土壤是陆地生态系统最大的有机碳库, 库的大小主要受到有机物质输入和土壤有机质分解过程的共同调节(Lal, 2004)。传统的观点一直认为, 土壤有机质分解过程主要依赖于碳库的大小、温度和湿度等非生物因子(Kuzyakov et al., 2000)。近年来, 随着对土壤激发效应(priming effect)的深入研究, 发现激发效应是土壤中一个普遍存在的自然现象, 主要是由根系分泌物输入和凋落物分解等过程相互作用引起的(Kuzyakov, 2010)。尽管土壤有机质的稳定性很强(Lützowet al., 2006), 但土壤激发效应可以加速它的分解。在全球尺度上, 植物-土壤间相互作用的根际过程控制着陆地生态系统CO2总释放量的50% (Schimel, 1995; Cheng et al., 2013; Hopkins et al., 2013), 并影响生态系统多个养分循环过程(Chapin et al., 2011)。
一些模型模拟研究认为, 在全球气候变化背景下, 随着大气CO2浓度和温度升高, 将会有更多的碳储存在植物生物量和土壤有机碳库中(Joos et al., 2001; Gerber et al., 2004), 同时也会增加根系分泌物和凋落物的输入(Paterson et al., 1997; Macdonald et al., 2011; Phillips et al., 2011), 加速土壤有机质分解, 从而对大气CO2浓度产生正反馈(Kuzyakov, 2010; Sayer et al., 2011; Bengtson et al., 2012)。这表明激发效应可以通过改变土壤有机碳库的大小而影响全球变化的进程, 因此阐明激发效应的发生机制将有助于深入理解生态系统地下过程对未来气候变化的响应与适应机理, 并将有助于改进陆地生态系统的过程模型, 准确地评估全球变化对陆地生态系统的深远影响。
因为根际是土壤激发效应发生的最主要部位, 所以本文就根际激发效应的研究历史、主要发生部位、发生机制和影响因素进行了详细的阐述, 在此基础上提出了根际激发效应的生态重要性, 并对未来根际激发效应研究进行了展望, 以期更好地从理论上探寻土壤碳、氮循环的内在机制, 推动对陆地生态系统地下过程的深入认识。
1 土壤激发效应的研究历史
尽管20世纪40年代同位素技术的发展和完善为激发效应的研究提供了重要的方法基础, 但是直到20世纪80年代和90年代, 激发效应才引起学者们的进一步关注, Jenkinson等(1985)和Kuzyakov等(2000)的两篇综述分别论述了激发效应在土壤氮转化和有机碳转化方面的研究进展, 提出了新的研究方向, 推动了激发效应在土壤碳、氮转化方面的研究。近年来, 多篇综述进一步综合分析了激发效应的机制(Blagodatskaya & Kuzyakov, 2008; Kuzyakov, 2010; Cheng et al., 2013; Dijkstra et al., 2013), 使激发效应研究成为当前土壤生态学研究的一个重要组成部分和生长点(Kuzyakov, 2010)。
2 土壤激发效应的发生部位
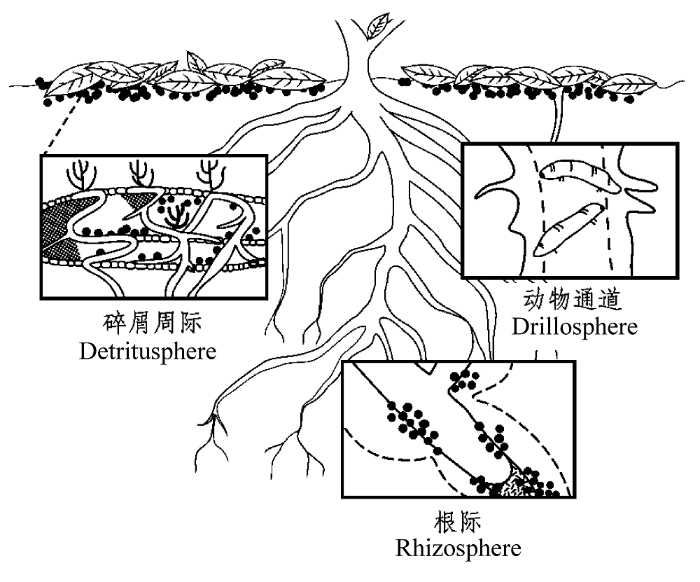
图1
根际(图1)是土壤中根系周边的狭小区域, 距离根系表面1-3 mm (Jones et al., 2004; Kuzyakov & Xu, 2013), 受植物根系与微生物活动的强烈影响(Herman et al., 2006)。植物通过根系分泌物和根系凋落物等向根际输入易于土壤微生物利用的含碳有机物(根际沉积物), 包括碳水化合物、有机酸、激素、维生素、死亡细胞和植物根系分泌的少量其他物质(Dijkstra & Cheng, 2007a), 改变了根际的营养环境(Toberman et al., 2011), 提供了大量可利用的碳和能量, 促进了微生物数量及其活性, 因此根际区域土壤微生物数量通常是非根际区域的19-32倍以上(Bodelier et al., 1997), 根际区域土壤微生物活性是非根际区域的10倍以上(Kuzyakov, 2010)。根际激发效应可增加3-5倍或减少10%-50%的土壤有机质的分解速率(Kuzyakov, 2002), 根际是土壤激发效应最重要的发生部位(Kuzyakov, 2010; Cheng et al., 2013), 因此本文主要阐述根际激发效应的发生机制及其生态重要性。
3 根际激发效应的方向与强度
由于根系分泌物、凋落物、死亡微生物等物质输入根际, 加速或抑制了根际土壤有机质分解, 而表现为正或负的根际激发效应, 此效应的强度依赖于植物物种(Fu & Cheng, 2002; Cheng et al., 2003; Bengtson et al., 2012)、根系构型(Kuzyakov, 2002)、菌根(Cheng et al., 2012)、光合作用(Kuzyakov & Cheng, 2001, 2004)、植物物候(Cheng et al., 2003; Cheng & Kuzyakov, 2005)、土壤类型(Dijkstra & Cheng, 2007b)、土壤可利用底物的数量和质量(Blagodatskaya & Kuzyakov, 2008; Bengtson et al., 2012; Guenet et al., 2012; Dijkstra et al., 2013; Drake et al., 2013)、土壤水分(Dijkstra & Cheng, 2007a)等。研究表明, 正的根际激发效应可促进有机质矿化增加3.8倍, 而负的根际激发效能减少50%的有机质矿化(Cheng et al., 2013)。
4 根际激发效应的发生机制
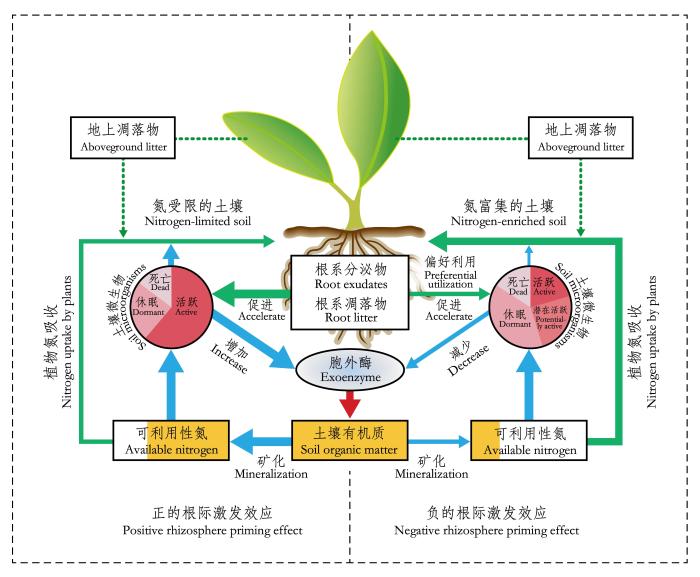
根际激发效应的产生是植物根系和微生物以及土壤有机质之间相互作用的结果, 是由根系分泌物驱动, 通过改变微生物量及其活性来实现的, 同时受其他生物与非生物因子的调控(Kemmitt et al., 2008; Kuzyakov, 2010)。虽然土壤中微生物种类繁多, 但通常仅有0.1%-2.0%的微生物处于活跃状态, 10%-40%由于能量缺乏而处于潜在活跃的状态(Blagodatskaya & Kuzyakov, 2013)。植物根系向根际区域输入易于被微生物利用的有机物质, 为“饥饿”的微生物提供碳和能量, 可在很短的时间内激活处于潜在活跃状态的微生物(de Nobiliet al., 2001), 改变微生物的群落结构和大小, 显著增加土壤微生物数量和活性(Fontaine et al., 2003)。当土壤养分缺乏时, 微生物可“投资” 1%-5%的同化产物用于产生胞外酶, 通过解聚合作用分解部分难以利用的有机质, 获取其所需养分(Burnset al., 2013), 因此根际的养分状况会影响微生物对其生长和胞外酶产生之间的能量分配(Fontaine et al., 2011)。此外, 微生物也可以直接分泌一些有机酸或酶, 通过化学反应改变根际养分状况(Kuzyakov et al., 2002)。虽然微生物的活性及土壤养分可利用性被认为是激发效应发生的重要因素(Kuzyakov, 2010; Dijkstra et al., 2013, Sullivan & Hart, 2013), 但是目前对根际激发效应的发生机制还未形成一致的结论(Kuzyakov, 2010; Sullivan & Hart, 2013)。根据近期的研究, 我们通过综合分析提出了一个具体的根际激发效应发生机制(图2)。
图2
图2
根际激发效应的发生机制: 当土壤氮受限时, 植物将较多的光合产物投资到地下, 根际微生物利用根际沉积物获取碳和能量, 增加微生物数量和活性, 促进微生物胞外酶的分泌, 分解有机质释放可利用氮, 产生正的根际激发效应(图左); 当土壤氮富集时, 减少了微生物对养分的需求, 根际微生物偏好利用根系分泌物, 减少胞外酶的分泌, 而植物也减少向地下的碳分配, 导致根际微生物数量减少和活性降低, 抑制了有机质分解, 产生负的根际激发效应(图右)。绿箭头、蓝箭头和红箭头分别代表由植物、土壤微生物和胞外酶主导的生态过程; 箭头的粗细表征通量的大小或过程的强弱。可利用性氮库中的黄色代表来自土壤有机质矿化作用产生的可利用性氮, 其色块面积大小代表来自有机质矿化作用的数量。
