植物功能性状是指一切对植物的定居、生存和适应有着潜在影响的, 或与获取、利用和保存资源的能力有关的属性, 并且这些属性本身或者属性之间的相互联系能够指示生态系统对环境变化的响应情况, 强烈影响生态过程(刘晓娟和马克平, 2015)。植物功能性状可以为预测生态机制如何影响群落多样性提供基础, 因此, 近年来不同环境条件下植物功能性状之间的权衡关系得到越来越多关注(Wright et al., 2004; Díaz et al., 2016; Song et al., 2018)。叶作为植物能量获取的主要器官, 其性状决定了植物的许多生态功能, 如光合作用、蒸腾作用、对极端温度的适应能力以及对叶内各结构的生物量投资等。另外, 叶功能性状还可以反映植物自身生物量的分配格局及其对环境的适应能力(Li et al., 2008; 杨冬梅等, 2012; Li et al., 2021), 如“收益递减”假说认为随着叶大小增加, 植物叶面积增长速率慢于叶生物量, 表明植物拦截光的结构成本随着叶面积增加增长得更快(Niklas et al., 2007), 并且这种异速生长关系会随环境条件发生变化(Zhu et al., 2019)。
光合固碳是植物碳收益的主要来源, 叶作为主要的光合器官, 其光合收益与必要支撑结构(如小枝)的生物量分配关系是近年来生态学与全球变化研究的重点(Westoby & Wright, 2003; Yan et al., 2013; Smith et al., 2016; Wang et al., 2019; Zhu et al., 2019; 尹凤娟等, 2021)。一片完整的叶子至少包含叶片与叶柄两部分, 叶片与同样具有支撑和运输功能的叶柄可能存在类似上述的相关关系, 但目前有关研究较少。理论上, 植物在进化过程中, 一方面会尽可能地扩展叶片尺寸以获取尽可能多的光合收益, 另一方面会通过减少对叶柄的生物量投资以节约成本, 从而使叶片总收益最大化(杨冬梅等, 2012)。然而这种最优化往往会受到叶柄结构与功能的限制, 因为大叶片更大的支撑与运输需求会导致其对叶柄的生物量投入相应增加(Niklas, 1999; 祝介东等, 2011), 因此柄叶结构之间可能存在典型的权衡关系(Niinemets et al., 2007b; Li et al., 2021)。受温度、水分、光照等外界因素的影响, 叶片大小常呈现有规律的变化趋势(Bazzaz & Carlson, 1982; Wright et al., 2017), 叶柄也会表现出相应的适应性特征, 如生长在高海拔的同种植物通常会投入更多生物量给叶柄(Li et al., 2008); 在强光照与强风环境中, 叶柄所占的生物量比例往往会增加(Niinemets & Kull, 1999; Niklas, 1999); 不同习性植物(常绿和落叶)叶内生物量分配比例也常常不同, 在给定叶柄干质量下, 常绿植物的叶片面积小于落叶植物, 但叶片生物量反而高于落叶植物(Li et al., 2008)。柄叶关系是研究植物光合效率和支持/保护成本之间功能权衡的核心(Li et al., 2021), 上述结果均表明叶片与叶柄性状之间存在显著的相关关系, 充分了解叶内各结构的权衡关系对探讨植物对环境的适应性, 揭示植物的生长策略及资源分配模式具有重要意义。
目前关于叶片与叶柄生物量分配关系的研究大多局限在乔木(Li et al., 2008; 潘少安等, 2015), 但不同生活型植物生活史对策通常不同(Niinemets et al., 2007a; 祝介东等, 2011), 因此针对不同生活型植物的柄叶权衡关系开展研究十分必要。此外, 有研究发现相较于植物小枝, 复叶的叶柄作为一次性(每年更换茎状叶柄)的支撑结构, 可以降低叶子着生的结构成本, 帮助植物以尽可能低的成本获取光源(Malhado et al., 2010)。目前关于叶型对植物功能性状影响的实验主要集中于乔木(Li et al., 2008; Song et al., 2018), 生活型及环境条件的差异同样可能导致复叶草本、灌木表现出与复叶乔木不同的叶特征, 因此扩大取样范围能使针对叶型的研究更具代表性。此外, 相同生活型植物也可能因不同功能特征(喜光和耐阴)在叶内生物量分配方面有所差异(龙嘉翼等, 2018)。物种耐阴性是种间竞争力差异的主要决定因素(Niinemets, 1996), 喜光和耐阴树种长期处于光照条件差异明显的环境中, 在进化过程中往往会形成不同的生存对策(Galia Selaya et al., 2008; He et al., 2019)。目前关于植物在昏暗环境中的权衡策略存在两种假说: 一是“压力耐受假说”, 支持者认为耐阴植物可能通过降低叶面积比率、潜在相对生长率(Kleiman & Aarssen, 2007), 增加比叶质量、枝密度(冯秋红等, 2008)的方式来增强植物养分存储与防御能力, 帮助植物更加适应昏暗环境(Poorter, 2009; Meng et al., 2015); 二是“碳增益假说”, 支持者认为耐阴植物为了适应昏暗环境, 会采取提高光截获能力、降低呼吸消耗的策略(Valladares & Niinemets, 2008; Meng et al., 2015)。因此, 植物在昏暗环境中的生存对策仍存在争议, 进一步探索不同耐阴性乔木叶内生物量分配模式将更直观地展示出喜光与耐阴树种的生活史对策, 有助于更好地理解乔木适应环境的生存策略。此外, 植物功能性状之间的权衡关系通常由大尺度种间性状数据检验, 但也有研究表明物种内部或者局部空间尺度上叶性状之间的相关性并不会更弱(Jiang et al., 2021), 因此不能简单地将大尺度的研究结果直接套用到较小的空间尺度上, 在局域尺度上进行植物柄叶权衡研究对扩大现有结论的普适性至关重要。
植物生活型、叶型、耐阴性均可作为植物功能型的划分标准(朱玉洁等, 2011), 因此从以上3个方面探讨植物柄叶权衡关系有助于揭示不同功能型植物叶内部的生长机制与生活史策略。本研究以小兴安岭阔叶红松(Pinus koraiensis)林优势或常见乔木、灌木、草本植物为研究对象, 分析其柄叶性状之间生物量分配的权衡关系, 探讨不同生活型、叶型植物以及不同耐阴性乔木对柄叶权衡关系的影响机制。本研究提出如下假设: (1)小兴安岭地区植物的叶片性状与叶柄性状存在显著权衡关系; (2)不同生活型植物叶柄-叶片性状的权衡关系均表现出差异性特征; (3)相同叶片大小, 复叶植物比单叶植物, 喜光树种比耐阴树种对叶柄生物量投入更多。
1 材料和方法
1.1 研究区概况
研究区位于黑龙江凉水国家级自然保护区(128.79°-128.96° E, 47.11°-47.27° N), 地处小兴安岭南坡达里带岭支脉东坡, 海拔280-707 m, 为典型的低山丘陵地貌。该区域森林覆盖率达96%, 其中红松林面积占80%, 是目前我国保存较为完整的原始阔叶红松混交林分布区之一。该区域属于温带大陆性季风气候, 雨热同期, 春秋两季气温变化剧烈, 夏季炎热短暂, 冬季寒冷漫长, 年平均气温-0.3 ℃, 年降水量676 mm。该区域的地带性顶极植被是阔叶红松林, 地带性土壤为暗棕壤。
1.2 采样方法
本研究所调查的物种均为阔叶红松林内代表性物种, 于2021年8月植物生长最旺盛时期采集共20种植物, 包括草本7种、灌木7种、乔木6种, 属17科19属(表1)。单叶和复叶植物均兼顾乔木、灌木、草本3种生活型。此外, 乔木树种按其耐阴性分为喜光树种: 白桦(Betula platyphylla)、硕桦(B. costata)和水曲柳(Fraxinus mandshurica); 耐阴树种: 裂叶榆(Ulmus laciniata)、五角枫(Acer pictum subsp. mono)和紫椴(Tilia amurensis)(Niinemets & Valladares, 2006; Wang et al., 2019)。不同耐阴性树种采样植株均为成年乔木, 样株生长情况可见附录I。
表1 小兴安岭不同功能型物种统计信息
Table 1
