

水曲柳雌雄株复叶类型及光合功能对不同生境的响应
收稿日期: 2022-05-26
录用日期: 2023-01-31
网络出版日期: 2023-02-03
基金资助
中央高校基本科研业务费专项资金项目(2572020DR05)
Response of compound leaf types and photosynthetic function of male and female Fraxinus mandschurica to different habitats
Received date: 2022-05-26
Accepted date: 2023-01-31
Online published: 2023-02-03
Supported by
Fundamental Research Funds for the Central Universities(2572020DR05)
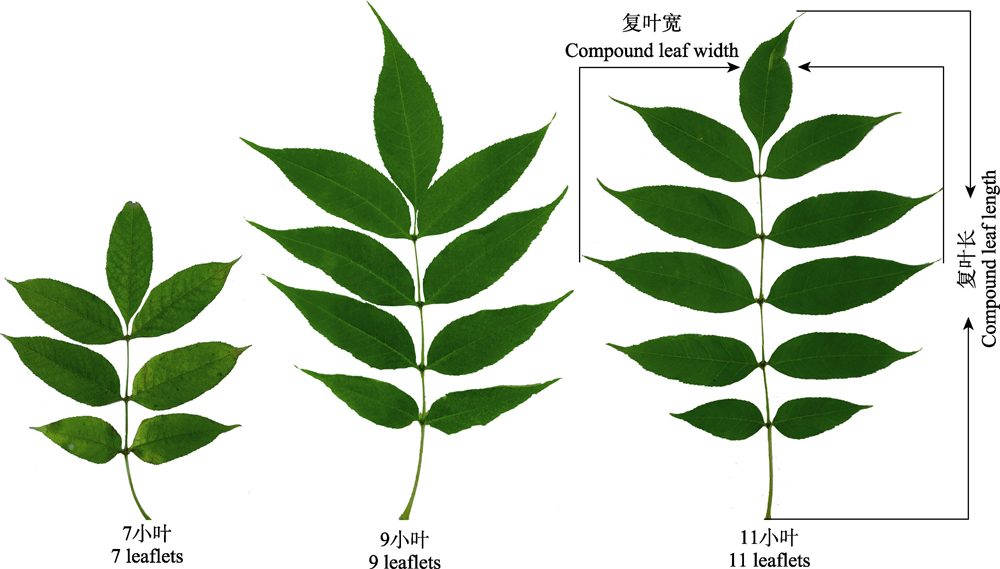
植物复叶形态和光合功能是对生境响应的最直观表现之一, 复叶光合能力和小叶数量直接反映出植物对生境的适应性。水曲柳(Fraxinus mandschurica)雌雄株存在一定的形态差异, 其复叶在不同生境下也可能存在不同的适应性, 为进一步分析雌雄异株型个体叶片对生境异质性的差异响应, 该研究以长期生长在干旱盐碱生境和适宜生境的水曲柳成熟雌株和雄株复叶为研究对象, 分析了两种生境中雌株和雄株复叶之间的性别差异以及不同小叶数量复叶间形态和光合功能性状的差异。研究发现: (1)和适宜生境相比, 干旱盐碱生境中水曲柳雌株和雄株同一小叶数量复叶的形态和光合能力虽未表现出性别差异, 但雌株9小叶型复叶数量占比的增加量高于雄株29.13%, 11小叶型复叶数量占比的下降量高于雄株33.74%, 雌株9小叶型复叶的光合速率下降量高于雄株10.98%。(2)在适宜生境中, 雄株复叶数量集中分布在9小叶型和11小叶型, 雌株复叶数量集中在11小叶型, 雌株和雄株11小叶型复叶的比叶面积大于7小叶型和9小叶型复叶, 干物质含量显著高于7小叶型和9小叶型复叶; 在干旱盐碱生境中, 雌株和雄株的复叶数量集中在9小叶型, 虽然雌雄株9小叶型复叶的比叶面积小于11小叶型复叶, 但9小叶型复叶的叶干质量高于11小叶型复叶; 两生境中雌株和雄株9小叶型和11小叶型复叶的净光合速率也无显著差异。(3)在复叶形态和气体交换能力指标中, 复叶的小叶数量占比、叶干质量、比叶面积和净光合速率具有较高的可塑性。综上所述, 在适宜生境下, 水曲柳雌雄株复叶的形态存在性别差异, 表现为雌株主要发育11小叶型复叶, 雄株主要发育9小叶型和11小叶型复叶, 雌雄株间的光合功能未表现出性别差异; 而在干旱盐碱生境下, 水曲柳雌雄株复叶的形态和光合功能未表现出性别差异, 雌株和雄株均主要发育9小叶型复叶。该研究为雌株和雄株复叶在不同生境下生长发育的性别差异提供了理论依据, 也为雌雄异株型乔木雌株和雄株复叶的生态适应性提供了数据支撑。
马常钦, 黄海龙, 彭政淋, 吴纯泽, 韦庆钰, 贾红涛, 卫星 . 水曲柳雌雄株复叶类型及光合功能对不同生境的响应[J]. 植物生态学报, 2023 , 47(9) : 1287 -1297 . DOI: 10.17521/cjpe.2022.0219
Aims The morphology and photosynthetic functions of compound leaf are one of the most intuitive manifestations of plant response to habitats. The changes of photosynthetic capacity and number of leaflets of compound leaf directly reflect the adaptability of plants to habitats. There are some morphological differences between male and female Fraxinus mandschurica, and their compound leaf may have different adaptabilities in various habitats.