Fig. 2
Mechanism of rhizosphere priming effects. At low nutrient levels (left), plants allocate more photosynthates to belowground and supply soil microorganisms with carbon and energy. As a result, microbial biomass and activities increase and enhance production of extracellular enzymes by the rhizosphere microorganisms to decompose soil organic matter and release nutrients. Therefore, a positive priming effect takes place. In contrast, at high nutrient levels (right), microorganisms have less demand for nutrients and thus preferentially utilize root exudates, leading to reduced production of extracellular enzymes. Moreover, as plants invest less photosynthates in belowground, microbial biomass and activities will decrease. As a result, the decomposition of soil organic matter slows down and a negative priming effect occurs. The green, blue, and red arrow-lines represent the ecological processes mediated by plants, soil microorganisms, and exoenzymes, respectively. The thickness of an arrow-line indicates its relative magnitude of a flux or intensity of a process. Yellow colour of the available nitrogen pool indicates mineral nitrogen derived from mineralzation of soil organic matter. The area of the yellow colour represents the amount of mineral nitrogen derived from mineralzation of soil organic matter.
根际激发效应的发生取决于土壤养分的可利用性, 主要与土壤氮的有效性有关(Dijkstra et al., 2013; Sullivan & Hart, 2013)。根际沉积物作为根际微生物的主要能量来源, 主要是含碳化合物(Merbach et al., 1999; Hütschet al., 2002), 通常其C:N高于根际微生物的C:N (Clevel & Liptzin, 2007)。由于根系和微生物对根际可利用氮的获取, 根际通常成为碳过剩而氮受限强烈的区域(Kuzyakov et al., 2002)。在氮受限的环境中, 植物会将较多的光合产物投资到地下(Dijkstra et al., 2008; Phillips et al., 2009, 2011), 通过增加根系分泌物输入促进微生物的生长和活性, 微生物周转加快, 导致根际有效氮被快速消耗。低的有效性氮促使微生物增加胞外酶的分泌(Fontaine et al., 2003; Bengtson et al., 2012; Burns et al., 2013), 加速土壤有机质的分解, 释放无机氮, 从中获取所需的氮和其他养分,从而导致正的根际激发效应的发生(图2), 以上微生物利用碳和能源获取难分解有机质中的氮的过程, 也被称为“nitrogen mining”机制(Craineet al., 2007; Fontaine et al., 2011), 在自然生态系统普遍存在。
虽然根际持续性的碳输入能促进微生物的活性, 但是在氮富集的条件下, 往往可观察到负的根际激发效应。这主要是由于在较高的氮有效性条件下, 根际微生物不需要通过分解难以利用的土壤有机质获取氮, 而是偏好利用易于分解的根系分泌物(Kuzyakov et al., 2000; Kuzyakov & Cheng, 2004), 减少了用于分解有机质的胞外酶分泌。同时, 较高的氮有效性还会减少植物向地下的碳分配(Phillips et al., 2009, 2011), 减小根际微生物数量和活性。根际微生物量的降低、胞外酶分泌的减少, 以及微生物对根系分泌物的偏好利用均抑制了土壤有机质的矿化, 从而产生负的根际激发效应(图2)(Fu et al., 2002; Blagodatskaya & Kuzyakov, 2008; Dijkstra et al., 2013), 此效应见于具有施氮肥等管理措施的农田生态系统。
因为根际土壤微生物活性和养分的可利用性常处于一种动态变化之中, 因此根际激发效应的正与负也常常交替出现。
以上根际激发效应是根际区域植物根系和微生物对资源竞争利用的结果。植物和土壤微生物几乎具有相同的养分需求, 在根际有限的资源环境中, 两者对氮的竞争尤为强烈(Kuzyakov & Xu, 2013), 常常会引起正的根际激发效应(图2)。但当植物和微生物同时受氮的强烈限制时, 也可能会引起负的根际激发效应(Dijkstra et al., 2013)。除了根系与微生物之间的竞争, 根际微生物之间也存在着强烈的竞争, 这种竞争也会引起不同的根际激发效应。当分泌物抵达根际时, 采用r生存策略的微生物(主要为细菌)首先利用根际沉积物和有效氮进行快速地生长和周转(Paterson et al., 2007; Moore-Kucera & Dick, 2008), 此时产生负的根际激发效应(Fontaine et al., 2003, 2011; Shahzad et al., 2012; Pascault et al., 2013)。随着根际资源的快速消耗, r生存策略的微生物活性降低, 而k生存策略的微生物数量增加、活性增强。因为k生存策略的微生物主要是真菌, 它们是影响土壤有机质分解的最主要类群(Talbot et al., 2008; Fontaine et al., 2011), 不仅可以通过菌丝从低有效性的底物中获取养分(Otten et al., 2001), 而且还可以分泌胞外酶分解难以被r生存策略的微生物利用的有机质(Blagodatskaya et al., 2007), 促进了土壤有机质分解, 而产生正的激发效应。
5 根际激发效应的影响因素
5.1 影响根际激发效应的生物因素