| 种 Species | 科 Family | 属 Genus | 生活型 Life form | 叶型 Leaf type | 耐阴性 Shade tolerance |
|---|---|---|---|---|---|
| 白桦 Betula platyphylla | 桦木科 Betulaceae | 桦木属 Betula | >乔木 Tree | 单叶 Simple leaf | 喜光 Shade intolerant |
| 硕桦 Betula costata | 桦木科 Betulaceae | 桦木属 Betula | >乔木 Tree | 单叶 Simple leaf | 喜光 Shade intolerant |
| 裂叶榆 Ulmus laciniata | 榆科 Ulmaceae | 榆属 Ulmus | >乔木 Tree | 单叶 Simple leaf | 耐阴 Shade tolerant |
| 五角枫 Acer pictum subsp. mono | 槭树科 Aceraceae | 槭属 Acer | >乔木 Tree | 单叶 Simple leaf | 耐阴 Shade tolerant |
| 紫椴 Tilia amurensis | 椴树科 Tiliaceae | 椴属 Tilia | >乔木 Tree | 单叶 Simple leaf | 耐阴 Shade tolerant |
| 水曲柳 Fraxinus mandshurica | 木樨科 Oleaceae | 梣属 Fraxinus | >乔木 Tree | 复叶 Compound leaf | 喜光 Shade intolerant |
| 东北茶藨子 Ribes mandshuricum | 虎耳草科 Saxifragaceae | 茶藨子属 Ribes | >灌木 Shrub | 单叶 Simple leaf | |
| 毛榛 Corylus mandshurica | 桦木科 Betulaceae | 榛属 Corylus | >灌木 Shrub | 单叶 Simple leaf | |
| 忍冬 Lonicera japonica | 忍冬科 Caprifoliaceae | 忍冬属 Lonicera | >灌木 Shrub | 单叶 Simple leaf | |
| 山梅花 Philadelphus incanus | 虎耳草科 Saxifragaceae | 山梅花属Philadelphus | >灌木 Shrub | 单叶 Simple leaf | |
| 溲疏 Deutzia scabra | 虎耳草科 Saxifragaceae | 溲疏属 Deutzia | >灌木 Shrub | 单叶 Simple leaf | |
| 卫矛 Euonymus alatus | 卫矛科 Celastraceae | 卫矛属 Euonymus | >灌木 Shrub | 单叶 Simple leaf | |
| 刺五加 Eleutherococcus senticosus | 五加科 Araliaceae | 五加属Acanthopanax | >灌木 Shrub | 复叶 Compound leaf | |
| 露珠草 Circaea cordata | 柳叶菜科 Onagraceae | 露珠草属 Circaea | >草本 Herb | 单叶 Simple leaf | |
| 荨麻 Urtica fissa | 荨麻科 Urticaceae | 荨麻属 Urtica | >草本 Herb | 单叶 Simple leaf | |
| 水金凤 Impatiens noli-tangere | 凤仙花科 Balsaminaceae | 凤仙花属 Impatiens | >草本 Herb | 单叶 Simple leaf | |
| 透茎冷水花 Pilea pumila | 荨麻科 Urticaceae | 冷水花属 Pilea | >草本 Herb | 单叶 Simple leaf | |
| 中国茜草 Rubia chinensis | 茜草科 Rubiaceae | 茜草属 Rubia | >草本 Herb | 单叶 Simple leaf | |
| 北野豌豆 Vicia ramuliflora | 豆科 Leguminosae | 野豌豆属 Vicia | >草本 Herb | 复叶 Compound leaf | |
| 升麻 Cimicifuga foetida | 毛茛科 Ranunculaceae | 升麻属 Cimicifuga | >草本 Herb | 复叶 Compound leaf |
所有物种均随机选择3株个体, 每个个体随机选择没有明显叶损伤的3-5个当年生小枝(即枝条末端到第一个终端节点与分枝分离的部分), 保证所有样枝上的叶子完整, 然后用封口袋密封带回实验室。在样枝采集后的6 h内对小枝上所有叶子的叶片性状与叶柄性状进行测量。
复叶被认为是一片完整的单叶, 其叶片面积、叶片鲜质量、叶片干质量均为各片小叶相应数据的加和值。复叶的叶柄、叶轴、小叶柄与单叶叶柄的功能类似, 均被视为柄状物, 统称为叶柄(Niinemets et al., 2006), 因此复叶叶柄干质量为叶柄、叶轴、小叶柄干质量的加和值。测量时以单片叶子为单位, 在实验室内将叶子分为叶片与叶柄两部分, 叶柄通常位于叶片的基部, 上端与叶片相连, 下端着生于茎上, 本实验所选植物叶片与叶柄的划分可见附录II。用Canon LiDE 400 (分辨率4 800 dpi)扫描仪扫描叶片, 并用Betch软件计算叶片面积。然后, 利用数显游标卡尺(精度0.01 mm)测量叶片厚度和叶柄基径, 刻度尺(精度0.1 cm)测量叶柄长度。将测量后的叶片与叶柄用电子天平(精度0.001 g)称量鲜质量, 之后分别放入75 ℃的烘箱中烘48 h至恒质量, 用电子天平分别记录干质量。
1.3 数据处理与分析
利用最小显著差异(LSD)法对柄叶性状进行单因素方差分析(α = 0.05), 比较不同生活型植物叶片、叶柄性状之间差异的显著性(Hess & Hess, 2017)。为了充分分析叶柄-叶片性状的相关关系, 采用y = bxa方程对叶片性状与叶柄性状进行拟合, x、y分别代表各功能性状。为使数据更符合检验的正态化要求, 本研究将所有性状值均进行对数转换(以10为底), 用方程lgy = lgb+ algx表达功能性状之间的相关关系。a = 1表示两性状为等速生长关系, 大于或小于1表示性状之间是异速生长关系(Niklas et al., 2007)。采取标准化主轴法(SMA)计算方程中斜率与截距的值, 比较不同生活型、叶型植物及不同耐阴性乔木斜率的异质性, 并对比斜率与1的差异。如果斜率同质则计算物种组之间的共同斜率, 并比较共同斜率下截距的差异。对相关关系中斜率和截距的数据分析均采用“SMATR”包实现(Warton et al., 2006)。所有统计分析均在R 3.4.4软件中进行。
2 结果
2.1 叶柄和叶片性状变异
表2 小兴安岭3种生活型植物叶功能性状差异(平均值±标准误)
Table 2
| 性状 Trait | 参数 Parameter | 生活型 Life form | ||
|---|---|---|---|---|
| 乔木 Tree | 灌木 Shrub | 草本 Herb | ||
| 叶片面积 Lamina area (cm2) | 样本量 No. of samples | 568 | 380 | 246 |
| 平均值 Mean | 75.353a | 42.500b | 26.668c | |
| 范围 Range | 3.960-512.455 | 2.600-262.338 | 1.912-240.509 | |
| 标准差 SD | 96.168 | 44.125 | 39.218 | |
| 标准误 SE | 4.035 | 2.264 | 2.500 | |
| 变异系数 CV (%) | 127.6 | 103.8 | 147.1 | |
