

Chin J Plan Ecolo ›› 2015, Vol. 39 ›› Issue (12): 1188-1197.DOI: 10.17521/cjpe.2015.0115
• Orginal Article • Previous Articles Next Articles
CHEN Qing-Qing1, LI De-Zhi1,2,3,*( )
)
Online:2015-12-01
Published:2015-12-31
Contact:
De-Zhi LI
About author:# Co-first authors
CHEN Qing-Qing,LI De-Zhi. Kin recognition in Setaria italica under the condition of root segregation[J]. Chin J Plan Ecolo, 2015, 39(12): 1188-1197.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2015.0115
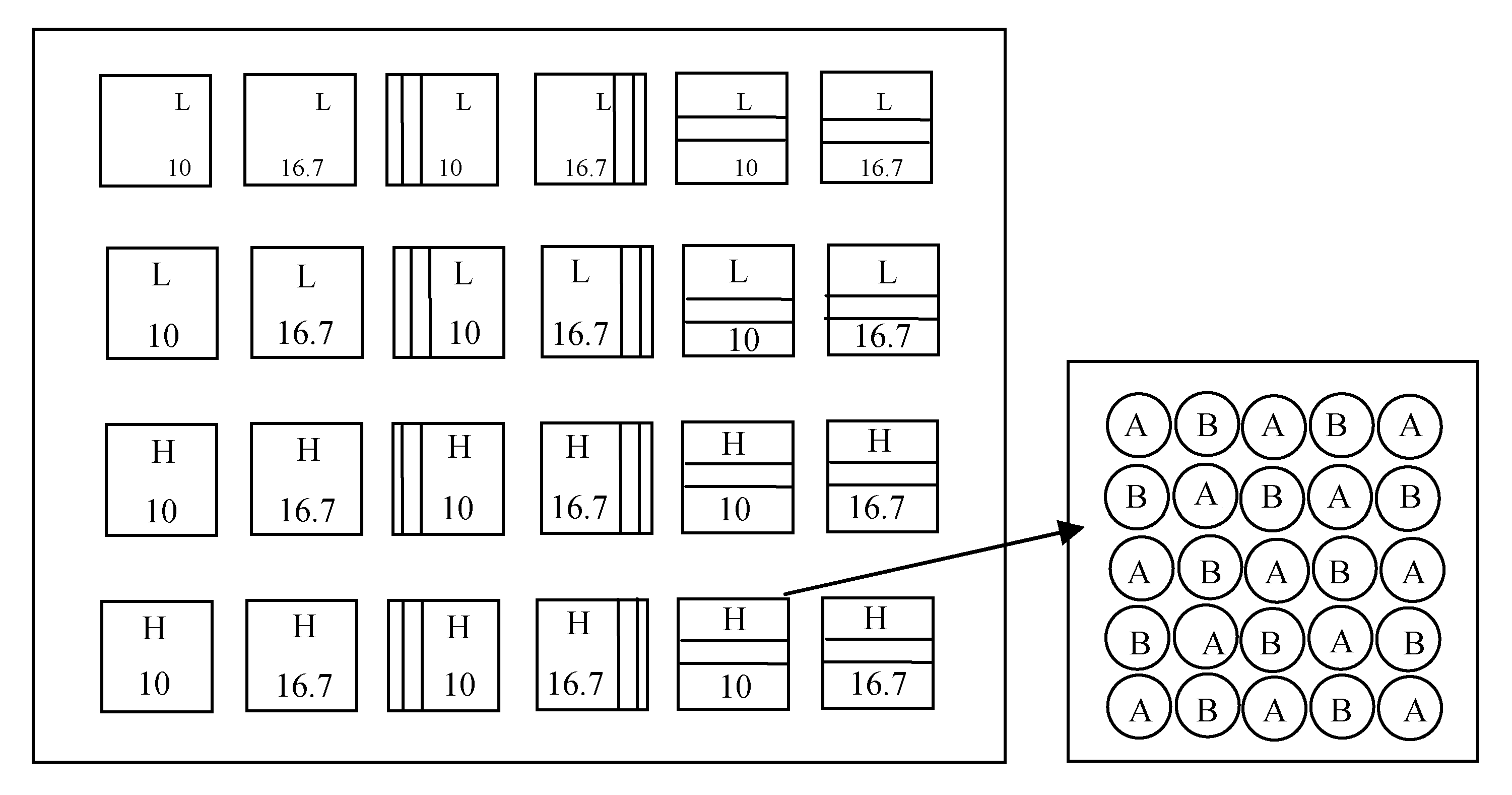
Fig. 1 Diagram of experimental design. Blank box, kin group; Vertical bars box, non-kin group; Stripes box, stranger group. H, high soil nutrient level; L, low soil nutrient level. 10 and 16.7, plant spacing (cm). A, mixed seeds of Setaria italica; B, mixed seed of Panicum miliaceum.
