

北京山区油松和元宝槭冠层气孔导度特征及其环境响应
收稿日期: 2021-05-25
录用日期: 2021-07-29
网络出版日期: 2021-08-26
基金资助
国家自然科学基金(31872711)
Canopy stomatal conductance characteristics of Pinus tabulaeformis and Acer truncatum and their responses to environmental factors in the mountain area of Beijing
Received date: 2021-05-25
Accepted date: 2021-07-29
Online published: 2021-08-26
Supported by
National Natural Science Foundation of China(31872711)
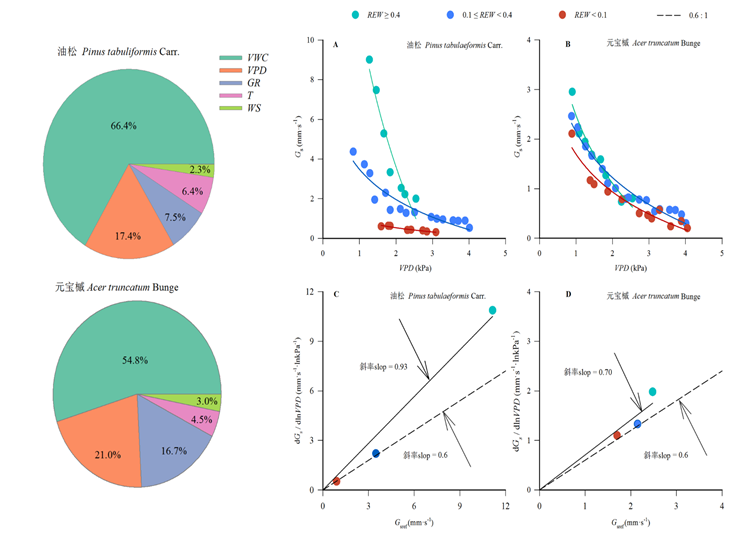
叶片气孔是植物进行水汽交换的通道, 影响着植物的蒸腾和光合作用。然而叶片气孔行为受环境条件和树种类型的影响, 不同树种冠层气孔导度对环境因子响应的差异性, 以及在生长季不同时期叶片气孔对冠层蒸腾的调节作用是否会发生改变, 仍不清楚。该研究目的是通过探究各环境因子对不同树种冠层气孔导度的相对贡献率以及叶片气孔对冠层蒸腾的调节作用, 为深入了解植物水分利用状况和山区森林经营提供参考依据。于2018年生长季以北京八达岭国家森林公园内的58年生油松(Pinus tabuliformis)和39年生元宝槭(Acer truncatum)为研究对象, 利用热扩散技术对其树干液流进行连续监测, 并同步监测环境因子。利用彭曼公式计算冠层气孔导度(Gs)。主要结果: (1)油松和元宝槭日间Gs在日、月时间尺度上存在明显差异。5-7月油松和元宝槭日动态Gs均随饱和水汽压差(VPD)和太阳辐射(GR)的增加呈上升趋势, 上升持续时间比8月和9月长; 在月尺度上, 随着VPD、GR的降低和土壤湿度(VWC)的升高, Gs从5月到9月整体上升。(2)利用增强回归树法分析得到VWC和VPD对Gs的贡献率最大, 其次是GR、气温和风速。VWC和VPD对油松Gs的贡献率分别为66.4%和17.4%, 对元宝槭Gs的贡献率分别为54.8%和21.0%。(3)油松和元宝槭的dGs/dlnVPD值与参考冠层气孔导度之间的斜率均显著高于0.6, 气孔调节作用相对较强。综上所述, 气孔对环境因子的响应在树种以及生长季不同时期之间存在差异, 为防止水分过度散失, 两树种在不同土壤水分条件下均通过严格的气孔调节控制蒸腾量。
陈胜楠, 陈左司南, 张志强 . 北京山区油松和元宝槭冠层气孔导度特征及其环境响应[J]. 植物生态学报, 2021 , 45(12) : 1329 -1340 . DOI: 10.17521/cjpe.2021.0198
Aims Leaf stomata are channels for plants to exchange water vapor that affects transpiration and photosynthesis. However, leaf stomatal behaviors are affected by environmental factors and tree species. It is still unclear whether the responses of canopy stomatal conductance to environmental factors differ between tree species and whether the stomatal regulations on canopy transpiration change with different periods of the growing season. The objective of this study was to explore the relative contribution of environmental factors to canopy stomatal conductance and the regulation of leaf stomata on canopy transpiration for different tree species, which could provide references for further understanding the water use status of trees and forest management in mountain areas.