| 叶片鲜质量 Lamina fresh mass (g) | 平均值 Mean | 1.288a | 0.501b | 0.230c |
| 范围 Range | 0.037-8.456 | 0.028-3.637 | 0.015-2.147 | |
| 标准差 SD | 1.918 | 0.600 | 0.321 | |
| 标准误 SE | 0.080 | 0.0308 | 0.020 | |
| 变异系数 CV (%) | 148.8 | 119.8 | 139.5 | |
| 叶片干质量 Lamina dry mass (g) | 平均值 Mean | 0.449a | 0.138b | 0.059c |
| 范围 Range | 0.014-3.035 | 0.006-0.864 | 0.005-0.484 | |
| 标准差 SD | 0.652 | 0.149 | 0.091 | |
| 标准误 SE | 0.027 | 0.008 | 0.006 | |
| 变异系数 CV (%) | 145.3 | 107.7 | 155.7 | |
| 叶柄干质量 Petiole dry mass (g) | 平均值 Mean | 0.061a | 0.012b | 0.007b |
| 范围 Range | 0.001-0.442 | 0.001-0.112 | 0.001-0.081 | |
| 标准差 SD | 0.109 | 0.020 | 0.013 | |
| 标准误 SE | 0.005 | 0.001 | 0.001 | |
| 变异系数 CV (%) | 178.4 | 167.4 | 193.7 | |
| 叶柄/叶干质量 Petiole/leaf dry mass ratio | 平均值 Mean | 0.085a | 0.060b | 0.086a |
| 范围 Range | 0.004-0.393 | 0.002-0.435 | 0.003-0.304 | |
| 标准差 SD | 0.049 | 0.052 | 0.051 | |
| 标准误 SE | 0.002 | 0.003 | 0.003 | |
| 变异系数 CV (%) | 58.0 | 86.4 | 59.5 | |
同一行中, 不同小写字母表示不同生活型的图基检验结果有显著差异(p < 0.05)。为使不同生活型植物的叶性状数据更具代表性, 采样时尽量选择叶片大小、叶柄长度有明显差异的物种。统计时, 复叶叶片面积、叶片鲜质量、叶片干质量均由各小叶叶性状加和所得, 复叶叶柄干质量包括复叶叶柄、叶轴、小叶柄干质量。叶干质量为叶片干质量与叶柄干质量之和。
In the same row, different lowercase letters indicate that the results of the Tukey test are significantly distinct from different life-form groups (p < 0.05). In order to make the leaf trait data from different life forms more representative, species with diverse leaf size and petiole length should be sampled as much as possible. The lamina area, lamina fresh mass and lamina dry mass of compound leaves were obtained by summing the trait values of each leaflet. The petiole dry mass of compound leaf includes the dry mass of petioles, rachises and petiolules. Leaf dry mass is the sum of lamina dry mass and petiole dry mass.
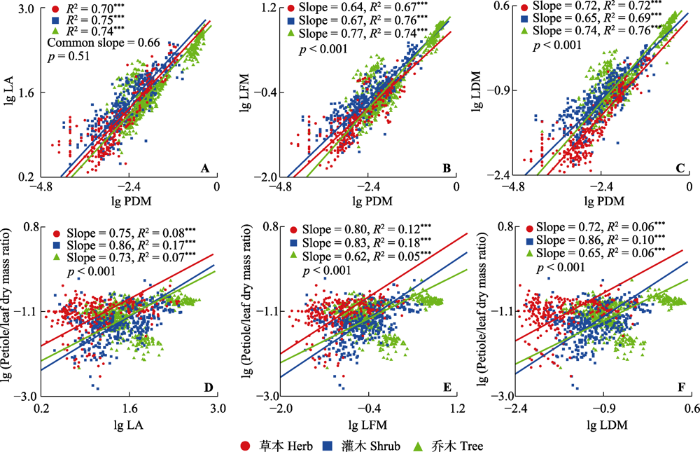
2.2 生活型对叶片性状与叶柄性状相关关系的影响
图1
图1
小兴安岭3种生活型植物叶柄-叶片性状相关关系及叶片性状-叶柄生物量分配比例相关关系在不同生活型植物间的差异。LA, 叶片面积; LDM, 叶片干质量; LFM, 叶片鲜质量; PDM, 叶柄干质量; Petiole/leaf dry mass ratio, 叶柄在叶中的生物量分配比例。p值代表斜率间差异的显著性。Common slope, 共同斜率; Slope, 斜率。***, p < 0.001。
Fig. 1
Differences in petiole-lamina trait correlations and lamina trait-petiole biomass allocation ratio correlations among three life-form plants in Xiao Hinggan Mountains. LA, lamina area; LDM, lamina dry mass; LFM, lamina fresh mass; PDM, petiole dry mass; Petiole/leaf dry mass ratio, petiole biomass allocation ratio in leaves. p value represents the significance of difference in slopes. ***, p < 0.001.
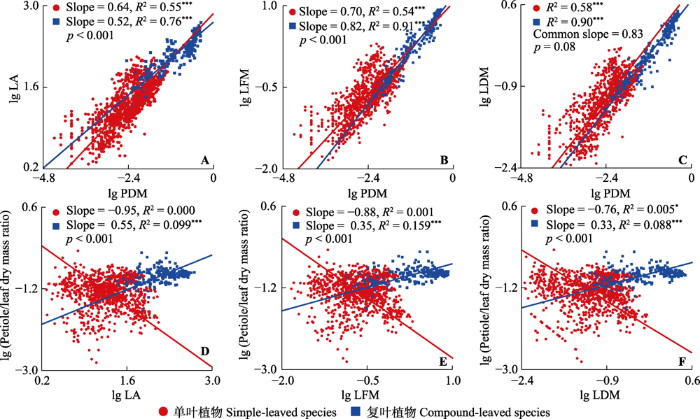
2.3 叶型对叶片性状与叶柄性状相关关系的影响
单叶植物叶片面积-叶柄干质量的斜率显著大于复叶植物(图2A), 表明增大相同的叶片面积, 复叶植物需要向叶柄投入更多的生物量。但叶型对叶片鲜质量与叶柄干质量相关关系的影响与前者相反, 复叶植物的斜率更大(图2B)。叶片干质量与叶柄干质量在单叶和复叶物种组中具有共同斜率0.83 (95%的CI是0.78-0.85), 显著小于1 (图2C), 并且单叶植物的截距显著高于复叶植物, 表明相同的叶柄干质量, 单叶植物叶片干质量更大。单叶植物叶柄生物量分配比例与叶片面积、叶片鲜质量及叶片干质量的相关关系并不显著(图2D-2F), 且单、复叶植物叶柄生物量分配比例与叶片大小相关关系拟合的效果较差(0≤ R2 ≤ 0.159)。
图2
图2
小兴安岭单叶植物和复叶植物叶柄-叶片性状相关关系及叶片性状-叶柄生物量分配比例相关关系的差异。LA, 叶片面积; LDM, 叶片干质量; LFM, 叶片鲜质量; PDM, 叶柄干质量; Petiole/leaf dry mass ratio, 叶柄在叶中的生物量分配比例。p值代表斜率间差异的显著性。Common slope, 共同斜率; Slope, 斜率。*, p < 0.05; ***, p < 0.001。
Fig. 2
Differences in petiole-lamina trait correlations and leaf trait-petiole biomass allocation ratio correlations between simple- and compound-leaved species in Xiao Hinggan Mountains. LA, lamina area; LDM, lamina dry mass; LFM, lamina fresh mass; PDM, petiole dry mass; Petiole/leaf dry mass ratio, petiole biomass allocation ratio in leaves. p value represents the significance of difference in slopes. *, p < 0.05; ***, p < 0.001.