| 变异来源 Source of variation | df | STB | LBA | SBA | RBA | H | EL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |||||||
| D | 1 | 129.80 | 0.000 | 0.32 | 0.573 | 15.66 | 0.000 | 12.15 | 0.001 | 13.98 | 0.000 | 5.42 | 0.022 | |||||
| SN | 1 | 12.74 | 0.001 | 7.61 | 0.007 | 43.24 | 0.000 | 5.48 | 0.022 | 0.02 | 0.878 | 47.99 | 0.000 | |||||
| NI | 2 | 10.82 | 0.000 | 5.76 | 0.004 | 4.53 | 0.013 | 0.19 | 0.828 | 5.54 | 0.005 | 0.32 | 0.725 | |||||
| D × SN | 1 | 0.04 | 0.850 | 0.40 | 0.530 | 3.03 | 0.084 | 0.01 | 0.920 | 12.29 | 0.001 | 4.49 | 0.036 | |||||
| D × NI | 2 | 3.53 | 0.032 | 4.15 | 0.018 | 0.68 | 0.508 | 0.20 | 0.817 | 2.42 | 0.093 | 0.84 | 0.432 | |||||
| SN × NI | 2 | 0.35 | 0.708 | 6.72 | 0.002 | 1.76 | 0.176 | 0.97 | 0.382 | 2.59 | 0.079 | 4.76 | 0.010 | |||||
| D × SN × NI | 2 | 0.19 | 0.828 | 3.63 | 0.029 | 2.27 | 0.107 | 0.40 | 0.669 | 1.75 | 0.177 | 3.78 | 0.026 | |||||
| STB | 1 | 195.88 | 0.000 | 54.96 | 0.000 | 64.39 | 0.000 | 161.32 | 0.000 | 12.61 | 0.001 | |||||||
Table 1 Analysis of covariance (using stem biomass as a covariate) for the morphology and biomass allocation of Setaria italica in response to neighbor identity (kin, non-kin or stranger neighbors) and its interaction with plant density (high or low) and soil nutrient level (high or low)
| 变异来源 Source of variation | df | STB | LBA | SBA | RBA | H | EL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |||||||