Methods During the growing season of 2018, Pinus tabuliformis (58-year-old) and Acer truncatum (39-year- old) at Badaling National Forest Park in Beijing were selected. Sap flow was measured by using the thermal dissipation method. Environmental factors were also measured synchronously. Canopy stomatal conductance (Gs) was estimated by using the Penman-Monteith equation.
Important findings (1) The daytime Gs of P. tabulaeformis and A. truncatum varied evidently at daily and monthly scales. From May to July, daily dynamic Gs of P. tabulaeformis and A. truncatum increased with vapor pressure deficit (VPD) and solar radiation (GR), in which the rising periods were longer than that during August and September. At the monthly scale, as VPD and GR decreased, soil moisture (VWC) increased, Gs generally increased from May to September. (2) VWC and VPD contributed to the major variation of the Gs, and then the GR, air temperature, and wind speed followed based on the boosted regression tree method. The relative contributions of VWC and VPDto Gs were 66.4% and 17.4% for P. tabulaeformis and 54.8% and 21.0% for A. truncatum, respectively. (3) The slopes between dGs/dlnVPD and the reference canopy stomatal conductance for both P. tabulaeformis and A. truncatum were significantly larger than 0.6, suggesting that their stomatal regulations were relatively strong. In summary, the response of stomata to environmental factors differs between tree species and different periods of the growing season. Under different soil water conditions, these two tree species could control transpiration through strict stomatal regulation to prevent excessive water loss.

| [1] | Aspinwall MJ, King JS, Domec JC, McKeand SE, Isik F (2011). Genetic effects on transpiration, canopy conductance, stomatal sensitivity to vapour pressure deficit, and cavitation resistance in loblolly pine. Ecohydrology, 4, 168-182. |
| [2] | Attia Z, Domec JC, Oren R, Way DA, Moshelion M (2015). Growth and physiological responses of isohydric and anisohydric poplars to drought. Journal of Experimental Botany, 66, 4373-4381. |
| [3] | Bovard BD, Curtis PS, Vogel CS, Su HB, Schmid HP (2005). Environmental controls on sap flow in a northern hardwood forest. Tree Physiology, 25, 31-38. |
| [4] | Brito P, Lorenzo JR, González-Rodríguez ÁM, Morales D, Wieser G, Jiménez MS (2015). Canopy transpiration of a semi-arid Pinus canariensis forest at a treeline ecotone in two hydrologically contrasting years. Agricultural and Forest Meteorology, 201, 120-127. |
| [5] | Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholz FG (2005). Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in neotropical savanna trees. Trees, 19, 296-304. |
| [6] | Campbell GS, Norman JM (1998). An Introduction to Environmental Biophysics. 2nd ed. Springer, New York. |
| [7] | Chen D, Wang YK, Liu SY, Wei XG, Wang X (2014). Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid Northwest China. Agricultural Water Management, 136, 23-33. |
| [8] | Chen SN, Kong Z, Chen LX, Liu QQ, Liu PS, Zhang ZQ (2020). The stand transpiration characteristics of Pinus tabulaeformis and its influential factors in a semi-arid urban environment. Acta Ecologica Sinica, 40, 1269-1280. |
| [8] | [ 陈胜楠, 孔喆, 陈立欣, 刘清泉, 刘平生, 张志强 (2020). 半干旱区城市环境下油松林分蒸腾特征及其影响因子. 生态学报, 40, 1269-1280.] |