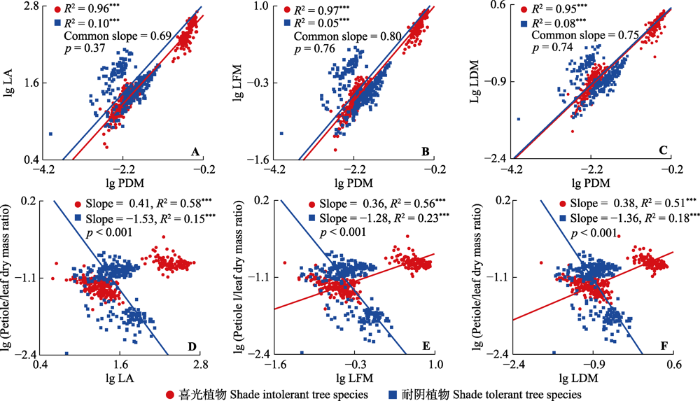
2.4 耐阴性对乔木叶片性状与叶柄性状关系的影响
不同耐阴性乔木叶片性状与叶柄干质量均表现出显著的异速生长关系(图3), 且具有共同斜率, 叶片面积、叶片鲜质量、叶片干质量与叶柄干质量的SMA共同斜率分别为0.69 (95%的CI是0.68-0.71)、0.80 (95%的CI是0.78-0.81)、0.75 (95%的CI是0.73-0.77)。其中在叶片面积、叶片鲜质量与叶柄干质量的相关关系中, 耐阴树种的截距显著高于喜光树种(图3A、3B), 表明在相同的叶柄干质量下, 耐阴树种的叶柄可以支撑更大的叶片面积与叶片鲜质量。不同耐阴性乔木在叶片干质量与叶柄干质量的相关关系中差异不显著(图3C, 截距差异不显著, p = 0.22)。不同耐阴性乔木叶柄生物量分配比例与叶片性状的相关关系均表现为喜光树种的斜率大于0, 耐阴树种的斜率小于0 (图3D-3F), 这表明随着叶片大小增加, 喜光树种倾向于将更多的生物量投资给叶柄, 耐阴树种则相反。
图3
图3
小兴安岭不同耐阴性乔木叶柄-叶片性状相关关系及叶片性状-叶柄生物量分配比例相关关系的差异。LA, 叶片面积; LDM, 叶片干质量; LFM, 叶片鲜质量; PDM, 叶柄干质量; Petiole/leaf dry mass ratio, 叶柄在叶中的生物量分配比例。p值代表斜率间差异的显著性。Common slope, 共同斜率; Slope, 斜率。***, p < 0.001。
Fig. 3
Differences in petiole-lamina trait correlations and lamina trait-petiole biomass allocation ratio relationships in different shade tolerant tree species in Xiao Hinggan Mountains. LA, lamina area; LDM, lamina dry mass; LFM, lamina fresh mass; PDM, petiole dry mass; Petiole/leaf dry mass ratio, petiole biomass allocation ratio in leaves. p value represents the significance of difference in slopes. ***, p < 0.001.
3 讨论
3.1 叶柄与叶片的权衡关系
总体上, 不同生活型、叶型植物以及不同耐阴性乔木叶片大小(叶片面积、叶片鲜质量、叶片干质量)与叶柄干质量均呈现出正向的异速生长关系(图1-3), 表明叶片性状的增长速度显著低于支撑结构叶柄的增长速度, 即表现出典型的“收益递减”效应, 这与前人的研究(Niinemets & Kull, 1999; Niinemets et al., 2007a; Li et al., 2008; Xiang et al., 2009; 祝介东等, 2011; 潘少安等, 2015)结果一致。这一结果可能源于: (1)根据“管道模型”理论, 为满足植物正常生命活动对水分的需求, 植物输导组织的横截面积与其所支持的叶片面积成正比, 除需行使运输功能外, 叶柄还需具有抵抗叶片自身重力及外界动态阻力的能力, 对支撑结构额外的生物量投资是造成柄叶异速生长的重要原因(Enquist, 2002); (2)伴随着叶片大小的增加, 叶片自身重力带给叶柄的支撑压力增大, 受到风等外力加附于叶片上的牵拉力更大, 植物必须追加对叶柄的生物量投资以满足其支撑需求(Niklas, 1999); (3)大叶往往具有更强的蒸腾作用, 但叶柄对水分的输导能力远低于与之相连的小枝, 因此水分运输受阻可能会使植物将更多的叶内生物量用于叶柄输导组织的建设(Enquist, 2002; Niinemets et al., 2007a; 杨冬梅等, 2012); (4)对叶柄的生物量投资增大是植物抵御被草食性动物取食的一种策略, 更小的叶片往往展叶效率更高, 这一策略会降低叶子的食用口感, 从而减少食草动物对叶子的取食; 而较大的叶子也有可能为了减少被食草动物取食而增大对较难食用的叶柄的生物量投资(Moles & Westoby, 2000); (5)叶片增大需要更长的叶柄以减少植物内部的自遮阴效应(杨冬梅等, 2012), 因而植物对叶柄的生物量投资比例也会相应增加。以上分析表明叶柄与叶片的权衡关系是植物适应环境的重要策略, 可能会受到多种因素影响, 从不同尺度研究柄叶权衡将有助于揭示植物内在的生长机制。
3.2 不同生活型植物柄叶权衡关系
本研究发现叶片性状与叶柄性状的权衡关系在不同生活型植物间差异显著(图1)。叶片面积可以反映叶子拦截光线的能力, 叶片面积与叶柄干质量之间的回归关系在具有共同斜率的基础上(图1A), y轴截距灌木>草本>乔木(p < 0.001), 表明在相同的叶柄干质量下, 灌木能够支撑更大的叶片面积。这一结果与不同生活型植物各自的生活史特征相适应: 乔木通常生长较大的叶片(表2), 在高温下强烈的蒸腾作用会使大叶比小叶面临更严重的水分胁迫风险(Huang et al., 2020), 乔木可能会采取加强对运输结构叶柄建设的方式保护光合收益结构叶片; 由于大部分光源为乔木叶片所截获, 林下灌木为争取光源, 可能以提高叶柄支撑效率的方式, 帮助叶片尽可能的扩展面积, 进而使其能够获得足够的光合收益; 位于森林最低层的草本植物, 则通过增加单位叶片面积的叶绿素含量的方式提高光合能力(Li et al., 2018), 帮助自身适应林下弱光环境。本研究结果还表明灌木叶内叶柄生物量分配比例与叶片面积的拟合斜率显著大于乔木和草本(图1D), 表明在叶片面积变化相同的情况下, 灌木对叶柄的生物量投资更灵活, 这一特征可能使灌木具备更高效的固碳能力, 是灌木适应林下弱光环境的一种生存策略。
叶片鲜质量代表叶片大小可以反映出叶柄承载叶片的机械载荷与生物力学效率(Niinemets et al., 2007a)。当以叶片鲜质量代表叶片大小, 比较不同生活型植物叶片鲜质量与叶柄干质量相关关系的差异性时, 在单位叶柄干质量下, 3种生活型中乔木叶柄承载叶片机械载荷的能力最强, 草本植物最弱(图1B), 即随着演替进程由草本向灌木、乔木推进, 植物叶柄对叶片的支撑效率不断提高。这可能是由于自然界植物为争夺光源总倾向于向高处生长, 随着草本、灌木、乔木叶片在林内所处高度不断增加, 植物所遭受风雨等外力的直接干扰逐渐增强, 导致高大乔木的叶柄被迫承载更大的动态载荷(Kazda et al., 2009), 因此提高叶柄支撑效率是帮助植物适应日趋复杂外部环境的重要进化特征。
叶片干质量代表叶片大小可以最直观地反映叶内生物量的分配情况。Niinemets (1996)发现随着比叶质量增加, 乔木用于支撑功能的生物量成本可能大于灌木。本研究结果与此结论相矛盾, 本研究发现单位叶片干质量, 乔木对叶柄的生物量投入远小于草本植物, 灌木的生物量投入最大(图1C), 这反映了各生活型植物生长策略的差异: (1)叶片表面积可以通过影响植物蒸腾速率进而影响导管的传输效率, 由于乔木的叶片面积普遍更大(表2), 其所受到的叶片的蒸腾拉力也更强烈, 因此导致乔木在叶水平上相对减少对运输结构的生物量投入(Niinemets et al., 2007a; 杨冬梅等, 2012)。并且由于乔木枝干运输能力强, 具备更优的木质部横截面积、导管及管胞数量(Enquist, 2002), 因此, 乔木叶柄中也可能具有更高效、成本更低的结构帮助乔木叶柄减少支持与运输成本(祝介东等, 2011)。(2)林下弱光环境限制了灌木的生长, 为适应这一环境, 灌木采取加大对叶柄生物量投资的策略(图1F), 以更长的叶柄帮助叶片获取更多的林下光源(Takenaka, 1994; Niinemets et al., 2007a)。(3)由于长叶柄策略会使得叶柄承受更强烈的牵拉力与扭转力(Niklas, 1999), 而草本植物叶内木质素等含量较低(Carins Murphy et al., 2016), 所以灌木适应林下弱光的策略并不适用于草本植物。草本植物可能通过增加单位叶片面积的叶绿素含量(Li et al., 2018)或增强植物光合活性的方式来适应林下环境(Wright et al., 2004)。