Methods This experiment took compound leaves male and female mature F. mandschurica growing in the drought saline-alkali habitat and suitable habitat as the research materials. The gender differences between female and male plants and the differences of morphology and photosynthetic function between compound leaf with different numbers of leaflets under two habitats were compared and analyzed.
Important findings The results showed that (1) compared with the suitable habitat, the morphology and photosynthetic capacity of compound leaf with the same number leaflets of female and male F. mandschurica under the drought saline-alkali habitat did not show gender difference, but the increment of percentages of compound leaf with 9 leaflets of female plants was 29.13% higher than that of the male plants, the decrement of percentages of compound leaf with 11 leaflets was 33.74% higher than that of the male plants, and the decreament of photosynthetic rate of compound leaf with 9 leaflets of female plants was 10.98% higher than that of the male plants. (2) Under the suitable habitat, the proportion of compound leaves of male plants was mainly concentrated within 9 and 11 leaflets, while the proportion of compound leaf of female plants was mainly concentrated within 11 leaflets. Meanwhile, the specific leaf area and leaf dry mass of compound leaves with 11 leaflets of female and male plants were greater than that of compound leaf with 7 and 9 leaflets. Under the drought saline-alkali habitat, the proportion of compound leaf of female and male plants was mainly concentrated in 9 leaflets. The specific leaf area of compound leaf with 9 leaflets of male and female plants was less than that of 11 leaflet compound leaf, whereas the leaf dry mass showed the opposite trend. There was also no significant difference in the net photosynthetic rate of compound leaf with 9 and 11 leaflets between female and male plants in the two habitats. (3) Among the indexes of compound leaf morphology and stomatal gas exchange capacity, the percentages of compound leaf with different number of leaflets, leaf dry mass, specific leaf area and net photosynthetic rate of compound leaf have high plasticity. Therefore, under the suitable habitat, there is no gender difference in the photosynthetic function between male and female plants, but there are gender differences in the morphology of compound leaf of male and female F. mandschurica, which are shown as follows: the female plants mainly developed the compound leaf with 11 leaflets, and the male plants predominantly developed the compound leaf with 9 leaflets and 11 leaflets. However, under the drought saline-alkali habitat, the morphology and photosynthetic function of compound leaf of male and female F. mandschurica did not show gender differences, and both female and male plants mainly developed the compound leaf with 9 leaflets. This study provides a theoretical basis for the gender difference in the growth and development of compound leaf in different habitats, and also provides data support for the ecological adaptability of compound leaf of dioecious trees.

