刘凯强, 刘文辉, 贾志锋, 马祥, 梁国玲 (2020). 干旱胁迫对‘青燕1号’燕麦器官生长及水分利用效率的影响. 草地学报, 28, 1552-1562.] |
| [27] | Liu M, Zhao Y, Wang YT, Korpelainen H, Li CY (2022). Stem xylem traits and wood formation affect sex-specific responses to drought and rewatering in Populus cathayana. Tree Physiology, 42, 1350-1363. |
| [28] | Mathobo R, Marais D, Steyn JM (2017). The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agricultural Water Management, 180, 118-125. |
| [29] | Mu Q, Dong MQ, Xu J, Cao YX, Ding YB, Sun SK, Cai HJ (2022). Photosynthesis of winter wheat effectively reflected multiple physiological responses under short-term drought-rewatering conditions. Journal of the Science of Food and Agriculture, 102, 2472-2483. |
| [30] | Nakamura K, Sakamoto K, Kido Y, Fujimoto Y, Suzuki T, Suzuki M, Yabu Y, Ohta N, Tsuda A, Onuma M, Kita K (2005). Mutational analysis of the Trypanosoma vivax alternative oxidase: the E(X)6Y motif is conserved in both mitochondrial alternative oxidase and plastid terminal oxidase and is indispensable for enzyme activity. Biochemical and Biophysical Research Communications, 334, 593-600. |
| [31] | Ni SL, Li XM, Wang YC, Ren GS (2018). Physiological development and water use efficiency of winter wheat after re-watering following drought stresses at different growth stages. Journal of Irrigation and Drainage, 37(11), 20-25. |
| [31] | [倪胜利, 李兴茂, 王亚翠, 任根深 (2018). 旱后复水对冬小麦生长发育及水分利用效率的影响. 灌溉排水学报, 37(11), 20-25.] |
| [32] | Peng SM, Jiang H, Zhang S, Chen LH, Li XG, Korpelainen H, Li CY (2012). Transcriptional profiling reveals sexual differences of the leaf transcriptomes in response to drought stress in Populus yunnanensis. Tree Physiology, 32, 1541-1555. |
| [33] | Renner SS (2014). The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. American Journal of Botany, 101, 1588-1596. |
| [34] | Rubin RL, van Groenigen KJ, Hungate BA (2017). Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant and Soil, 416, 309-323. |
| [35] | Song XY, Zhou GS, He QJ (2021). Critical leaf water content for maize photosynthesis under drought stress and its response to rewatering. Sustainability, 13, 7218. DOI: 10.3390/su13137218. |
| [36] | Tardieu F, Tuberosa R (2010). Dissection and modelling of abiotic stress tolerance in plants. Current Opinion in Plant Biology, 13, 206-212. |
| [37] | Tian K, Wang Y, Chen D, Cao M, Luo J (2022). Influence of drought stress and post-drought rewatering on phytoremediation effect of Arabidopsis thaliana. Bulletin of Environmental Contamination and Toxicology, 108, 594-599. |
| [38] | Wang J, Vanlerberghe GC (2013). A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiologia Plantarum, 149, 461-473. |
| [39] | Wang YL, Sun JW, Xun SH, Zang DK, Fang XX, Zhang T (2017). Effects of drought stress on photosynthetic characteristics, fluorescence parameters and relative water content of ‘Dong Yue Hong’ leaves. Shandong Agricultural Sciences, 49(4), 46-50. |
| [39] | [王玉丽, 孙居文, 荀守华, 臧德奎, 方晓晓, 张涛 (2017). 干旱胁迫对东岳红光合特性、叶绿素荧光参数及叶片相对含水量的影响. 山东农业科学, 49(4), 46-50.] |
| [40] | Xu X, Yang F, Xiao XW, Zhang S, Korpelainen H, Li CY (2008). Sex-specific responses of Populus cathayana to drought and elevated temperatures. Plant, Cell & Environment, 31, 850-860. |
| [41] | Xu XY, Sun S, Jin LQ, Liu MJ, Gao HY (2015). Up-regulation of the mitochondrial alternative oxidase pathway enhances photoprotection in Malus hupehensis leaves under drought stress. Plant Physiology Journal, 51, 2119-2125. |
| [41] | [徐秀玉, 孙山, 金立桥, 刘美君, 高辉远 (2015). 干旱胁迫下平邑甜茶叶片交替呼吸途径上调对光破坏的防御作用. 植物生理学报, 51, 2119-2125.] |
| [42] | Yang B, Liu ZZ, Peng FR, Cao F, Chen T, Deng QJ, Chen WJ (2017). Growth and photosynthetic characteristics for pecan cultivars during drought stress and recovery. Journal of Zhejiang A&F University, 34, 991-998. |
| [42] | [杨标, 刘壮壮, 彭方仁, 曹凡, 陈涛, 邓秋菊, 陈文静 (2017). 干旱胁迫和复水下不同薄壳山核桃品种的生长和光合特性. 浙江农林大学学报, 34, 991-998.] |
| [43] | Yu SY, Zhou YF, Huang W, Wang X, Gao XQ, Fu BZ (2021). Effects of drought, salt, and drought-salt combined stress on photosynthetic and physiological characteristics of Agropyron mongolicum. Acta Agrestia Sinica, 29, 2399-2406. |
| [43] | [余淑艳, 周燕飞, 黄薇, 王星, 高雪芹, 伏兵哲 (2021). 干旱、盐及旱盐复合胁迫对沙芦草光合和生理特性的影响. 草地学报, 29, 2399-2406.] |
| [44] | Zhang S, Chen FG, Peng SM, Ma WJ, Korpelainen H, Li CY (2010). Comparative physiological, ultrastructural and proteomic analyses reveal sexual differences in the responses of Populus cathayana under drought stress. Proteomics, 10, 2661-2677. |
| [45] | Zhang S, Chen LH, Duan BL, Korpelainen H, Li CY (2012). Populus cathayana males exhibit more efficient protective mechanisms than females under drought stress. Forest Ecology and Management, 275, 68-78. |
| [46] | Zhang ZH, Cao BL, Gao S, Xu K (2019). Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma, 256, 1013-1024. |
| [47] | Zhao WH, Liu LZ, Shen Q, Yang JH, Han XY, Tian F, Wu JJ (2020). Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water, 12, 2127. DOI: 10.3390/w12082127. |
| [48] | Zhao YQ, Gao MQ, Li T, Wang WF (2020). Effects of water stress on leaf gas exchange and biomass allocation of Populus × popularis ‘35-44’ cuttings. Acta Ecologica Sinica, 40, 1683-1689. |
| [48] | [赵瑜琦, 高苗琴, 李涛, 王卫锋 (2020). 干旱胁迫对群众杨光合特性与器官干物质分配的影响. 生态学报, 40, 1683-1689.] |