Niklas等(2007)的研究表明, 不同生活型植物叶面积增长速率普遍低于叶干质量的增长速率, 并提出这种“收益递减”是由于光合能力较差的质量成分(叶组织密度或叶厚度或两者)积累的结果。本实验不仅印证了这一观点, 并为该结果的解释补充了另一种思路, 即执行支持与运输功能的叶柄也属于光合能力低下的质量成分(Niinemets & Kull, 1999; Niinemets et al., 2006), 这种低效能结构的生长速度显著高于光合收益结构(叶片), 是限制叶片大小增加的关键因素。本研究在一定程度上扩充了限制叶片大小增大的因素, 有助于丰富“收益递减”理论。在演替过程中, 不同生活型植物出现的次序通常依次为草本、灌木、乔木, 因此对上述不同生活型植物柄叶权衡关系的研究, 不仅能够反映出位于森林内不同高度位置上植物各自的生活史策略, 还能反映出植物在进化过程中各性状之间权衡关系的演变过程。
3.3 不同叶型植物柄叶权衡关系
不同叶型植物叶功能性状及其权衡关系(如叶面积与叶干质量、元素含量等)的差异引起了国内外诸多学者的关注(Warman et al., 2011; Wu et al., 2019)。不同叶型植物叶片面积与叶柄干质量的相关关系表明, 在相同的叶柄干质量下, 单叶植物叶片面积的增长速率更快(图2A)。这可能是由于复叶植物除具有与单叶类似的小叶柄外, 还存在用于支撑的叶轴(Niinemets et al., 2006)。对于复叶植物而言, 叶轴与小枝的功能类似, 不仅能够支撑小叶占据更广的空间位置, 还具有一定的运输功能。叶轴的存在虽然增加了植物叶内整体支撑结构的生物量投资, 但却以远低于枝的生物量成本, 在小枝的基础上进一步扩展了叶片的分布空间, 帮助叶片获得相对更多的光源(Niinemets & Kull, 1999; Niinemets et al., 2006; Li et al., 2021), 这在光照资源竞争激烈的原始森林中十分重要。单复叶植物叶片干质量与叶柄干质量具有共同斜率(图2C), 表明叶片与叶柄的相对生长速率与叶型无关, 即叶内生物量分配策略与叶型无关。但由于单叶植物的截距显著高于复叶植物, 表明构造不同叶型植物所需要的初始生物量成本存在很大差异, 复叶植物特殊的叶片结构使其叶片在长度与宽度延伸的同时, 柄状结构所受到的载重与扭转增加, 从而使复叶叶柄承受更大的静态载荷与动态阻力(Niinemets et al., 2006; Li et al., 2008, 2021)。这种特殊的构造就要求复叶植物增加对柄状结构初始生物量成本的投入。叶片鲜质量与叶柄干质量的相关关系(图2B)表明, 复叶叶柄的支撑效率显著高于单叶植物。有研究表明, 在相同的叶柄横截面积下, 植物为了满足对水分的需求, 大叶片叶柄内通常会发育少量的大导管而不是大量的小导管(Li et al., 2021)。普遍认为复叶植物的叶子大于单叶植物, 那么复叶植物的叶柄中可能也存在类似结构。由于密度小的大导管比密度大的小导管具有更小的运输阻力(Sack & Frole, 2006; Lintunen & Kalliokoski, 2010; Gebauer et al., 2016; Li et al., 2021), 从而保证这种大导管植物在自然界中正常生存时, 导管内水分提供的膨压能够支撑足够面积的叶片(Niinemets & Kull, 1999)。
3.4 不同耐阴性乔木柄叶权衡关系
由于长期处于不同的光照条件下, 耐阴植物和喜光植物往往表现出相异的适应特征(龙嘉翼等, 2018; He et al., 2019), 探究柄叶性状在不同耐阴性乔木间的权衡关系, 有助于深入了解不同耐阴性乔木对环境的适应及响应策略, 对预测不同环境下乔木的资源分配及调节机制具有重要意义。本研究发现喜光与耐阴树种叶片性状与叶柄性状均表现出相似的斜率关系(图3A-3C), 并且在叶片面积-叶柄干质量、叶片鲜质量-叶柄干质量关系中, 耐阴树种的截距显著高于喜光树种。这一结果表明在相同的叶柄干质量下, 耐阴树种的叶柄能够支撑面积、自身重力更大的叶片, 这与“碳增益假说”相契合, 即耐阴树种在昏暗环境中的投资偏好向着提高光截获能力方向倾斜(龙嘉翼等, 2018)。本研究还发现叶柄生物量分配比例与叶片性状的斜率均表现为喜光树种>0, 耐阴树种<0 (图3D-3F), 说明随着叶片大小的增加, 耐阴树种叶柄生物量分配比例会相应减少, 喜光树种则相反。这一结果也印证了上述假说, 耐阴树种通常在昏暗环境中倾向于加大对叶片的投资。此外, 我们发现叶片干质量与叶柄干质量的相关关系在耐阴树种和喜光树种之间的差异不明显(图3C), 这与Niinemets等(2007)关于“耐阴植物出于对光环境的适应以及对生物力学的需要, 叶子往往会相应地增加叶柄生物量”的观点相矛盾。这可能是植物结构与生理可塑性权衡后的结果。一方面, 耐阴树种的生活环境往往是茂密的演替后期林(Yan et al., 2013), 相对密闭的空间使得耐阴树种比喜光树种所承受的外来干扰更少(Lee et al., 2010), 即叶柄受到风等外力的拉扯对耐阴树种的影响最小, 这种相对收益可能与大叶片导致的更多支出相抵消, 从而使得耐阴树种与喜光树种叶内生物量分配模式看似一致。另一方面, 喜光树种的叶片常常暴露于高温、强光环境中, 容易受水分胁迫, 因此植物会采取加大对运输组织投资的策略以避免或者减少生理胁迫(Kikuzawa et al., 1996; Meng et al., 2015)。
致谢
感谢黑龙江凉水国家级自然保护区管理局以及黑龙江凉水森林生态系统国家定位观测研究站在样品采集过程中给予的大力支持。
附录I 小兴安岭不同耐阴性乔木胸径、树高、第一活枝高信息概况表
Supplement I Information of diameter at breast height, tree height and height of first living branch for different shade tolerant tree species in Xiao Hinggan Mountains
附录II 小兴安岭20种植物叶子示意图
Supplement II Leaf diagram of 20 plant species in Xiao Hinggan Mountains
参考文献
Photosynthetic acclimation to variability in the light environment of early and late successional plants
DOI:10.1007/BF00379999
PMID:28309954
[本文引用: 1]

Fourteen plant species from early-, mid-, and late-successional habitats were grown for a period of 25 to 50 days in each of two light environments, i.e. full sunlight and in deep shade. The rate of photosynthesis for newly formed leaves was measured as a function of light intensity for plants from each light environment. Photosynthetic flexibility, measured as the difference in response between sun- and shade-grown plants, was determined for each of 5 parameters including dark respiration, quantum yield, light compensation, half-saturating irradiance for photosynthesis, and the photosynthetic rate at 1,400 μE m s. We found photosynthetic flexibility to be high for early successional annuals, intermediate for midsuccessional species, and low for late successional species.