因为土壤微生物是根际激发效应发生的“主角”, 所以大量研究在探寻微生物在根际激发效应中所起的作用。初始的研究集中在土壤微生物生物量在激发效应产生中的作用(Kuzyakovet al., 2000), 但由于总的微生物生物量中包含了许多处于休眠状态的微生物(Blagodatskaya & Kuzyakov, 2013), 因此有些研究开始探讨不同土壤微生物类群在土壤有机质分解中的作用, 研究发现: 随着碳利用性的增加, 与土壤有机质分解有关的真菌、革兰氏阳性细菌、革兰氏阴性细菌活性都会增强(Nottingham et al., 2009; Bird et al., 2011; Fontaine et al., 2011; Garcia-Pausas & Paterson, 2011; Dijkstra et al., 2013)。限于目前的研究手段, 当前大多数研究仅限于细菌、真菌和放线菌在土壤有机质分解中的作用, 初步明确了r生存策略的微生物和k生存策略的微生物在激发效应产生中的作用(Fontaine et al., 2003; Pascault et al., 2013)。微生物的活性, 尤其是胞外酶的活性对根际激发效应的影响逐渐受到较多的关注, 但是尚缺乏普遍性的研究结论(Marxsen & Witzel, 1991; Fontaine & Barot, 2005; Blagodatskaya & Kuzyakov, 2008; Burns et al., 2013)。考虑到根际激发效应的产生主要是活性微生物作用的结果, 未来这方面的研究迫切需要发展量化处于活性状态的各类微生物种群的新技术(Blagodatskaya & Kuzyakov, 2013), 阐明它们在根际激发效应产生中的作用。
土壤微生物的活性受到可利用底物的制约, 在根际主要取决于根系分泌物的数量和质量(Dijkstra et al., 2013)。植物根系分泌物一般占光合产物的10%, 与植物营养状况密切相关(Zhang et al., 2004), 因此光合作用强度、植物生长状况、植物种类和物候期等都会改变根系分泌物的数量和质量, 从而改变根际激发效应的方向和强度(Kuzyakov & Cheng, 2001, 2004; Kuzyakov, 2002; Gärdenäset al., 2011)。在普通光照强度下可产生正的根际激发效应, 当光照停止后根际激发效应逐渐减少为0, 而在光照恢复4天后却观察到负的激发效应(Kuzyakov & Cheng, 2001)。不同种类植物的生长状况不同, 其光合作用的效率存在差异, 可进一步影响根际激发效应的大小和方向。
植物地上、地下部分的生长情况也是影响根系分泌物数量和质量的重要因素, 尤其是根系的生长, 通常在根毛和根冠之间有最大值(Kuzyakov, 2002)。由于根尖的移动, 沿根系存在不同类型的根际沉积区域, 相应的微生物种群及其活性存在空间上的差异, 根际激发效应的强度和方向也会随之变化。通常新生的根产生的根系分泌物比成熟的根多, 从而引起较强的激发效应。此外, 根系的密度也影响根际激发效应的强度和方向(Kuzyakov, 2010)。在土壤有效氮不足时, 根系可以通过改变根的构型和增加细根的周转、根系分泌物等促进激发效应的发生(Hodge et al., 1996; Paterson & Sim, 1999; Kuzyakov, 2002)。因为根系的生物量与根际激发强度成正比(Fu & Cheng, 2002), 所以植物发达的根系有可能产生较强的根际激发效应。此外, 根系与微生物的共生, 如豆科植物与根瘤固氮菌或菌根等可能对激发效应产生影响, 例如, 研究发现在成熟期具根瘤的大豆根系比不具根瘤的根系能产生更强的根际激发效应(Zhu & Cheng, 2012); 丛枝状菌根真菌能够促进土壤有机质的分解(Hodge & Fitter, 2010; Cheng et al., 2013)。
双子叶植物的根际激发效应比单子叶植物强烈(Cheng & Kuzyakov, 2005)。C3植物和C4植物因为生理上的差异引起根系分泌物的组成和质量不同, 也会导致根际激发效应的差异(Fu et al., 2002)。与禾本科的春小麦(Triticum aestivum)相比, 豆科植物大豆(Glycine max)引发了较高的激发效应(Cheng, 2009)。近来的研究还发现, 作物混种的不同搭配也会产生不同的根际激发效应(Pausch et al., 2013)。然而, 以上都是初步的结果, 还需要进行大量的研究来明确植物类型对根际激发效应的影响, 比如低等植物——蕨类、苔藓, 和高等植物不同类群——裸子植物、被子植物等根际激发效应间的差异, 相同科属的物种之间根际激发效应的差异也需要进一步调查。
植物在不同的物候期, 根系分泌物的数量和组成存在差异, 也会影响根际激发效应的强度和方向。春季植物生长旺盛, 对氮的需求量较大, 此时植物分配较多的光合产物到根际, 产生正的根际激发效应; 在夏季或秋季至植物成熟期后, 植物对氮需求减少, 根系生长和根系分泌物量也逐渐下降, 从而导致较低的或负的根际激发效应。对于一年生植物, 在生长初期, 根际激发效应不明显或为负的根际激发效应; 而在生长盛期则产生明显的正的根际激发效应(Kuzyakov et al., 2001; Warembourg & Esterlich, 2001); 在开花期根际激发效应可达到最大值(Cheng & Kuzyakov, 2005; Cheng et al., 2013; Pausch et al., 2013)。根际沉积物的季节性变化对一年生植物的影响比对多年生植物的影响明显(Gärdenäset al., 2011)。
5.2 影响根际激发效应的非生物因素
土壤的结构和质地(Blagodatskaya & Kuzyakov, 2008; Cheng et al., 2013; Zhang et al., 2013)、矿质性质(Rasmussen et al., 2007)等均可对根际激发效应产生影响。因为土壤有机质的累积和矿化常依赖于土壤团粒结构大小, 大团粒结构(macroaggregates)中的碳通常比小团粒结构(microaggregates)中的碳易于分解(Six & Jastrow, 2002), 意味着不同团粒结构对有机质具有不同的保护作用而产生不同的激发效应(Blagodatskaya & Kuzyakov, 2008)。例如, 在中等大小团粒(1-2 mm)中观察到正的激发效应, 而在0.25-1 mm的团粒中观察到负的激发效应(Degens & Sparling, 1996)。这可能是由于不同微生物类群的需求定位不同, 通常真菌偏向于利用大团粒结构内的资源, 而细菌优先利用小团粒结构的资源(Guggenberger et al., 1999)。
土壤的养分含量决定根际激发效应的方向和强度(Kuzyakov et al., 2000), 其中氮和磷是影响植物和微生物生长的最重要的两个大量元素。土壤中可利用性氮含量直接影响微生物活性及根系与微生物对养分的竞争。虽然有研究认为土壤中较高的氮有效性可以引起较强的根际激发效应(Azam et al., 1993; Nyborg et al., 1995; Soon, 1998), 但更多的研究认为土壤的低氮有效性更能促进正的根际激发效应发生(Fontaine et al., 2003, 2011; Zhang & Wang, 2012; Dijkstra et al., 2013), 而高的氮输入会抑制激发效应的发生(Nottingham et al., 2012)。尽管研究表明根际激发效应可能与磷的有效性有关(Nottingham et al., 2012), 但更多研究认为微生物可以直接固持土壤中的无机磷或通过分泌胞外酶水解有机磷中的磷离子基团, 很少促进有机质的分解(Dakora & Phillips, 2002; George et al., 2011; Dijkstra et al., 2013)。因此, 根际激发效应主要与氮的有效性相关, 而与磷的有效性的关系不显著(Dijkstra et al., 2013; Sullivan & Hart, 2013)。
土壤水分可以直接改变微生物活性和植物的生长状况, 影响根际的激发效应, 通常较高的土壤含水量能产生更强烈的根际激发效应(Dijkstra & Cheng, 2007a)。植物的蒸腾作用能使根际发生干湿交替, 短时间和高频率的干湿交替可以促进土壤有机质矿化(Fierer & Schimel, 2003; Dijkstra & Cheng, 2007a; Cheng, 2009)。Cheng (2009)指出大豆的根际激发效应比小麦强烈, 可能是由于大豆比小麦具有持续、更高的蒸腾速率。土壤水分还能通过影响土壤微生物和植物对氮素的竞争(Lodge et al., 1994)、根系分泌物的含量(Gorissen et al., 2004)、酶活性(Bell & Henry, 2011; Geisseler et al., 2011; Burns et al., 2013)等对根际激发效应产生影响。