| [1] | Blein T, Hasson A, Laufs P (2010). Leaf development: What it needs to be complex. Current Opinion in Plant Biology, 13, 75-82. |
| [2] | Bongers FJ, Olmo M, Lopez-Iglesias B, Anten NPR, Villar R (2017). Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biology, 19, 386-395. |
| [3] | Bradshaw AD (2006). Unravelling phenotypic plasticity—Why should we bother? New Phytologist, 170, 644-648. |
| [4] | Chen XQ (1983). Study on soil genesis and soil classification on Harbin experimental tree farm. Journal of Northeast Forestry University, 11(3), 12-19. |
| [4] | [陈喜全 (1983). 关于哈尔滨实验林场土壤发生分类的探讨. 东北林学院学报, 11(3), 12-19.] |
| [5] | Dai AG (2013). Increasing drought under global warming in observations and models. Nature Climate Change, 3, 52-58. |
| [6] | DeMason DA, Chawla R (2004). Roles for auxin during morphogenesis of the compound leaves of pea (Pisum sativum). Planta, 218, 435-448. |
| [7] | Dong TF, Feng YL, Lei YB, Zhang LK (2012). Comparison on leaf functional traits of main dominant woody species in wet and dry habitats. Chinese Journal of Ecology, 31, 1043-1049. |
| [7] | [董廷发, 冯玉龙, 类延宝, 张丽坤 (2012). 干旱和湿润生境中主要优势树种叶片功能性状的比较. 生态学杂志, 31, 1043-1049.] |
| [8] | Du H, Liu H, Xiong L (2013). Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Frontiers in Plant Science, 4, 397. DOI: 10.3389/fpls.2013.00397. |
| [9] | Feng S, Sun HW, Ma HP, Zhang X, Ma SR, Qiao K, Zhou AM, Bu YY, Liu SK (2020). Sexual differences in physiological and transcriptional responses to salinity stress of Salix linearistipularis. Frontiers in Plant Science, 11, 517962. DOI: 10.3389/fpls.2020.517962. |
| [10] | Funk JL, Larson JE, Ames GM, Butterfield BJ, Cavender-Bares J, Firn J, Laughlin DC, Sutton-Grier AE, Williams L, Wright J (2017). Revisiting the Holy Grail, using plant functional traits to understand ecological processes. Biological Reviews, 92, 1156-1173. |
| [11] | Gage JL, Jarquin D, Romay C, Lorenz A, Buckler ES, Kaeppler S, Alkhalifah N, Bohn M, Campbell DA, Edwards J, Ertl D, Flint-Garcia S, Gardiner J, Good B, Hirsch CN, et al. (2017). The effect of artificial selection on phenotypic plasticity in maize. Nature Communications, 8, 1-11. |
| [12] | Gao L, Yang J, Liu RX (2009). Effects of soil moisture levels on photosynthesis, transpiration, and moisture use efficiency of female and male plants of Hippophae rhamnoides ssp. sinensis. Acta Ecologica Sinica, 29, 6025-6034. |
| [12] | [高丽, 杨劼, 刘瑞香 (2009). 不同土壤水分条件下中国沙棘雌雄株光合作用、蒸腾作用及水分利用效率特征. 生态学报, 29, 6025-6034.] |
| [13] | Gao L, Yang J, Liu RX (2010). Leaf morphological structure and physiological and biochemical characteristics of female and male Hippophae Rhamnoides subsp. sinensis under different soil moisture condition. Chinese Journal of Applied Ecology, 21, 2201-2208. |
| [13] | [高丽, 杨劼, 刘瑞香 (2010). 不同土壤水分条件下中国沙棘雌雄株叶片形态结构及生理生化特征. 应用生态学报, 21, 2201-2208.] |
| [14] | Gao S, Song HF (2021). Sex-related response of Salicaceae to drought stress. Chinese Journal of Applied and Environmental Biology, 27, 495-502. |
| [14] | [高爽, 宋海凤 (2021). 杨柳科植物对干旱胁迫的性别响应差异. 应用与环境生物学报, 27, 495-502.] |
| [15] | Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology, 21, 394-407. |
| [16] | Gianoli E, González-Teuber M (2005). Environmental heterogeneity and population differentiation in plasticity to drought in Convolvulus chilensis (Convolvulaceae). Evolutionary Ecology, 19, 603-613. |
| [17] | Henn JJ, Buzzard V, Enquist BJ, Halbritter AH, Klanderud K, Maitner BS, Michaletz ST, P?tsch C, Seltzer L, Telford RJ, Yang Y, Zhang L, Vandvik V (2018). Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Frontiers in Plant Science, 9, 1548. DOI: 10.3389/fpls.2018.01548. |