Cell expansion not cell differentiation predominantly co-ordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade
DOI:10.1093/aob/mcw167 URL [本文引用: 1]
The global spectrum of plant form and function
DOI:10.1038/nature16489 URL [本文引用: 1]
Universal scaling in tree and vascular plant allometry: toward a general quantitative theory linking plant form and function from cells to ecosystems
A general theory of allometric scaling that predicts how the proportions of vascular plants and the characteristics of plant communities change or scale with plant size is outlined. The theory rests, in part, on the assumptions of (1) minimal energy dissipation in the transport of fluid through space-filling, fractal-like, branching vascular networks; and (2) the absence of scaling with plant size in the anatomical and physiological attributes of leaves and xylem. The theory shows how the scaling of metabolism with plant size is central to the scaling of whole-plant form and function. It is shown how allometric constraints influence plant populations and, potentially, processes in plant evolution. Rapidly accumulating evidence in support of the general allometric model is reviewed and new evidence is presented. Current work supports the notion that scaling of how plants utilize space and resources is central to the development of a general synthetic and quantitative theory of plant form, function, ecology and diversity.
Response of plant functional traits to environment and its application
植物功能性状对环境的响应及其应用
Biomass allocation and leaf life span in relation to light interception by tropical forest plants during the first years of secondary succession
DOI:10.1111/j.1365-2745.2008.01441.x URL [本文引用: 1]
Petiole and leaf traits of poplar in relation to parentage and biomass yield
DOI:10.1016/j.foreco.2015.11.036 URL [本文引用: 1]
Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements
DOI:10.1111/nph.15803 URL [本文引用: 2]
Understanding tests of the association of categorical variables: the Pearson Chi-square test and Fisher's exact test
DOI:10.1111/trf.14057 URL [本文引用: 1]
Increase in absolute leaf water content tends to keep pace with that of leaf dry mass-Evidence from bamboo plants
DOI:10.3390/sym12081345 URL [本文引用: 1]
Individual-level leaf trait variation and correlation across biological and spatial scales
DOI:10.1002/ece3.7425
PMID:34026011
[本文引用: 1]

Even with increasing interest in the ecological importance of intraspecific trait variation (ITV) for better understanding ecological processes, few studies have quantified ITV in seedlings and assessed constraints imposed by trade-offs and correlations among individual-level leaf traits. Estimating the amount and role of ITV in seedlings is important to understand tree recruitment and long-term forest dynamics. We measured ten different size, economics, and whole leaf traits (lamina and petiole) for more than 2,800 seedlings (height ≥ 10 cm and diameter at breast height < 1 cm) in 283 seedling plots and then quantified the amount of ITV and trait correlations across two biological (intraspecific and interspecific) and spatial (within and among plots) scales. Finally, we explored the effects of trait variance and sample size on the strength of trait correlations. We found about 40% (6%-63%) variation in leaf-level traits was explained by ITV across all traits. Lamina and petiole traits were correlated across biological and spatial scales, whereas leaf size traits (e.g., lamina area) were weakly correlated with economics traits (e.g., specific lamina area); lamina mass ratio was strongly related to the petiole length. Trait correlations varied among species, plots, and different scales but there was no evidence that the strength of trait relationships was stronger at broader than finer biological and spatial scales. While larger trait variance increased the strength of correlations, the sample size was the most important factor that was negatively related to the strength of trait correlations. Our results showed that a large amount of trait variation was explained by ITV, which highlighted the importance of considering ITV when using trait-based approaches in seedling ecology. In addition, sample size was an important factor that influenced the strength of trait correlations, which suggests that comparing trait correlations across studies should consider the differences in sample size.© 2021 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Optimisation of spatial allocation patterns in lianas compared to trees used for support
DOI:10.1007/s00468-008-0277-9 URL [本文引用: 1]
Some evidence for an adaptive linkage between leaf phenology and shoot architecture in sapling trees
DOI:10.2307/2389850 URL [本文引用: 1]
The leaf size/number trade-off in trees
DOI:10.1111/j.1365-2745.2006.01205.x URL [本文引用: 1]
A laser scanning system for estimating wind velocity reduction through tree windbreaks
DOI:10.1016/j.compag.2010.03.007 URL [本文引用: 1]
Allometric relationships between lamina area, lamina mass and petiole mass of 93 temperate woody species vary with leaf habit, leaf form and altitude
DOI:10.1111/j.1365-2435.2008.01407.x URL [本文引用: 7]
Factors influencing leaf chlorophyll content in natural forests at the biome scale
DOI:10.3389/fevo.2018.00064 URL [本文引用: 2]
Geographic variation in the petiole-lamina relationship of 325 eastern Qinghai-Tibetan woody species: analysis in three dimensions
DOI:10.3389/fpls.2021.748125 URL [本文引用: 7]
The effect of tree architecture on conduit diameter and frequency from small distal roots to branch tips in Betula pendula, Picea abies and Pinus sylvestris
DOI:10.1093/treephys/tpq085
PMID:21030407
[本文引用: 1]

We studied the effect of tree architecture on xylem anatomy in three Betula pendula Roth., three Picea abies (L.) H. Karst. and three Pinus sylvestris (L.) trees (mean age 35 years). First, the analysis of conduit anatomy in different tree parts showed that conduits tapered and their frequency increased from roots (≥ 2 mm) to stem, from stem to branches and further to leaf petioles in B. pendula. Conduit anatomy in lateral and main roots, as well as lateral and main branches, significantly differed from each other in all the studied species. The increase in conduit diameter and decrease in frequency from the pith to the bark were clear aboveground, but variable patterns were observed belowground. In the leaf petioles of B. pendula, conduit diameter increased and conduit frequency decreased with increasing individual leaf area. Second, the results concerning the scaling of conduit diameter were compared with the predictions of the general vascular scaling model (WBE model) and Murray's law. The scaling parameter values at the tree level corresponded with the predictions of the WBE model in all the studied trees except for one tree of both conifer species. However, the scaling parameter values changed from one tree compartment to another rather than remaining uniform inside a tree, as assumed by the WBE model. The assumptions of the WBE model of a constant conductivity ratio, constant tapering and an unchanged total number of conduits were not fulfilled. When the conductivity ratio and relative tapering were plotted together, the results aboveground corresponded quite well with Murray's law: the conductivity ratio increased when relative tapering decreased. Our results support the theory that trees adjust both their macro- and microstructure to maximize their water transport efficiency, but also to prevent embolism and ensure mechanical safety.