根际激发效应的大小也受土壤pH的影响(Rukshana et al., 2012, 2013)。一般土壤pH为5-8之间时微生物和胞外酶有较高的活性(Blagodatskaya & Anderson, 1998), 此时可产生较强的根际激发效应(Blagodatskaya & Kuzyakov, 2008)。土壤pH还能通过影响植物根系的生长和伸长(Edwards & Scott, 1974)、菌根的共生(Read & Perez-Moreno, 2003)、根际微生物的组成(Blagodatskaya & Kuzyakov, 2008; Rukshana et al., 2012)而影响根际激发效应的方向和强度。
许多研究表明, CO2浓度升高能促进植物的光合作用和净初级生产力(Amthor, 1995), 增加植物对地下的碳分配(de Graaff et al., 2006; Phillips et al., 2011), 并对根际分泌物的组成、数量和质量产生影响(Kuzyakov, 2002), 从而促进了根际激发效应(Phillips et al., 2012; Dijkstra et al., 2013)。同时, CO2浓度升高增加了植物和微生物对氮的获取(Drake et al., 2013), 降低了根际氮素的可利用性(Gill et al., 2002; Reich et al., 2006), 改变了植物根系和微生物对氮的竞争, 从而可间接影响根际激发效应的发生(Phillips et al., 2011, 2012)。
6 根际激发效应的生态学意义
在大多数陆地生态系统中, 大部分氮被固定在土壤有机质中, 植物生长受到氮素的限制(LeBauer & Treseder, 2008), 土壤有机质中可利用氮的释放需经微生物的矿化作用, 然而土壤微生物因为碳和能源的缺乏, 大部分处于非活跃状态(Blagodatskaya & Kuzyakov, 2013)。在生态系统中植物和土壤微生物的共存必须解决两者各自所面对的矛盾, 而在漫长的进化过程中, 植物和微生物成功地解决了这个难题。在氮受限的环境中, 植物通过根系向根际分泌有机物质, 为根际微生物提供碳和能量, 激活处于休眠和潜在活跃状态的微生物生产胞外酶, 加速有机质分解, 释放土壤有机质所固定的养分, 为微生物和植物提供可利用性氮, 缓解植物和土壤微生物对氮的竞争, 最终导致正的根际激发效应。在可利用氮富集的生态系统中, 微生物与植物对氮素的竞争很小, 此时植物虽然减少了对地下的投资, 但是根系分泌物的输入导致微生物对其偏好利用, 并固持了多余的氮素, 从而使养分不会因为淋溶等过程而流失(Kuzyakov & Xu, 2013), 但结果却产生了负的根际激发效应。正是根际这种微生物和植物的相互作用, 通过根际激发效应改变土壤碳、氮可利用性, 调节了养分的可利用性, 并可能长期地影响土壤有机质的累积和释放, 维持根际养分的正常周转, 对生态系统养分循环发挥着极为重要的作用。
因此, 根际激发效应的强度和方向能够反映生态系统土壤碳、氮动态过程和系统内养分的盈亏。尽管根际激发效应可通过影响植物、微生物等对养分的获取策略和竞争关系维持生态系统碳、氮循环和生态系统各组分间的养分平衡, 但是在不同的生态系统是否存在差异, 以及养分平衡的阈值如何, 尚需进一步研究(Kuzyakov, 2010)。
7 对根际激发效应的展望
综上所述, 我们已经对根际激发效应的发生机制、发生部位、方向与强度, 以及影响因素等有了一定程度的认知, 但是大多数研究仍然侧重于有机物质输入对碳分解或氮矿化的影响, 很少研究涉及对碳和氮平衡的评估(Qiao et al., 2013), 因而缺乏对根际激发效应生态重要性的综合评价。因此, 未来有关根际激发效应的研究应当侧重于以下几个 方面:
(1)加强不同生态系统根际激发效应的原位研究。尽管激发效应已经被确认为是生态系统普遍存在的一个自然现象, 但是由于研究方法的限制, 目前大多数根际激发效应的研究是在温室内进行的(Kuzyakov, 2010), 所获得的认识能否真实地反映生态系统原位的真实情况, 还需要加强野外原位控制实验, 以及长期的观测和数据积累。特别是从物种水平、群落水平和生态系统水平上深刻揭示根际激发效应相应的发生过程与机理。
(2)通过研究根际激发效应过程中的碳平衡与养分平衡, 综合评价根际激发效应过程中植物和微生物各自的收益, 阐明根际激发效应的生态重要性。尽管植物和微生物有相似的养分需求, 但是两者还是存在一定的差异, 前者更多地受养分限制, 后者则主要受碳和能源的限制(Kuzyakov, 2002)。根据进化理论, 根际激发效应很可能是植物和土壤微生物对土壤环境的一种适应策略。植物通过根系分泌物为根际微生物提供了碳和能源, 而根际微生物通过加速土壤有机质的降解回报植物以矿质养分。因此, 植物会根据自身需求和不同的养分条件, 调节根系分泌物的输出, 通过影响微生物活动来控制根际激发效应的发生(Liljeroth et al., 1994; Kuzyakov et al., 2001, 2002)。因此, 我们可以通过调控根际激发效应的大小和方向, 研究不同根际激发效应过程中植物和根际微生物各自的养分获取以及碳的分配与利用, 从而量化根际激发效应的生态重要性。尽管大多数生态系统受氮素限制, 但是磷和铁等大量元素也时常短缺。因此, 在评价根际激发效应过程中植物和土壤微生物各自的收益时, 需要同时关注植物和土壤微生物对碳、氮、磷等养分的获取, 才能全面评价根际激发效应的生态学意义。
(3)探明根际来源的碳在土壤中的去向, 综合评价根际激发效应对土壤有机碳的深远影响。尽管很多研究已经揭示根际激发效应能够促进土壤原有有机质的分解(Kuzyakov, 2010), 但是引发根际激发效应的这部分碳并没有完全被微生物分解释放到大气, 依然有一部分残留在土壤而弥补因激发效应引起的碳损失(Fontaine et al., 2004a, 2004b; Hamer & Marschner, 2005; Ohm et al., 2007; Qiao et al., 2013)。因此, 这部分碳在土壤中的去向至关重要, 如果根际激发效应促进了土壤中的原有有机碳, 而残留的根源性碳滞留在易分解碳库中, 那么土壤有机碳的增加可能是一种假象, 随着可利用碳库的消耗, 这部分碳还是很快被释放到大气中, 因而根际激发效应对土壤有机碳的影响依然较大。相反, 如果这部分碳进入了难分解的碳库, 那么根际激发效应并没有因促进土壤有机碳的分解而影响土壤有机碳的截留(Qiao et al., 2013)。
(4)继续深化研究根际激发效应的微生物学机制。活的有机体和死的有机物之间的相互作用在根际激发效应的产生中具有重要的作用(Kuzyakov, 2010), 特别是根际微生物的活性一直被认为是根际激发效应产生的驱动者。然而, 由于土壤中微生物种类繁多, 而且大量微生物处于休眠状态(Morita, 1990; Stenströmet al., 2001; Blagodatskaya & Kuzyakov, 2013), 究竟是哪些类群的微生物参与了根际激发效应? 通过什么方式参与的? 胞外酶在根际激发效应的产生中到底起什么样的作用? 有关研究目前依然缺乏充分的实验数据。近年来, 随着微生物分子技术的迅速发展, 阐明根际激发效应的微生物学机制及其控制因素已经成为本领域内的核心科学问题。
(5)根际激发效应与全球变化。土壤有机质分解对温度的敏感性是预测陆地生态系统碳循环对全球变暖影响的关键环节(Craine et al., 2013), 但目前的研究尚未达成一致的认识(von Lützow & Kögel-Knabner,2009; Conant et al., 2011), 而根际激发效应具有改变土壤有机碳的巨大潜势而对气候变暖产生正的反馈, 因此根际激发效应对温度的敏感性更值得关注(Cheng et al., 2013)。特别是在全球变化背景下, 大气CO2浓度增加和气温升高同时发生, 而大气CO2浓度增加又能潜在地促进根际激发效应, 因此阐明根际激发效应、大气CO2浓度增加和气温升高之间的相互作用是探究陆地生态系统对气候变化响应的重要前提。目前已经有研究开始探讨根际激发效应对温度的敏感性(Zhu & Cheng, 2011; Thiessen et al., 2013), 但仍需要大量研究去破解其内在的机制, 为生态系统过程模型的精确模拟预测提供基石。