| D | 1 | 129.80 | 0.000 | 0.32 | 0.573 | 15.66 | 0.000 | 12.15 | 0.001 | 13.98 | 0.000 | 5.42 | 0.022 | |||||
| SN | 1 | 12.74 | 0.001 | 7.61 | 0.007 | 43.24 | 0.000 | 5.48 | 0.022 | 0.02 | 0.878 | 47.99 | 0.000 | |||||
| NI | 2 | 10.82 | 0.000 | 5.76 | 0.004 | 4.53 | 0.013 | 0.19 | 0.828 | 5.54 | 0.005 | 0.32 | 0.725 | |||||
| D × SN | 1 | 0.04 | 0.850 | 0.40 | 0.530 | 3.03 | 0.084 | 0.01 | 0.920 | 12.29 | 0.001 | 4.49 | 0.036 | |||||
| D × NI | 2 | 3.53 | 0.032 | 4.15 | 0.018 | 0.68 | 0.508 | 0.20 | 0.817 | 2.42 | 0.093 | 0.84 | 0.432 | |||||
| SN × NI | 2 | 0.35 | 0.708 | 6.72 | 0.002 | 1.76 | 0.176 | 0.97 | 0.382 | 2.59 | 0.079 | 4.76 | 0.010 | |||||
| D × SN × NI | 2 | 0.19 | 0.828 | 3.63 | 0.029 | 2.27 | 0.107 | 0.40 | 0.669 | 1.75 | 0.177 | 3.78 | 0.026 | |||||
| STB | 1 | 195.88 | 0.000 | 54.96 | 0.000 | 64.39 | 0.000 | 161.32 | 0.000 | 12.61 | 0.001 | |||||||
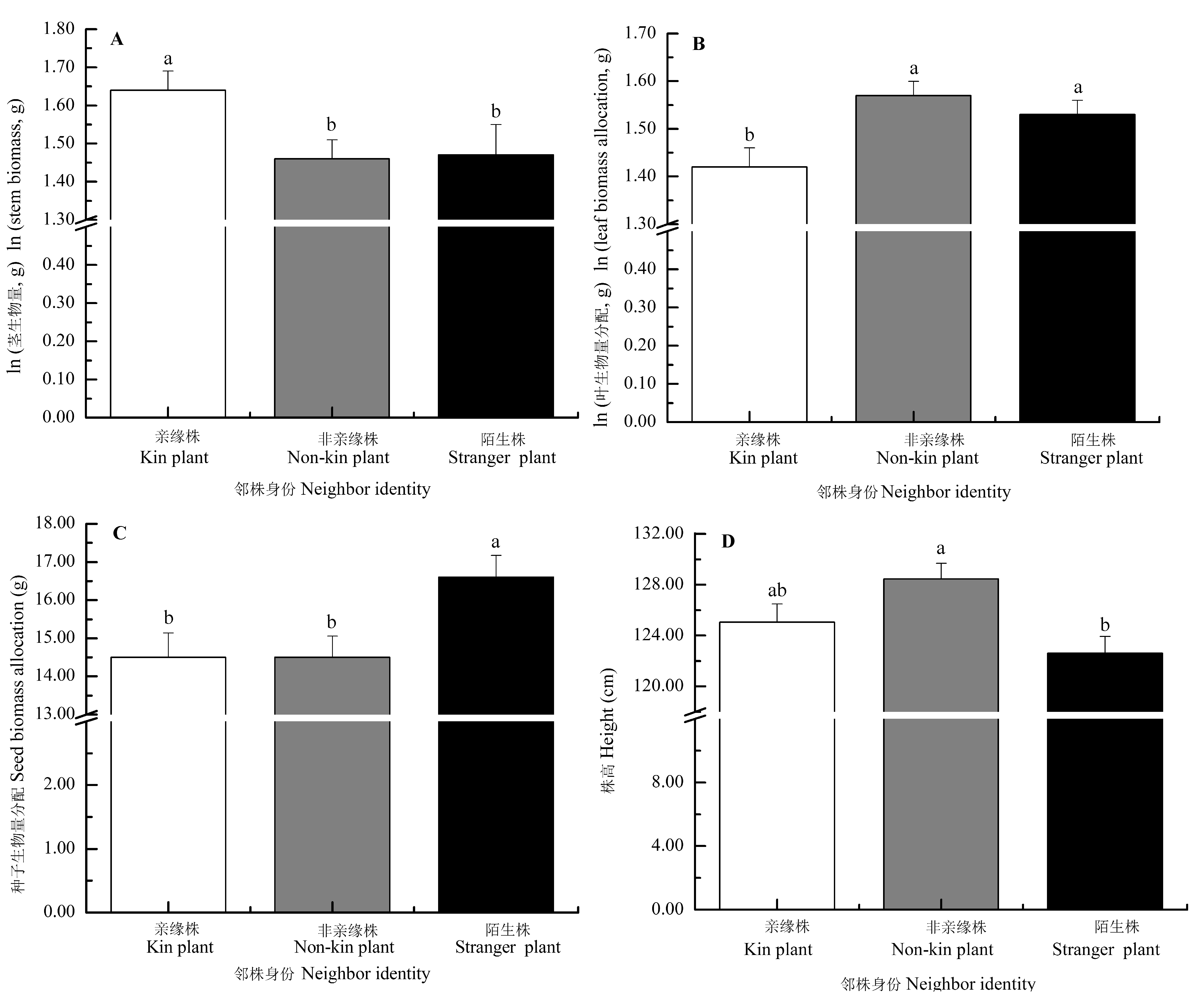
Fig. 2 Effects of neighbor identity on the morphology and biomass allocation of Setaria italica (mean ± SE). The traits (except for stem biomass) were predicted from the models with stem biomass as a covariate, by which the effects of the differences of stem biomass on trait means were accounted for. Different letters indicated significant differences among different treatments (p < 0.05, LSD).