Plant functional traits-Concepts, applications and future directions
植物功能性状研究进展
Trade-offs between twig and leaf traits of ornamental shrubs grown in shade
观赏灌木小枝和叶性状在林下庇荫环境中的权衡关系
Are compound leaves an adaptation to seasonal drought or to rapid growth? Evidence from the Amazon rain forest
DOI:10.1111/j.1466-8238.2010.00567.x URL [本文引用: 1]
Growth synchrony between leaves and stems during twig development differs among plant functional types of subtropical rainforest woody species
DOI:10.1093/treephys/tpv021 URL [本文引用: 3]
Do small leaves expand faster than large leaves, and do shorter expansion times reduce herbivore damage?
DOI:10.1034/j.1600-0706.2000.900310.x URL [本文引用: 1]
Plant growth-form alters the relationship between foliar morphology and species shade-tolerance ranking in temperate woody taxa
DOI:10.1007/BF00045490 URL [本文引用: 2]
Biomass investment in leaf lamina versus lamina support in relation to growth irradiance and leaf size in temperate deciduous trees
Foliar biomass investment in support and assimilative compartments was studied in four temperate deciduous tree species along a natural light gradient across the canopy. The species ranked according to shade tolerance as Betula pendula Roth. < Populus tremula L. < Fraxinus excelsior L. < Tilia cordata Mill. Long-term light conditions at sampling locations were characterized as seasonal mean integrated quantum flux density (Q(int), mol m(-2) day(-1)) estimated by a method combining hemispherical photography and light measurements with quantum sensors. Leaf morphology was altered by Q(int) in all species. Both lamina and petiole dry mass per lamina area (LMA and PMA, respectively) increased with increasing Q(int). Shade-tolerant species had lower LMA at low Q(int) than shade-intolerant species; however, PMA was not related to shade tolerance. Across species, the ratio of petiole dry mass to lamina dry mass (PMR) varied from 0.07 to 0.21. It was independent of Q(int) in the simple-leaved species, but decreased with increasing Q(int) in the compound-leaved F. excelsior, which also had the largest foliar biomass investment in petioles. Differences in leaf mass and area, ranging over four orders of magnitude, provided an explanation for the interspecific variability in PMR. Species with large leaves also had greater biomass investments in foliar support than species with smaller leaves. This relationship was similar for both simple- and compound-leaved species. There was a negative relationship between PMR and petiole N concentration, suggesting that petioles had greater carbon assimilation rates and paid back a larger fraction of their construction cost in species with low PMR than in species with high PMR. This was probably the result of a negative relationship between PMR and petiole surface to volume ratio. Nevertheless, petioles had lower concentrations of mineral nutrients than laminas. Across species, the ratio of petiole N to lamina N varied from only 3 to 6%, demonstrating that petiole costs are less in terms of nutrients than in terms of total biomass, and that the petiole contribution to carbon assimilation is disproportionately lower than that of the lamina contribution.
Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy
DOI:10.1093/aob/mcm107 URL [本文引用: 6]
Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants
The implications of extensive variation in leaf size for biomass distribution between physiological and support tissues and for overall leaf physiological activity are poorly understood. Here, we tested the hypotheses that increases in leaf size result in enhanced whole-plant support investments, especially in compound-leaved species, and that accumulation of support tissues reduces average leaf nitrogen (N) content per unit dry mass (N(M)), a proxy for photosynthetic capacity. Leaf biomass partitioning among the lamina, mid-rib and petiole, and whole-plant investments in leaf support (within-leaf and stem) were studied in 33 simple-leaved and 11 compound-leaved species. Support investments in mid-ribs and petioles increased with leaf size similarly in simple leaves and leaflets of compound leaves, but the overall support mass fraction within leaves was larger in compound-leaved species as a result of prominent rachises. Within-leaf and within-plant support mass investments were negatively correlated. Therefore, the total plant support fraction was independent of leaf size and lamina dissection. Because of the lower N(M) of support biomass, the difference in N(M) between the entire leaf and the photosynthetic lamina increased with leaf size. We conclude that whole-plant support costs are weakly size-dependent, but accumulation of support structures within the leaf decreases whole-leaf average N(M), potentially reducing the integrated photosynthetic activity of larger leaves.
Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: a neglected source of leaf physiological differentiation?
Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs
DOI:10.1890/0012-9615(2006)076[0521:TTSDAW]2.0.CO;2 URL [本文引用: 1]
A mechanical perspective on foliage leaf form and function
DOI:10.1046/j.1469-8137.1999.00441.x URL [本文引用: 4]
“Diminishing returns” in the scaling of functional leaf traits across and within species groups
More than 5,000 measurements from 1,943 plant species were used to explore the scaling relationships among the foliar surface area and the dry, water, and nitrogen/phosphorus mass of mature individual leaves. Although they differed statistically, the exponents for the relationships among these variables were numerically similar among six species groups (ferns, graminoids, forbs, shrubs, trees, and vines) and within 19 individual species. In general, at least one among the many scaling exponents was <1.0, such that increases in one or more features influencing foliar function (e.g., surface area or living leaf mass) failed to keep pace with increases in mature leaf size. Thus, a general set of scaling relationships exists that negatively affects increases in leaf size. We argue that this set reflects a fundamental property of all plants and helps to explain why annual growth fails to keep pace with increases in total body mass across species.
Canonical rules for plant organ biomass partitioning and annual allocation
DOI:10.3732/ajb.89.5.812
PMID:21665681
[本文引用: 1]

Here we review a general allometric model for the allometric relationships among standing leaf, stem, and root biomass (M(L), M(S), and M(R), respectively) and the exponents for the relationships among annual leaf, stem, and root biomass production or "growth rates" (G(L), G(S), and G(R), respectively). This model predicts that M(L) ∝ M(S)(3/4) ∝ M(R)(3/4) such that M(S) ∝ M(R) and that G(L) ∝ G(S) ∝ G(R). A large synoptic data set for standing plant organ biomass and organ biomass production spanning ten orders of magnitude in total plant body mass supports these predictions. Although the numerical values for the allometric "constants" governing these scaling relationships differ between angiosperms and conifers, across all species, standing leaf, stem, and root biomass, respectively, comprise 8%, 67%, and 25% of total plant biomass, whereas annual leaf, stem, and root biomass growth represent 30%, 57%, and 13% of total plant growth. Importantly, our analyses of large data sets confirm the existence of scaling exponents predicted by theory. These scaling "rules" emerge from simple biophysical mechanisms that hold across a remarkably broad spectrum of ecologically and phyletically divergent herbaceous and tree-sized monocot, dicot, and conifer species. As such, they are likely to extend into evolutionary history when tracheophytes with the stereotypical "leaf," "stem," and "root" body plan first appeared.