| [9] | Chen ZSN (2020). Influences of Stand Density and Age on Canopy Transpiration, Nocturnal Sap Flow, and Their Biophysical Controls. PhD dissertation, Beijing Forestry University, Beijing. |
| [9] | [ 陈左司南 (2020). 不同密度/林龄油松和元宝枫人工林冠层蒸腾和夜间液流特征及机制研究. 博士学位论文, 北京林业大学, 北京.] |
| [10] | Chen ZSN, Zhang ZQ, Chen LX, Cai YM, Zhang HQ, Lou JP, Xu Z, Xu H, Song CH (2020). Sparse Pinus tabuliformis stands have higher canopy transpiration than dense stands three decades after thinning. Forests, 11, 70. DOI: 10.3390/f11010070. |
| [11] | Daley MJ, Phillips NG (2006). Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiology, 26, 411-419. |
| [12] | Elith J, Leathwick JR, Hastie T (2008). A working guide to boosted regression trees. Journal of Animal Ecology, 77, 802-813. |
| [13] | Ewers BE, MacKay DS, Gower ST, Ahl DE, Burrows SN, Samanta SS (2002). Tree species effects on stand transpiration in northern Wisconsin. Water Resources Research, 38, 11103. DOI: 10.1029/2001WR000830. |
| [14] | Ewers BE, MacKay DS, Samanta S (2007). Interannual consistency in canopy stomatal conductance control of leaf water potential across seven tree species. Tree Physiology, 27, 11-24. |
| [15] | Franks PJ, Drake PL, Froend RH (2007). Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant, Cell & Environment, 30, 19-30. |
| [16] | Ge Y, Wang MX, Sun XW, Qi JD (2017). Variation analysis of daily PM2.5 concentrations based on boosted regression tree: a case study in Changzhou. Environmental Science, 38, 485-494. |
| [16] | [ 葛跃, 王明新, 孙向武, 齐今笛 (2017). 基于增强回归树的城市PM2.5日均值变化分析: 以常州为例. 环境科学, 38, 485-494.] |
| [17] | Gillner S, Korn S, Hofmann M, Roloff A (2017). Contrasting strategies for tree species to cope with heat and dry conditions at urban sites. Urban Ecosystems, 20, 853-865. |
| [18] | Granier A (1985). A new method of sap flow measurement in tree stems. Annales des Sciences Forestières, 42, 193-200. |
| [19] | Granier A, Bréda N, Biron P, Villette S (1999). A lumped water balance model to evaluate duration and intensity of drought constraints in forest stands. Ecological Modelling, 116, 269-283. |
| [20] | Grossiord C, Buckley TN, Cernusak LA, Novick KA, Poulter B, Siegwolf RTW, Sperry JS, McDowell NG (2020). Plant responses to rising vapor pressure deficit. New Phytologist, 226, 1550-1566. |
| [21] | Gu DX, Wang Q, Otieno D (2017). Canopy transpiration and stomatal responses to prolonged drought by a dominant desert species in central Asia. Water, 9, 404. DOI: 10.3390/W9060404. |
| [22] | Hochberg U, Rockwell FE, Holbrook NM, Cochard H (2018). Iso/anisohydry: a plant-environment interaction rather than a simple hydraulic trait. Trends in Plant Science, 23, 112-120. |
| [23] | Jiao L, Lu N, Fang WW, Li ZS, Wang J, Jin Z (2019). Determining the independent impact of soil water on forest transpiration: a case study of a black locust plantation in the Loess Plateau, China. Journal of Hydrology, 572, 671-681. |
| [24] | Klein T, Shpringer I, Fikler B, Elbaz G, Cohen S, Yakir D (2013). Relationships between stomatal regulation, water- use, and water-use efficiency of two coexisting key Mediterranean tree species. Forest Ecology and Management, 302, 34-42. |