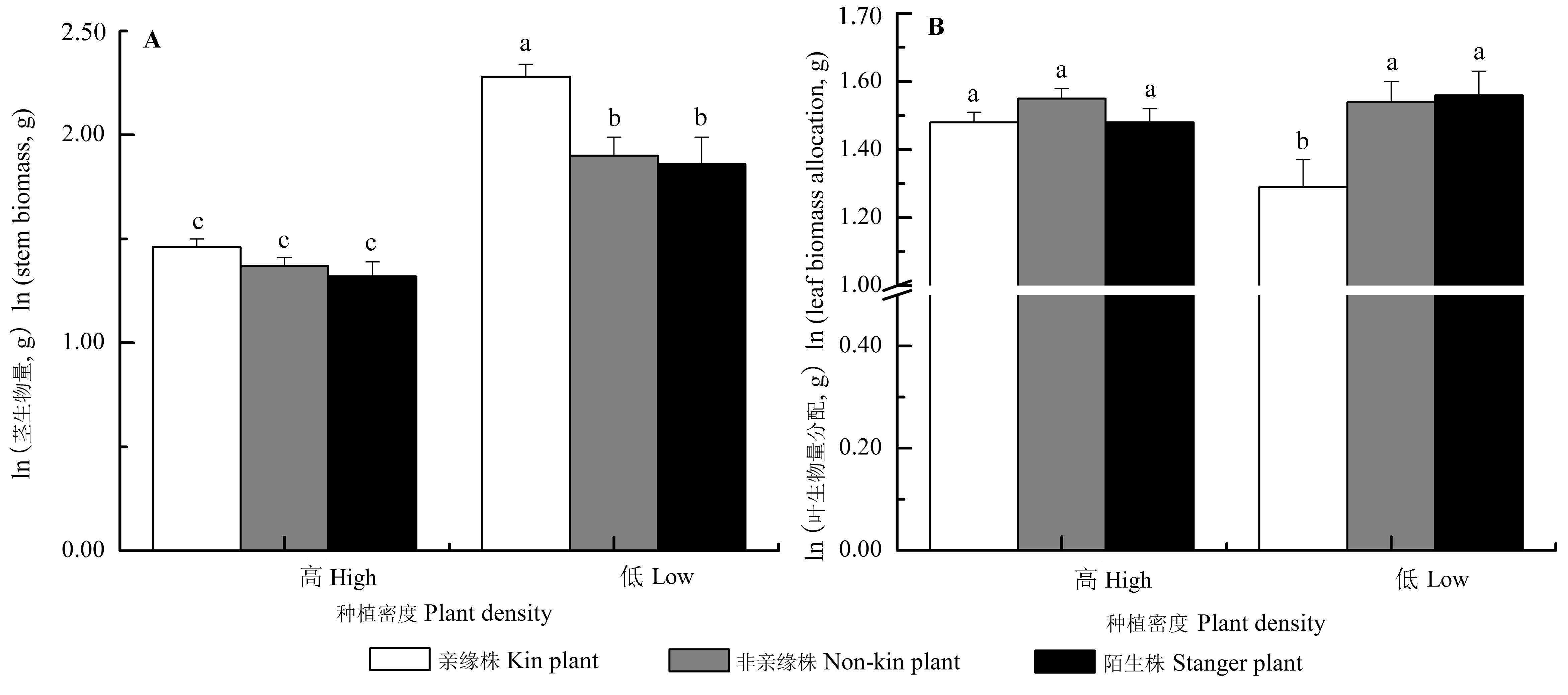
Fig. 3 Interaction effects of neighbor identity and plant density on the morphology and biomass allocation of Setaria italica (mean ± SE). The traits (except for stem biomass) were predicted from the models with stem biomass as a covariate, by which the effects of the differences of stem biomass on the trait means were accounted for. Different letters indicated significant differences among different treatments (p < 0.05, LSD).