Variations in leaf morphological and chemical traits in response to life stages, plant functional types, and habitat types in an old-growth temperate forest
DOI:10.1016/j.baae.2020.09.010 URL [本文引用: 1]
Biomass allocation strategies within a leaf: implication for leaf size optimization
DOI:10.17521/cjpe.2015.0094 URL [本文引用: 4]
从叶内生物量分配策略的角度理解叶大小的优化
DOI:10.17521/cjpe.2015.0094
[本文引用: 4]

叶大小的变化是许多因素综合作用的结果, 对叶大小优化机制的研究有助于我们更好地理解植物的适应进化和生活史策略。该研究通过对浙江省清凉峰常绿阔叶混交林中的19个常绿阔叶物种和30个落叶阔叶物种叶水平上的相关性状进行分析, 探讨叶内生物量分配策略对叶大小优化的限制性影响。研究结果显示: 无论叶大小用面积还是质量表示, 常绿物种和落叶物种均呈现出叶内生物量分配到支撑结构的比例随着叶大小的增加而增加的规律, 这主要是由叶柄大小与叶片大小之间显著的异速生长关系导致的。这种异速生长关系在常绿物种和落叶物种中普遍存在。然而, 由于常绿物种对叶柄具有较高的机械以及抵抗冰冻栓塞等不利环境的需求, 在某一给定的叶面积下, 常绿物种比落叶物种具有更高的叶柄生物量投资。这些结果表明: 作为整个植株支撑投资的一个重要组成部分, 叶内支撑投资所占的生物量比例对叶大小的优化具有一定的限制性影响。
Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests
DOI:10.1111/j.1469-8137.2008.02715.x URL [本文引用: 1]
Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees
DOI:10.1890/05-0710 URL [本文引用: 1]
Convergence in leaf size versus twig leaf area scaling: Do plants optimize leaf area partitioning?
DOI:10.1093/aob/mcw231 URL [本文引用: 1]
Correlation between leaf size and hydraulic architecture in five compound-leaved tree species of a temperate forest in NE China
DOI:10.1016/j.foreco.2017.08.005 URL [本文引用: 2]
Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot
DOI:10.1007/BF02347485 URL [本文引用: 1]
Shade tolerance, a key plant feature of complex nature and consequences
DOI:10.1146/annurev.ecolsys.39.110707.173506 URL [本文引用: 1]
Variation and relationships between twig and leaf traits of species across successional status in temperate forests
DOI:10.1080/02827581.2019.1674917 URL [本文引用: 2]
Not so simple after all: searching for ecological advantages of compound leaves
DOI:10.1111/j.1600-0706.2010.19344.x URL [本文引用: 1]
Bivariate line-fitting methods for allometry
Fitting a line to a bivariate dataset can be a deceptively complex problem, and there has been much debate on this issue in the literature. In this review, we describe for the practitioner the essential features of line-fitting methods for estimating the relationship between two variables: what methods are commonly used, which method should be used when, and how to make inferences from these lines to answer common research questions. A particularly important point for line-fitting in allometry is that usually, two sources of error are present (which we call measurement and equation error), and these have quite different implications for choice of line-fitting method. As a consequence, the approach in this review and the methods presented have subtle but important differences from previous reviews in the biology literature. Linear regression, major axis and standardised major axis are alternative methods that can be appropriate when there is no measurement error. When there is measurement error, this often needs to be estimated and used to adjust the variance terms in formulae for line-fitting. We also review line-fitting methods for phylogenetic analyses. Methods of inference are described for the line-fitting techniques discussed in this paper. The types of inference considered here are testing if the slope or elevation equals a given value, constructing confidence intervals for the slope or elevation, comparing several slopes or elevations, and testing for shift along the axis amongst several groups. In some cases several methods have been proposed in the literature. These are discussed and compared. In other cases there is little or no previous guidance available in the literature. Simulations were conducted to check whether the methods of inference proposed have the intended coverage probability or Type I error. We identified the methods of inference that perform well and recommend the techniques that should be adopted in future work.
The leaf size-twig size spectrum and its relationship to other important spectra of variation among species
There is a spectrum from species with narrow, frequently branched twigs carrying small leaves and other appendages, to species with thick twigs carrying large leaves and appendages. Here we investigate the allometry of this spectrum and its relationship to two other important spectra of ecological variation between species, the seed mass-seed output spectrum and the specific leaf area-leaf lifespan spectrum. Our main dataset covered 33 woody dicotyledonous species in sclerophyll fire-prone vegetation on low nutrient soil at 1,200 mm annual rainfall near Sydney, Australia. These were phylogenetically selected to contribute 32 evolutionary divergences. Two smaller datasets, from 390 mm annual rainfall, were also examined to assess generality of cross-species patterns. There was two to three orders of magnitude variation in twig cross-sectional area, individual leaf size and total leaf area supported on a twig across the study species. As expected, species with thicker twigs had larger leaves and branched less often than species with thin twigs. Total leaf area supported on a twig was mainly driven by leaf size rather than by the number of leaves. Total leaf area was strongly correlated with twig cross-section area, both across present-day species and across evolutionary divergences. The common log-log slope of 1.45 was significantly steeper than 1. Thus on average, species with tenfold larger leaves supported about threefold more leaf area per twig cross-section, which must have considerable implications for other aspects of water relations. Species at the low rainfall site on loamy sand supported about half as much leaf area, at a given twig cross-section, as species at the low rainfall site on light clay, or at the high rainfall site. Within sites, leaf and twig size were positively correlated with seed mass, and negatively correlated with specific leaf area. Identifying and understanding leading spectra of ecological variation among species is an important challenge for plant ecology. The seed mass-seed output and specific leaf area-leaf lifespan spectra are each underpinned by a single, comprehensible trade-off and their consequences are fairly well understood. The leaf-size-twig-size spectrum has obvious consequences for the texture of canopies, but we are only just beginning to understand the costs and benefits of large versus small leaf and twig size.
Global climatic drivers of leaf size
DOI:10.1126/science.aal4760
PMID:28860384
[本文引用: 1]

Leaf size varies by over a 100,000-fold among species worldwide. Although 19th-century plant geographers noted that the wet tropics harbor plants with exceptionally large leaves, the latitudinal gradient of leaf size has not been well quantified nor the key climatic drivers convincingly identified. Here, we characterize worldwide patterns in leaf size. Large-leaved species predominate in wet, hot, sunny environments; small-leaved species typify hot, sunny environments only in arid conditions; small leaves are also found in high latitudes and elevations. By modeling the balance of leaf energy inputs and outputs, we show that daytime and nighttime leaf-to-air temperature differences are key to geographic gradients in leaf size. This knowledge can enrich "next-generation" vegetation models in which leaf temperature and water use during photosynthesis play key roles.Copyright © 2017, American Association for the Advancement of Science.
The worldwide leaf economics spectrum
DOI:10.1038/nature02403 URL [本文引用: 2]
Differences in leaf functional traits between simple and compound leaves of Canavalia maritime
DOI:10.15244/pjoes/85946 URL [本文引用: 1]
Within-twig biomass allocation in subtropical evergreen broad-leaved species along an altitudinal gradient: allometric scaling analysis
DOI:10.1007/s00468-008-0308-6 URL [本文引用: 2]
Scaling relationships among twig size, leaf size and leafing intensity in a successional series of subtropical forests
DOI:10.1093/treephys/tpt042 URL [本文引用: 2]
Leaf and twig functional traits of woody plants and their relationships with environmental change: a review
木本植物茎叶功能性状及其关系随环境变化的研究进展
Trade-off between twig and leaf of Pinus koraiensis at different life history stages
红松不同生活史阶段的枝叶权衡
“Diminishing Returns” in the scaling between leaf area and twig size in three forest communities along an elevation gradient of Wuyi Mountain, China
DOI:10.3390/f10121138 URL [本文引用: 2]
Within-leaf allometric relationships of mature forests in different bioclimatic zones vary with plant functional types
DOI:10.3724/SP.J.1258.2011.00687 URL [本文引用: 4]
不同气候带间成熟林植物叶性状间异速生长关系随功能型的变异