基金项目 国家自然科学基金(41071209)和2012年度中国科学院国际合作局俄乌白科技合作专项补助经费。
参考文献
Protozoa, Nematoda and Lumbricidae in the rhizosphere of Hordelymus europaeus (Poaceae): faunal interactions, response of micro-organisms and effects on plant growth
Interactions among protozoa (mixed cultures of ciliates, flagellates and naked amoebae), bacteria-feeding nematodes (Pellioditis pellio Schneider) and the endogeic earthworm species Aporrectodea caliginosa (Savigny) were investigated in experimental chambers with soil from a beechwood (Fagus sylvatica L.) on limestone. Experimental chambers were planted with the grass Hordelymus europeaus L. (Poaceae) and three compartments separated by 45-mum mesh were established: rhizosphere, intermediate and non-rhizosphere. The experiment lasted for 16 weeks and the following parameters were measured at the end of the experiment: shoot and root mass of H. europaeus, carbon and nitrogen content in shoots and roots, density of ciliates, amoebae, flagellates and nematodes, microbial biomass (SIR), basal respiration, streptomycin sensitive respiration, ammonium and nitrate contents, phosphate content of soil compartments. In addition, leaching of nutrients (nitrogen and phosphorus) and leachate pH were measured at regular intervals in leachate obtained from suction cups in the experimental chambers. Protozoa stimulated the recovery of nitrifying bacteria following defaunation (by chloroform fumigation) and increased nitrogen losses as nitrate in leachate. In contrast, protozoa and nematodes reduced leaching of phosphate, an effect ascribed to stimulation of microbial growth early in the experiment. Earthworms strongly increased the amount of extractable mineral nitrogen whereas it was strongly reduced by protozoa and nematodes. Both protozoa and nematodes reduced the stimulatory effect of earthworms on nitrogen mineralization. Microbial biomass, basal respiration, and numbers of protozoa and nematodes increased in the vicinity of the root. Protozoa generally caused a decrease in microbial biomass whereas nematodes and earthworms reduced microbial biomass only in the absence of protozoa. None of the animals studied significantly affected basal respiration, but specific respiration of microorganisms (O2 consumption per unit biomass) was generally higher in animal treatments. The stimulatory effect of nematodes and earthworms, however, occurred only in the absence of protozoa. The sensitivity of respiration to streptomycin suggested that protozoa selectively grazed on bacterial biomass but the bacterial/fungal ratio appeared to be unaffected by grazing of P. pellio. Earthworms reduced root biomass of H. europaeus, although shoot biomass remained unaffected, and concentrations of nitrogen in shoots and particularly in roots were strongly increased by earthworms. Both nematodes and protozoa increased plant biomass, particularly that of roots. This increase in plant biomass was accompanied by a marked decrease in nitrogen concentrations in roots and to a lesser extent in shoots. Generally, the effects of protozoa on plant growth considerably exceeded those of nematodes. It is concluded that nematodes and protozoa stimulated plant growth by non-nutritional effects, whereas the effects of earthworms were caused by an increase in nutrient supply to H. europaeus.