| [25] | Kong Z, Chen SN, Lü J, Chen LX, Zhang ZQ (2020). Characteristics of Populus euramericana sap flow over day and night and its influencing factors. Scientia Silvae Sinicae, 56(3), 8-20. |
| [25] | [ 孔喆, 陈胜楠, 律江, 陈立欣, 张志强 (2020). 欧美杨单株液流昼夜组成及其影响因素分析. 林业科学, 56(3), 8-20.] |
| [26] | Kumagai T, Tateishi M, Shimizu T, Otsuki K (2008). Transpiration and canopy conductance at two slope positions in a Japanese cedar forest watershed. Agricultural and Forest Meteorology, 148, 1444-1455. |
| [27] | Li CL, Liu M, Hu YM, Xu YY, Sun FY (2014). Driving forces analysis of urban expansion based on boosted regression trees and Logistic regression. Acta Ecologica Sinica, 34, 727-737. |
| [27] | [ 李春林, 刘淼, 胡远满, 徐岩岩, 孙凤云 (2014). 基于增强回归树和Logistic回归的城市扩展驱动力分析. 生态学报, 34, 727-737.] |
| [28] | Litvak E, McCarthy HR, Pataki DE (2012). Transpiration sensitivity of urban trees in a semi-arid climate is constrained by xylem vulnerability to cavitation. Tree Physiology, 32, 373-388. |
| [29] | MacKay SL, Arain MA, Khomik M, Brodeur JJ, Schumacher J, Hartmann H, Peichl M (2012). The impact of induced drought on transpiration and growth in a temperate pine plantation forest. Hydrological Processes, 26, 1779-1791. |
| [30] | Martin TA, Brown KJ, Cermák J, Ceulemans R, Kucera J, Meinzer FC, Rombold JS, Sprugel DG, Hinckley TM (1997). Crown conductance and tree and stand transpiration in a second-growth Abies amabilis forest. Canadian Journal of Forest Research, 27, 797-808. |
| [31] | Martin-Stpaul N, Delzon S, Cochard H (2017). Plant resistance to drought depends on timely stomatal closure. Ecology Letters, 20, 1437-1447. |
| [32] | McCarthy HR, Pataki DE (2010). Drivers of variability in water use of native and non-native urban trees in the greater Los Angeles area. Urban Ecosystems, 13, 393-414. |
| [33] | McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178, 719-739. |
| [34] | Oishi AC, Hawthorne DA, Oren R (2016). Baseliner: an open-source, interactive tool for processing sap flux data from thermal dissipation probes. SoftwareX, 5, 139-143. |
| [35] | Oren R, Pataki DE (2001). Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia, 127, 549-559. |
| [36] | Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KVR (1999). Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell & Environment, 22, 1515-1526. |
| [37] | Pataki DE, McCarthy HR, Litvak E, Pincetl S (2011). Transpiration of urban forests in the Los Angeles metropolitan area. Ecological Applications, 21, 661-677. |
| [38] | Peters EB, McFadden JP, Montgomery RA (2010). Biological and environmental controls on tree transpiration in a suburban landscape. Journal of Geophysical Research: Biogeosciences, 115, G04006. DOI: 10.1029/2009JG001266. |
| [39] | Pivovaroff AL, Cook VMW, Santiago LS (2018). Stomatal behaviour and stem xylem traits are coordinated for woody plant species under exceptional drought conditions. Plant, Cell & Environment, 41, 2617-2626. |
| [40] | Prasad AM, Iverson LR, Liaw A (2006). Newer classification and regression tree techniques: bagging and random forests for ecological prediction. Ecosystems, 9, 181-199. |
| [41] | Saito T, Kumagai T, Tateishi M, Kobayashi N, Otsuki K, Giambelluca TW (2017). Differences in seasonality and temperature dependency of stand transpiration and canopy conductance between Japanese cypress (Hinoki) and Japanese cedar (Sugi) in a plantation. Hydrological Processes, 31, 1952-1965. |