| [18] | Ibrahimova U, Zivcak M, Gasparovic K, Rastogi A, Allakhverdiev SI, Yang XH, Brestic M (2021). Electron and proton transport in wheat exposed to salt stress: Is the increase of the thylakoid membrane proton conductivity responsible for decreasing the photosynthetic activity in sensitive genotypes? Photosynthesis Research, 150, 195-211. |
| [19] | Jin Y, Wang CK (2015). Trade-offs between plant leaf hydraulic and economic traits. Chinese Journal of Plant Ecology, 39, 1021-1032. |
| [19] | [金鹰, 王传宽 (2015). 植物叶片水力与经济性状权衡关系的研究进展. 植物生态学报, 39, 1021-1032.] |
| [20] | Juvany M, Munné-Bosch S (2015). Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. Journal of Experimental Botany, 66, 6083-6092. |
| [21] | Koch G, Rolland G, Dauzat M, Bédiée A, Baldazzi V, Bertin N, Guédon Y, Granier C (2018). Are compound leaves more complex than simple ones? A multi-scale analysis. Annals of Botany, 122, 1173-1185. |
| [22] | Letts MG, Phelan CA, Johnson DRE, Rood SB (2008). Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiology, 28, 1037-1048. |
| [23] | Li JG, Pu LJ, Han MF, Zhu M, Zhang RS, Xiang YZ (2014). Soil salinization research in China: advances and prospects. Journal of Geographical Sciences, 24, 943-960. |
| [24] | Liao J, Song H, Tang D, Zhang S (2019). Sexually differential tolerance to water deficiency of Salix paraplesia—A female-biased alpine willow. Ecology and Evolution, 9, 8450-8464. |
| [25] | Liu YY, Song J, Wang M, Li N, Niu CY, Hao GY (2015). Coordination of xylem hydraulics and stomatal regulation in keeping the integrity of xylem water transport in shoots of two compound-leaved tree species. Tree Physiology, 35, 1333-1342. |
| [26] | Lu RK (2000). Soil Agriculture Chemistry Analytical Methods. Chinese Agricultural Science and Technology Press, Beijing. |
| [26] | [鲁如坤 (2000). 土壤农业化学分析方法. 中国农业科技出版社, 北京.] |
| [27] | Ma JL, Shi JC, Jing FM (1991). Site classification for Manchurian ash. Journal of Northeast Forestry University, 19(S1), 62-68. |
| [27] | [马建路, 石家琛, 景凤鸣 (1991). 水曲柳立地区划. 东北林业大学学报, 19(S1), 62-68.] |
| [28] | Malhado ACM, Whittaker RJ, Malhi Y, Ladle RJ ter Steege H, Phillips O, Arag?o LEOC, Baker TR, Arroyo L, Almeida S, Higuchi N, Killeen TJ, Monteagudo A, Pitman NCA, Prieto A, et al. (2010). Are compound leaves an adaptation to seasonal drought or to rapid growth? Evidence from the Amazon rain forest. Global Ecology and Biogeography, 19, 852-862. |
| [29] | Montesinos D, Villar-Salvador P, García-Fayos P, Verdú M (2012). Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytologist, 193, 705-712. |
| [30] | Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, van Kleunen M (2010). Plant phenotypic plasticity in a changing climate. Trends in Plant Science, 15, 684-692. |
| [31] | Nybakken L, Julkunen-Tiitto R (2013). Gender differences in Salix myrsinifolia at the pre-reproductive stage are little affected by simulated climatic change. Physiologia Plantarum, 147, 465-476. |
| [32] | Pan T, Liu MM, Kreslavski VD, Zharmukhamedov SK, Nie CR, Yu M, Kuznetsov VV, Allakhverdiev SI, Shabala S (2021). Non-stomatal limitation of photosynthesis by soil salinity. Critical Reviews in Environmental Science and Technology, 51, 791-825. |
| [33] | Qi CJ, Tang GG (2005). Dendrology. China Forestry Publishing House, Beijing. |
| [33] | [祁承经, 汤庚国 (2005). 树木学. 中国林业出版社, 北京.] |
| [34] | Song J, Yang D, Niu CY, Zhang WW, Wang M, Hao GY (2018). Correlation between leaf size and hydraulic architecture in five compound-leaved tree species of a temperate forest in NE China. Forest Ecology and Management, 418, 63-72. |
| [35] | Stowe LG, Brown JL (1981). A geographic perspective on the ecology of compound leaves. Evolution, 35, 818-821. |