Terrestrial higher-plant response to increase- ing atmospheric [CO 2] in relation to the global carbon cycle
Mineralization of N from plant residues and its interaction with native soil N.
A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling
In: Collins HP, Robertson GP, Klug MJ eds. The Significance and Regulation of Soil Biodiversity.
Fine scale variability in soil extracellular enzyme activity is insensitive to rain events and temperature in a mesic system
Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects
Increased temperatures and concomitant changes in vegetation patterns are expected to dramatically alter the functioning of northern ecosystems over the next few decades. Predicting the ecosystem response to such a shift in climate and vegetation is complicated by the lack of knowledge about the links between aboveground biota and belowground process rates. Current models suggest that increasing temperatures and rising concentrations of atmospheric CO(2) will be partly mitigated by elevated C sequestration in plant biomass and soil. However, empirical evidence does not always support this assumption, as elevated temperature and CO(2) concentrations also accelerate the belowground C flux, in many cases extending to increased decomposition of soil organic matter (SOM) and ultimately resulting in decreased soil C stocks. The mechanism behind the increase has remained largely unknown, but it has been suggested that priming might be the causative agent. Here, we provide quantitative evidence of a strong coupling between root exudation, SOM decomposition, and release of plant available N caused by rhizosphere priming effects. As plants tend to increase belowground C allocation with increased temperatures and CO(2) concentrations, priming effects need to be considered in our long-term analysis of soil C budgets in a changing environment. The extent of priming seems to be intimately linked to resource availability, as shifts in the stoichiometric nutrient demands of plants and microorganisms will lead to either cooperation (resulting in priming) or competition (no priming will occur). The findings lead us on the way to resolve the varying response of primary production, SOM decomposition, and release of plant available N to elevated temperatures, CO(2) concentrations, and N availability.
A call to investigate drivers of soil organic matter retention vs. mineralization in a high CO 2 world
The effect of the addition of organic materials on the decomposition of an organic soil
Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil
The priming effects induced by earthworm mucus on mineralization and humification of plant residues
Active microorg- anisms in soil: critical review of estimation criteria and approaches
Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and QCO 2of microbial communities in forest soils
Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies
Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review
Effects of photoperiod on growth of and denitrification by Pseudomonas chlororaphis in the root zone of Glyceria maxima, studied in a gnotobiotic microcosm
Soil enzymes in a changing environment: current knowledge and future directions
Advances in research on priming effect of soil organic carbon
土壤有机碳激发效应研究进展
Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO 2
Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C-N budgets
Rhizosphere effects on decomposition: controls of plant species, phenology, and fertilization
Root effects on soil organic matter decomposition
In: Zobel RW, Wright SF eds. Roots and Soil Management: Interactions Between Roots and the Soil. Agronomy Monograph No. 48, American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, USA.
Synthesis and modeling perspectives of rhizosphere priming
C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass?
Temperature and soil organic matter decomposition rates―synthesis of current knowledge and a way forward
Reduction of the temperature sensitivity of soil organic matter decomposition with sustained temperature increase
Microbial nitrogen limitation increases decomposition
Root exudates as mediators of mineral acquisition in low-nutrient environments
Interactions between plant growth and soil nutrient cycling under elevated CO 2: a meta-analysis
Soil microbial biomass is triggered into activity by trace amounts of substrate
Changes in aggregation do not correspond with changes in labile organic C fractions in soil amended with C 14-glucose
Rhizosphere priming: a nutrient perspective
Interactions between soil and tree roots accelerate long-term soil carbon decomposition
Moisture modulates rhizosphere effects on C decomposition in two different soil types
Long-term enhancement of N availability and plant growth under elevated CO 2 in a semi-arid grassland
Stoichiometry constrains microbial response to root exudation―insights from a model and a field experiment in a temperate forest
Rapid growth responses of corn root segments: effect of pH on elongation
A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil
Carbon input to soil may decrease soil carbon content
Mechanisms of the priming effect in a savannah soil amended with cellulose
Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation
Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect
The priming effect of organic matter: a question of microbial competition?
Rhizosphere priming effects on the decomposition of soil organic matter in C 4 and C 3 grassland soils
Rhizosphere respiration varies with plant species and phenology: a greenhouse pot experiment
Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon
Knowledge gaps in soil carbon and nitrogen interactions―from molecular to global scale
Soil moisture and plant residue addition interact in their effect on extracellular enzyme activity
Phosphorus nutrition: rhizosphere processes, plant response and adaptations
In: Bünemann E, Oberson A, Frossard E eds. Phosphorus in Action.
Sensitivity of a dynamic global vegetation model to climate and atmospheric CO 2
Nonlinear grassland responses to past and future atmospheric CO 2
Climate change affects carbon allocation to the soil in shrublands
Evidence that stable C is as vulnerable to priming effect as is more labile C in soil
Microbial contributions to the aggregation of a cultivated grassland soil amended with starch
Priming effects in soils after combined and repeated substrate additions
The nature and impact of teachers’ formative assessment practices. CSE Technical report 703
Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling
A novel method for characterisation and quantification of plant root exudates
Uncertainties in the temperature sensitivity of decomposition in tropical and subtropical ecosystems: implications for models
Ecosystem- level controls on root-rhizosphere respiration
Priming effect of soil carbon pools
土壤碳库激发效应
Plant rhizodeposition―an important source for carbon turnover in soils
Interactions between fertilizer nitrogen and soil nitrogen the so-called “priming” effect
Plant and mycorrhizal regulation of rhizodeposition
Global warming feedbacks on terrestrial carbon uptake under the Intergovernmental Panel on Climate Change (IPCC) emission scenarios
Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass―a new perspective
Tillage systems and soil ecology
Review: factors affecting rhizosphere priming effects
Priming effects: interactions between living and dead organic matter
Photosynthesis controls of rhizosphere respiration and organic matter decomposition
Photosynthesis controls of CO 2 efflux from maize rhizosphere
Carbon partitioning and below-ground translocation by Lolium perenne
Review of mechanisms and quantification of priming effects
Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature
Effect of nitrogen fertilisation on below-ground carbon allocation in lettuce
Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance
Soil carbon sequestration impacts on global climate change and food security
The carbon sink capacity of the world's agricultural and degraded soils is 50 to 66% of the historic carbon loss of 42 to 78 gigatons of carbon. The rate of soil organic carbon sequestration with adoption of recommended technologies depends on soil texture and structure, rainfall, temperature, farming system, and soil management. Strategies to increase the soil carbon pool include soil restoration and woodland regeneration, no-till farming, cover crops, nutrient management, manuring and sludge application, improved grazing, water conservation and harvesting, efficient irrigation, agroforestry practices, and growing energy crops on spare lands. An increase of 1 ton of soil carbon pool of degraded cropland soils may increase crop yield by 20 to 40 kilograms per hectare (kg/ha) for wheat, 10 to 20 kg/ha for maize, and 0.5 to 1 kg/ha for cowpeas. As well as enhancing food security, carbon sequestration has the potential to offset fossil fuel emissions by 0.4 to 1.2 gigatons of carbon per year, or 5 to 15% of the global fossil-fuel emissions.