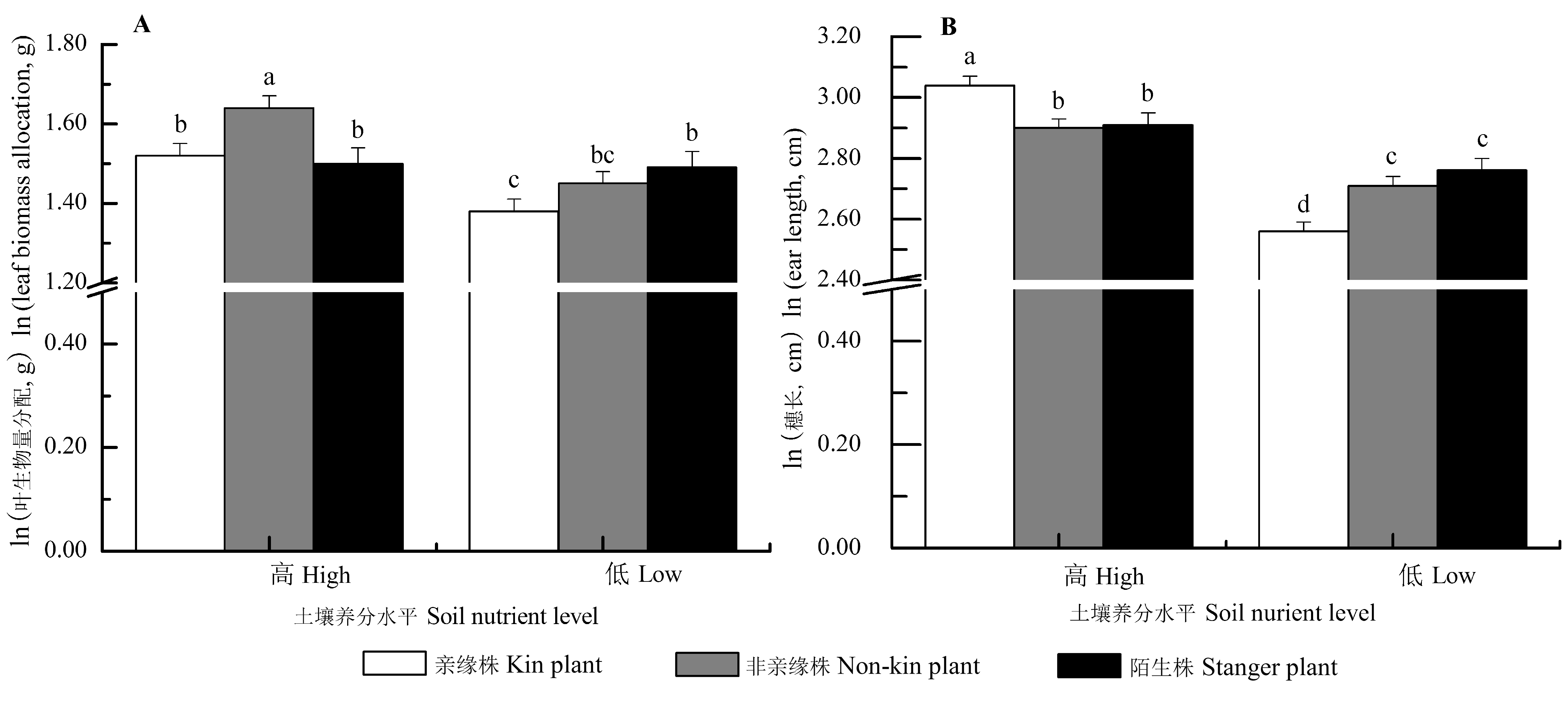
Fig. 4 Interaction effects of neighbor identity and soil nutrient level on the morphology and biomass allocation of Setaria italica (mean ± SE). The traits (except for stem biomass) were predicted from the models with stem biomass as a covariate, by which the effects of the differences of stem biomass on trait means were accounted for. Different letters indicated significant differences among different treatments (p < 0.05, LSD).