| [42] | Salleo S, Nardini A, Pitt F, Gullo MAL (2000). Xylem cavitation and hydraulic control of stomatal conductance in Laurel (Laurus nobilis L.). Plant, Cell & Environment, 23, 71-79. |
| [43] | Schäfer KVR, Oren R, Tenhunen JD (2000). The effect of tree height on crown level stomatal conductance. Plant, Cell & Environment, 23, 365-375. |
| [44] | She DL, Xia YQ, Shao MG, Peng SZ, Yu SG (2013). Transpiration and canopy conductance of Caragana korshinskii trees in response to soil moisture in sand land of China. Agroforestry Systems, 87, 667-678. |
| [45] | Sperry JS (2000). Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology, 104, 13-23. |
| [46] | Spicer R, Gartner BL (2001). The effects of cambial age and position within the stem on specific conductivity in Douglas- fir (Pseudotsuga menziesii) sapwood. Trees, 15, 222-229. |
| [47] | Tang JW, Bolstad PV, Ewers BE, Desai AR, Davis KJ, Carey EV (2006). Sap flux-upscaled canopy transpiration, stomatal conductance, and water use efficiency in an old growth forest in the Great Lakes region of the United States. Journal of Geophysical Research: Biogeosciences, 111, G02009. DOI: 10.1029/2005JG000083. |
| [48] | Tinoco-Ojanguren C, Pearcy RW (1993). Stomatal dynamics and its importance to carbon gain in two rainforest piper species. Oecologia, 94, 395-402. |
| [49] | Wolf A, Anderegg WRL, Pacala SW (2016). Optimal stomatal behavior with competition for water and risk of hydraulic impairment. Proceedings of the National Academy of Sciences of the United States of America, 113, E7222-E7230. |
| [50] | Wu X, Tang YK, Chen YM, Wen J, Xie YL, Lu SB (2018). Sap flow characteristics and responses to summer rainfall for Pinus tabulaeformis and Hippophae rhamnoides in the Loess hilly region of China. Ecology and Evolution, 8, 617-630. |
| [51] | Yan CR, Han XG, Chen LZ (2000). The relationship between the ecophysiological feature and leaf characteristics of some woody plants in Beijing mountain zone. Acta Ecologica Sinica, 20, 54-61. |
| [51] | [ 严昌荣, 韩兴国, 陈灵芝 (2000). 北京山区落叶阔叶林优势种叶片特点及其生理生态特性. 生态学报, 20, 54-61.] |
| [52] | Zhang T, Hong XL, Sun LW, Liu YJ (2017). Particle-retaining characteristics of six tree species and their relations with micro-configurations of leaf epidermis. Journal of Beijing Forestry University, 39(6), 70-77. |
| [52] | [ 张桐, 洪秀玲, 孙立炜, 刘玉军 (2017). 6种植物叶片的滞尘能力与其叶面结构的关系. 北京林业大学学报, 39(6), 70-77.] |
| [53] | Zhang Y, Oren R, Kang S (2012). Spatiotemporal variation of crown-scale stomatal conductance in an arid Vitis vinifera L. cv. Merlot vineyard: direct effects of hydraulic properties and indirect effects of canopy leaf area. Tree Physiology, 32, 262-279. |
| [54] | Zhang YJ, Meinzer FC, Qi JH, Goldstein G, Cao KF (2013). Midday stomatal conductance is more related to stem rather than leaf water status in subtropical deciduous and evergreen broadleaf trees. Plant, Cell & Environment, 36, 149-158. |
| [55] | Zhu LW, Zhao P, Wang Q, Ni GY, Niu JF, Zhao XH, Zhang ZZ, Zhao PQ, Gao JG, Huang YQ, Gu DX, Zhang ZF (2015). Stomatal and hydraulic conductance and water use in a eucalypt plantation in Guangxi, Southern China. Agricultural and Forest Meteorology, 202, 61-68. |
/
| 〈 |
|
〉 |