Mutualism and biodiversity in soils
Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed
Our meta-analysis of 126 nitrogen addition experiments evaluated nitrogen (N) limitation of net primary production (NPP) in terrestrial ecosystems. We tested the hypothesis that N limitation is widespread among biomes and influenced by geography and climate. We used the response ratio (R approximately equal ANPP(N)/ANPP(ctrl)) of aboveground plant growth in fertilized to control plots and found that most ecosystems are nitrogen limited with an average 29% growth response to nitrogen (i.e., R = 1.29). The response ratio was significant within temperate forests (R = 1.19), tropical forests (R = 1.60), temperate grasslands (R = 1.53), tropical grasslands (R = 1.26), wetlands (R = 1.16), and tundra (R = 1.35), but not deserts. Eight tropical forest studies had been conducted on very young volcanic soils in Hawaii, and this subgroup was strongly N limited (R = 2.13), which resulted in a negative correlation between forest R and latitude. The degree of N limitation in the remainder of the tropical forest studies (R = 1.20) was comparable to that of temperate forests, and when the young Hawaiian subgroup was excluded, forest R did not vary with latitude. Grassland response increased with latitude, but was independent of temperature and precipitation. These results suggest that the global N and C cycles interact strongly and that geography can mediate ecosystem response to N within certain biome types.
Priming effect of ammonium nitrogen fertilizer on soil nitrogen in typical soils of Loess Plateau
铵态氮肥对黄土高原典型土壤氮素激发效应的影响
Carbon translocation to the rhizosphere of maize and wheat and influence on the turnover of native soil organic matter at different soil nitrogen levels
A global perspective on belowground carbon dynamics under nitrogen enrichment
Litter decomposition and nutrient release as affected by soil nitrogen availability and litter quality in a semiarid grassland ecosystem
Nitrogen availability is critically important to litter decomposition, especially in arid and semiarid areas where N is limiting. We studied the relative contributions of litter quality and soil N to litter decomposition of two dominant grassland species, Stipa krylovii and Artemisia frigida, in a semiarid typical steppe ecosystem in Inner Mongolia, China. The study had four different rates of N addition (0, 8, 32, and 64 g N m(-2) year(-1)), and litter samples were decomposed under varying site conditions and by litter types. Litter-mixing effects of the two species were also examined. We found that N addition increased litter N concentration and thus enhanced litter decomposition by improving substrate quality. This increase, however, was offset by the negative effect of increased soil N, resulting in a diminished effect of increased soil N availability on in situ litter decomposition. The positive effects of improved litter quality slightly out-performed the negative effects of increased soil N. Our further analysis revealed that the negative effect of increasing soil N on litter decomposition could be partially explained by reduced soil microbial biomass and activity. Decomposition was significantly faster for litters of a two-species mixture than litters of the single species, but the rate of litter decomposition did not differ much between the two species, suggesting that compositional balance, rather than changes in the dominance between Stipa and Artemisia, is more critical for litter decomposition, hence nutrient cycling in this ecosystem. This semiarid steppe ecosystem may become more conservative in nutrient use with switching of dominance from Artemisia to Stipa with increasing soil N, because Stipa has a slower decomposition rate and a higher nutrient retention rate than Artemisia.
The importance of nutrient pulses in tropical forests
Recent research shows that nutrient fluxes are often pulsed In tropical forests, and that pulsed versus gradual inputs have different effects on the fates of nutrients in the ecosystem. Synchrony of nutrient mineralization with plant uptake can lower competition between microbes and plants for limiting nutrients while maintaining tight nutrient cycling, whereas asynchrony can lead to losses of nutrients from the system. Thus, nutrient pulses may play a critical role in maintaining productivity in tropical forests with tight nutrient cycling.
Stabiliza- tion of organic matter in temperate soils: mechanisms and their relevance under different soil conditions―a review
Root growth into litter layer and its impact on litter decomposition: a review
根系在凋落物层中的生长及其对凋落物分解的影响
Role of nitrogen in carbon mitigation in forest ecosystems
Significance of extracellular enzymes for organic matter degradation and nutrient regeneration in small streams
In: Ryszard JC ed. Microbial Enzymes in Aquatic Environments.
Release of carbon and nitrogen compounds by plant roots and their possible ecological importance
Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids
The starvation-survival state of microorganisms in nature and its relationship to the bioavailable energy
Microbial diversity and soil functions
Soil priming by sugar and leaf-litter substrates: a link to microbial groups
Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility
Fertiliser N, crop residue, and tillage alter soil C and N content in a decade
In: Lal R, Kimble J, Levine E, Stewart BA eds. Soil Management and Greenhouse Effect.
Priming effects in soil size fractions of a podzol Bs horizon after addition of fructose and alanine
Soil physics, fungal epidemiology and the spread of Rhizoctonia solani
Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect
Rhizodeposition shapes rhizosphere microbial community structure in organic soil
Effect of elevated CO 2 on rhizosphere carbon flow and soil microbial processes
Rhizodeposition and C-partitioning of Lolium perenne in axenic culture affected by nitrogen supply and defoliation
Plant inter-species effects on rhizosphere priming of soil organic matter decomposition
Elevated CO 2 increases root exudation from loblolly pine ( Pinus taeda) seedlings as an N-mediated response
The degree to which forest ecosystems provide a long-term sink for increasing atmospheric CO(2) depends upon the capacity of trees to increase the availability of growth-limiting resources. It has been widely speculated that trees exposed to CO(2) enrichment may increase the release of root exudates to soil as a mechanism to stimulate microbes to enhance nutrient availability. As a first test to examine how the atmospheric CO(2) and nitrogen availability affect the rates of root exudation, we performed two experiments in which the exudates were collected from loblolly pine (Pinus taeda L.) seedlings that were grown in controlled growth chambers under low and high CO(2) and at low and high rates of N supply. Despite the differences in experimental design between the two studies, plants grown at high CO(2) were larger, and thus whole plant exudation rates were higher under elevated CO(2) (P = 0.019), but the magnitude of this response depended on the N level in both studies. Seedlings increased mass-specific exudation rates in response to elevated CO(2) in both experiments, but only at low N supply. Moreover, N supply had a greater impact on the exudation rates than did CO(2), with mass-specific exudation rates significantly greater (98% and 69% in Experiments 1 and 2, respectively) in the seedlings grown at low N supply relative to high N supply. These results provide preliminary evidence that loblolly pines alter exudation rates in response to both CO(2) concentration and N supply, and support the hypothesis that increased C allocation to root exudates may be a mechanism by which trees could delay progressive N limitation in forested ecosystems.
Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO 2 fumigation
Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO 2
A common finding in multiple CO2 enrichment experiments in forests is the lack of soil carbon (C) accumulation owing to microbial priming of old soil organic matter (SOM). However, soil C losses may also result from the accelerated turnover of young microbial tissues that are rich in nitrogen (N) relative to bulk SOM. We measured root-induced changes in soil C dynamics in a pine forest exposed to elevated CO2 and N enrichment by combining stable isotope analyses, molecular characterisations of SOM and microbial assays. We find strong evidence that the accelerated turnover of root-derived C under elevated CO2 is sufficient in magnitude to offset increased belowground inputs. In addition, the C losses were associated with accelerated N cycling, suggesting that trees exposed to elevated CO2 not only enhance N availability by stimulating microbial decomposition of SOM via priming but also increase the rate at which N cycles through microbial pools.