| [29] | (in Chinese with English abstract) [李洁, 孙庚, 胡霞, 张洪轩, 刘琳, 吴宁 (2014). 植物的亲缘选择. 生态学报, 34, 3827-3838.] |
| [30] | Liu B, Zhao M, Wang XR, Zhang LY, Zhao YK (2013). Effects of different nitrogen fertilizer rate on yield and economic benefits millet.Culture with Planting, (5), 11-12. |
| (in Chinese with English abstract) [刘斌, 赵敏, 王显瑞, 张立媛, 赵禹凯 (2013). 不同施氮量对谷子产量和效益的影响. 耕作与栽培, (5), 11-12.] | |
| [31] | Liu EK, Duan XS, Liu HX, Zhang DR, Liu YP, Liu H, Hou SL, Zhang M, Song YF, Wang XY, Zhou HZ (2013). The analysis of quantitative relationships between the planting density and yield in spring foxtail millet.Chinese Agricultural Science Bulletin, 29(30), 118-123. |
| (in Chinese with English abstract) [刘恩魁, 段喜顺, 刘红霞, 张德荣, 刘永平, 刘环, 侯升林, 张敏, 宋银芳, 王新玉, 周汉章 (2013). 春谷种植密度与产量的数量关系及其分析. 中国农学通报, 29(30), 118-123.] | |
| [32] | Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009). Refining the stress-gradient hypothesis for competition and facilitation in plant communities.Journal of Ecology, 97, 199-205. |
| [33] | Markham J, Halwas S (2011). Effect of neighbour presence and soil volume on the growth of Andropogon gerardii Vitman.Plant Ecology & Diversity, 4, 265-268. |
| [34] | Mercer CA, Eppley SM (2014). Kin and sex recognition in a dioecious grass.Plant Ecology, 215, 845-852. |
| [35] | Milla R, Forero DM, Escudero A, Iriondo JM (2009). Growing with siblings: A common ground for cooperation or for fiercer competition among plants?Proceedings of the Royal Society B: Biological Sciences, 276, 2531-2540. |
| [36] | Milla R, Vélez Del Burgo A, Escudero A, Iriondo JM (2012). Kinship rivalry does not trigger specific allocation strategies in Lupinus angustifolius.Annals of Botany, 110, 165-175. |
| [37] | Mohana GS, Shaanker RU, Ganeshaiah KN, Dayanandan S (2001). Genetic relatedness among developing seeds and intra fruit seed abortion in Dalbergia sissoo (Fabaceae).American Journal of Botany, 88, 1181-1188. |
| [38] | Murphy GP, Dudley SA (2009). Kin recognition: Competition and cooperation in Impatiens (Balsaminaceae).American Journal of Botany, 96, 1990-1996. |
| [39] | Murphy GP, File AL, Dudley SA (2013). Differentiating the effects of pot size and nutrient availability on plant biomass and allocation.Botany, 91, 799-803. |
| [40] | Pakkasmaa S, Laurila A (2004). Are the effects of kinship modified by environmental conditions in Rana temporaria tadpoles?Annales Zoologici Fennici, 41, 413-420. |
| [41] | Sadras VO (2007). Evolutionary aspects of the trade-off between seed size and number in crops.Field Crops Research, 100, 125-138. |
| [42] | Semchenko M, Saar S, Lepik A (2014). Plant root exudates mediate neighbour recognition and trigger complex behavioural changes.New Phytologist, 204, 631-637. |
| [43] | Silvertown J (2004). Plant coexistence and the niche.Trends in Ecology & Evolution, 19, 605-611. |
| [44] | Tonsor SJ (1989). Relatedness and intraspecific competition in Plantago lanceolata.The American Naturalist, 134, 897-906. |
| [45] | Weiner J (2004). Allocation, plasticity and allometry in plants.Perspectives in Plant Ecology, Evolution and Systematics, 6, 207-215. |
| [46] | Weinig C (2000). Differing selection in alternative competitive environments: Shade-avoidance responses and germination timing.Evolution, 54, 124-136. |
| [47] | Willson MF, Hoppes WG, Goldman DA, Thomas PA, Katusic- Malmborg PL, Bothwell JL (1987). Sibling competition in plants: An experimental study.The American Naturalist, 129, 304-311. |
| [48] | Yang YJ, Wang HF, Guo PY, Wang YG, Yuan XY, Xing GF, Shao DH, Qi X, Xie LL, Nie ME, Guo J, Ning N (2013). Effects of fertilization and density on photosynthetic characteristics and yield of hybrid foxtail millet.Plant Nutrition and Fertilizer Science, 19, 566-576. |
| [1] | Axelrod R, Hamilton WD (1981). The evolution of cooperation.Science, 211, 1390-1396. |
| [2] | Bais HP (2015). Shedding light on kin recognition response in plants.New Phytologist, 205, 4-6. |
| [3] | Bertness MD, Callaway RM (1994). Positive interactions in communities.Trends in Ecology and Evolution, 9, 191-193. |
| [4] | Bhatt MV, Khandelwal A, Dudley SA (2011). Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs.New Phytologist, 189, 1135-1142. |