| [36] | Sun JJ (2020). Variations of Leaf and Fine Root Functional Traits of 15 Woody Species in Two Habitats. PhD dissertation, Northeast Forestry University, Harbin. 18. |
| [36] | [孙婧珏 (2020). 两种生境下15种木本植物叶和细根功能性状的差异. 博士学位论文, 东北林业大学, 哈尔滨. 18.] |
| [37] | Valladares F, Gianoli E, Gómez JM (2007). Ecological limits to plant phenotypic plasticity. New Phytologist, 176, 749-763. |
| [38] | Valladares F, Sanchez-Gomez D, Zavala MA (2006). Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology, 94, 1103-1116. |
| [39] | van Kleunen M, Fischer M (2005). Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytologist, 166, 49-60. |
| [40] | Velikova V, Arena C, Izzo LG, Tsonev T, Koleva D, Tattini M, Roeva O, de Maio A, Loreto F (2020). Functional and structural leaf plasticity determine photosynthetic performances during drought stress and recovery in two Platanus orientalis populations from contrasting habitats. International Journal of Molecular Sciences, 21, E3912. DOI: 10.3390/ijms21113912. |
| [41] | Verelst W, Bertolini E, de Bodt S, Vandepoele K, Demeulenaere M, Pè ME, Inzé D (2013). Molecular and physiological analysis of growth-limiting drought stress in Brachypodium distachyon leaves. Molecular Plant, 6, 311-322. |
| [42] | Wang H, Jones B, Li ZG, Frasse P, Delalande C, Regad F, Chaabouni S, Latche? A, Pech JC, Bouzayen M (2005). The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. The Plant Cell, 17, 2676-2692. |
| [43] | Wang JW, Liu Y, Xu YX, Chen WJ, Han YN, Wang GG, Jin SH (2022). Sexual differences in gas exchange and chlorophyll fluorescence of Torreya grandis under drought stress. Trees, 36, 283-294. |
| [44] | Ward JK, Dawson TE, Ehleringer JR (2002). Responses of Acer negundo genders to interannual differences in water availability determined from carbon isotope ratios of tree ring cellulose. Tree Physiology, 22, 339-346. |
| [45] | Wu BD, Liu J, Jiang K, Zhou JW, Wang CY (2019). Differences in leaf functional traits between simple and compound leaves of Canavalia maritime. Polish Journal of Environmental Studies, 28, 1425-1432. |
| [46] | Xu F, Guo WH, Xu WH, Wei YH, Wang RQ (2009). Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves? Progress in Natural Science, 19, 1789-1798. |
| [47] | Xu X, Peng GQ, Wu CC, Korpelainen H, Li CY (2008). Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiology, 28, 1751-1759. |
| [48] | Yang D, Zhang YJ, Song J, Niu CY, Hao GY (2019). Compound leaves are associated with high hydraulic conductance and photosynthetic capacity: evidence from trees in Northeast China. Tree Physiology, 39, 729-739. |
| [49] | Yang XY, Lu MQ, Wang YF, Wang YR, Liu ZJ, Chen S (2021). Response mechanism of plants to drought stress. Horticulturae, 7, 50. DOI: 10.3390/horticulturae7030050. |
| [50] | Zhang S, Chen LH, Duan BL, Korpelainen H, Li CY (2012). Populus cathayana males exhibit more efficient protective mechanisms than females under drought stress. Forest Ecology and Management, 275, 68-78. |
| [51] | Zhang WX, Cao FL (2002). Influence of drought stress on photosynthesis and photochemistry efficiency in leaves of Ginkgo biloba during high temperature days. Forest Research, 15, 672-679. |
| [51] | [张往祥, 曹福亮 (2002). 高温期间水分对银杏光合作用和光化学效率的影响. 林业科学研究, 15, 672-679.] |
| [52] | Zhao WL, Zhang JL, Zhang YJ, Cao KF (2019). Analysis of photosynthesis-water relationship between simple-and compound-leafed leguminous trees. Plant Science Journal, 37, 628-636. |
| [52] | [赵万里, 张教林, 章永江, 曹坤芳 (2019). 豆科复叶和单叶树种的光合-水分关系分析. 植物科学学报, 37, 628-636.] |
| [53] | Zheng WJ (1985). Records of Chinese Trees. China Forestry Publishing House, Beijing. |
| [53] | [郑万钧 (1985). 中国树木志. 中国林业出版社, 北京.] |
/
| 〈 |
|
〉 |