Labile carbon retention compensates for CO 2 released by priming in forest soils
Ecological communities are increasingly exposed to multiple interacting stressors. For example, warming directly affects the physiology of organisms, eutrophication stimulates the base of the food web, and harvesting larger organisms for human consumption dampens top-down control. These stressors often combine in the natural environment with unpredictable results. Bacterial communities in coastal ecosystems underpin marine food webs and provide many important ecosystem services (e.g. nutrient cycling and carbon fixation). Yet, how microbial communities will respond to a changing climate remains uncertain. Thus, we used marine mesocosms to examine the impacts of warming, nutrient enrichment, and altered top-predator population size structure (common shore crab) on costal microbial biofilm communities in a crossed experimental design. Warming increased bacterial alpha-diversity (18% increase in species richness and 67% increase in evenness), but this was countered by a decrease in alpha-diversity with nutrient enrichment (14% and 21% decrease for species richness and evenness respectively). Thus, we show some effects of these stressors could cancel each other out under climate change scenarios. Warming and top-predator population size structure both affected bacterial biofilm community composition, with warming increasing the abundance of bacteria capable of increased mineralization of dissolved and particulate organic matter, such as Flavobacteriia, Sphingobacteriia, and Cytophagia. However, the community shifts observed with warming depended on top-predator population size structure, with Sphingobacteriia increasing with smaller crabs and Cytophagia increasing with larger crabs. These changes could alter the balance between mineralization and sequestration of carbon in coastal ecosystems, leading to a positive feedback loop between warming and CO2 production. Our results highlight the potential for warming to disrupt microbial communities and biogeochemical cycling in coastal ecosystems, and the importance of studying these effects in combination with other environmental stressors.
Soil mineralogy affects conifer forest soil carbon source utilization and microbial priming
Mycorrhizas and nutrient cycling in ecosystems―a journey towards relevance?
Nitrogen limitation constrains sustainability of ecosystem response to CO 2
Model organic compounds differ in priming effects on alkalinity release in soils through carbon and nitrogen mineralisation
Soil organic carbon contributes to alkalinity priming induced by added organic substrates
Soil carbon release enhanced by increased tropical forest litterfall
Terrestrial ecosystems and the carbon cycle
Plant clipping decelerates the mineralization of recalcitrant soil organic matter under multiple grassland species
Influence of FACE experiment on soft organic matter pools and priming effect
FACE试验对土壤有机质库组分及其激发效应的影响
Organic matter turnover
In: Lal R ed. Encyclopedia of Soil Science.
Crop residue and fertilizer management effects on some biological and chemical properties of a Dark Grey Solod
Reversible transition between active and dormant microbial states in soil
The rate of respiration obtained in the substrate-induced respiration (SIR) method can be divided into the respiration rate of growing (r) and non-growing (K) microorganisms. The fraction of r is generally small (5-20%) in soils with no recent addition of substrates, but can be 100% in soils with high substrate availability. This suggests that substrate availability determines the proportion of biomass between these groups, and implies that transitions between them can take place reversibly. These hypotheses were tested by adding three different amounts of glucose which induced first-order, zero-order, and growth-associated respiration kinetics to three soils at four pre-incubation times (4, 12, 27, and 46 days) before the SIR measurement. An abiotic flush of CO(2) in the SIR measurement was detected and corrected for before data analysis. Accumulated CO(2)-C over 4 days after glucose addition, corrected for the respiration in unamended controls, corresponded to 41-50% mineralization of the glucose-C, and the relative amount mineralized by each soil was independent of the glucose amount added. The high glucose concentration gave an increased SIR, which reverted to the initial value within 27-46 days. In a specific sample, the maximum respiration rate induced during the pre-incubation, and the amount of organisms transformed from the K to the r state, as quantified in respiration rate units in the SIR measurement, were identical to each other, and these parameters were also highly correlated to the initial glucose concentration. The K-->r transition was very fast, probably concurrent with the instantaneous increase in the respiration rate obtained by the glucose amendment. Thereafter, a slow first-order back-transition from the r to the K state ensued, with half-lives of 12, 23, and 70 days for the three soils. The results suggest the existence of community-level controls by which growth within or of the whole biomass is inhibited until it has been completely transformed into the r state. The data also suggest that the microbial specific activity is not related to the availability of exogenous substrate in a continuous fashion, rather it responds as a sharp transition between dormant and fully active. Furthermore, the inherent physiological state of the microbial biomass is strongly related to its history. It is proposed that the normal dynamics of the soil microbial biomass is an oscillation between active and dormant physiological states, while significant growth occurs only at substantial substrate amendment.
Evaluation of mechanisms controlling the priming of soil carbon along a substrate age gradient
Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change
Both priming and temperature sensitivity of soil organic matter decomposition depended on microbial biomass―an incubation study
Rhizosphere effects on soil nutrient dynamics and microbial activity in an Australian tropical lowland rainforest
Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies
Nitrogen (N) enrichment is an element of global change that could influence the growth and abundance of many organisms. In this meta-analysis, I synthesized responses of microbial biomass to N additions in 82 published field studies. I hypothesized that the biomass of fungi, bacteria or the microbial community as a whole would be altered under N additions. I also predicted that changes in biomass would parallel changes in soil CO2 emissions. Microbial biomass declined 15% on average under N fertilization, but fungi and bacteria were not significantly altered in studies that examined each group separately. Moreover, declines in abundance of microbes and fungi were more evident in studies of longer durations and with higher total amounts of N added. In addition, responses of microbial biomass to N fertilization were significantly correlated with responses of soil CO2 emissions. There were no significant effects of biomes, fertilizer types, ambient N deposition rates or methods of measuring biomass. Altogether, these results suggest that N enrichment could reduce microbial biomass in many ecosystems, with corresponding declines in soil CO2 emissions.
Rapid ex- change between soil carbon and atmospheric carbon diox- ide driven by temperature change
Temperature sensitivity of soil organic matter decomposition―What do we know?
Effect of enclosure on soil C mineralization and priming effect in Stipa grandis grassland of Inner Mongolia
围封对内蒙古大针茅草地土壤碳矿化及其激发效应的影响
Plant phenology and soil fertility effects on below-ground carbon allocation for an annual ( Bromus madritensis) and a perennial ( Bromus erectus) grass species
An overview of rhizosphere processes related with plant nutrition in major cropping systems in China
Effects of NH 4 +and NO 3 -on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China
Addition of external organic carbon and native soil organic carbon decom- position: a meta-analysis
15d). In addition, the incubation temperature and the addition rate of organic matter significantly influenced the native SOC decomposition in response to the addition of external organic C.]]>
Rhizosphere priming effect increases the temperature sensitivity of soil organic matter decomposition
Nodulated soybean enhances rhizosphere priming effects on soil organic matter decomposition more than non-nodulated soybean