| [5] | Biedrzycki ML, Bais HP (2010). Kin recognition in plants: A mysterious behaviour unsolved.Journal of Experimental Botany, 61, 4123-4128. |
| [6] | Biedrzycki ML, Jilany TA, Dudley SA, Bais HP (2010). Root exudates mediate kin recognition in plants.Communicative & Integrative Biology, 3, 28-35. |
| [7] | Biernaskie JM (2011). Evidence for competition and cooperation among climbing plants.Proceedings of the Royal Society B: Biological Sciences, 278, 1989-1996. |
| [8] | Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JMJ, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R (2008). Facilitation in plant communities: The past, the present, and the future.Journal of Ecology, 96, 18-34. |
| [9] | Cahill JF (2003). Lack of relationship between below-ground competition and allocation to roots in 10 gressland species.Journal of Ecology, 91, 532-540. |
| [10] | Cahill JF Jr, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St Clair CC (2010). Plants integrate information about nutrients and neighbors.Science, 328, 1657-1657. |
| [11] | Callaway RM (2007). Positive Interactions and Interdependence in Plant Communities. Springer, Dordrecht, The Netherlands. |
| [12] | Casal JJ (2013). Photoreceptor signaling networks in plant responses to shade.Annual Review of Plant Biology, 64, 403-427. |
| [13] | Cavieres LA, Peñaloza A (2012). Facilitation and interference at the intraspecific level: Recruitment of Kageneckia angustifolia D. Don (Rosaceae) in the montane sclerophyllous woodland of central Chile.Perspectives in Plant Ecology, Evolution and Systematics, 14, 13-19. |
| [14] | Chen BJW, During HJ, Anten NPR (2012). Detect thy neighbor: Identity recognition at the root level in plants.Plant Science, 195, 157-167. |
| [15] | Cheplick GP (1992). Sibling competition in plants.Journal of Ecology, 80, 567-575. |
| [16] | Clara Castellanos M, Donat-Caerols S, González-Martínez SC, Verdú M (2014). Can facilitation influence the spatial genetics of the beneficiary plant population?Journal of Ecology, 102, 1214-1221. |
| [17] | Crepy MA, Casal JJ (2015). Photoreceptor-mediated kin recognition in plants.New Phytologist, 205, 329-338. |
| [18] | Dudley SA, File AL (2007). Kin recognition in an annual plant.Biology Letters, 3, 435-438. |
| [19] | Dudley SA, Murphy GP, File AL (2013). Kin recognition and competition in plants.Functional Ecology, 27, 898-906. |
| [20] | Fajardo A, McIntire EJB (2011). Under strong niche overlap conspecifics do not compete but help each other to survive: Facilitation at the intraspecific level.Journal of Ecology, 99, 642-650. |
| [21] | Fang S, Clark RT, Zheng Y, Iyer-Pascuzzi AS, Weitz JS, Kochian LV, Edelsbrunner H, Liao H, Benfey PN (2013). Genotypic recognition and spatial responses by rice roots.Proceedings of the National Academy of Sciences of the United States of America, 110, 2670-2675. |
| [22] | Farrer EC, Goldberg DE (2011). Patterns and mechanisms of conspecific and heterospecific interactions in a dry perennial grassland.Journal of Ecology, 99, 265-276. |
| [23] | File AL, Murphy GP, Dudley SA (2012). Fitness consequences of plants growing with siblings: Reconciling kin selection, niche partitioning and competitive ability.Proceedings of the Royal Society B: Biological Sciences, 279, 209-218. |
| [24] | Gundel PE, Pierik R, Mommer L, Ballare CL (2014). Competing neighbors: Light perception and root function.Oecologia, 176, 1-10. |
| [25] | Hamilton WD (1964a). The genetical evolution of social behaviour. I.Journal of Theoretical Biology, 7, 1-16. |
| [26] | Hamilton WD (1964b). The genetical evolution of social behaviour. II.Journal of Theoretical Biology, 7, 17-52. |
| [27] | Hess L, de Kroon H (2007). Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination.Journal of Ecology, 95, 241-251. |
| [28] | Lepik A, Abakumova M, Zobel K, Semchenko M (2012). Kin recognition is density-dependent and uncommon among temperate grassland plants.Functional Ecology, 26, 1214-1220. |
| [48] | (in Chinese with English abstract) [杨艳君, 王宏富, 郭平毅, 王玉国, 原向阳, 邢国芳, 邵东红, 祁祥, 解丽丽, 聂萌恩, 郭俊, 宁娜 (2013). 施肥和密度对张杂谷5号光合特性及产量的影响. 植物营养与肥料学报, 19, 566-576.] |
| [29] | Li J, Sun G, Hu X, Zhang HX, Liu L, Wu N (2014). Kin selection in plants.Acta Ecologica Sinica, 34, 3827-3838. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn