

亚热带天然常绿阔叶林乔木树种与林下灌木树种根-叶功能性状协调性及差异
收稿日期: 2024-05-06
录用日期: 2024-11-12
网络出版日期: 2024-11-14
基金资助
国家自然科学基金(31422012)
Coordination and differences in root-leaf functional traits between tree species and understory shrub species in a subtropical natural evergreen broadleaf forest
Received date: 2024-05-06
Accepted date: 2024-11-12
Online published: 2024-11-14
Supported by
National Natural Science Foundation of China(31422012)
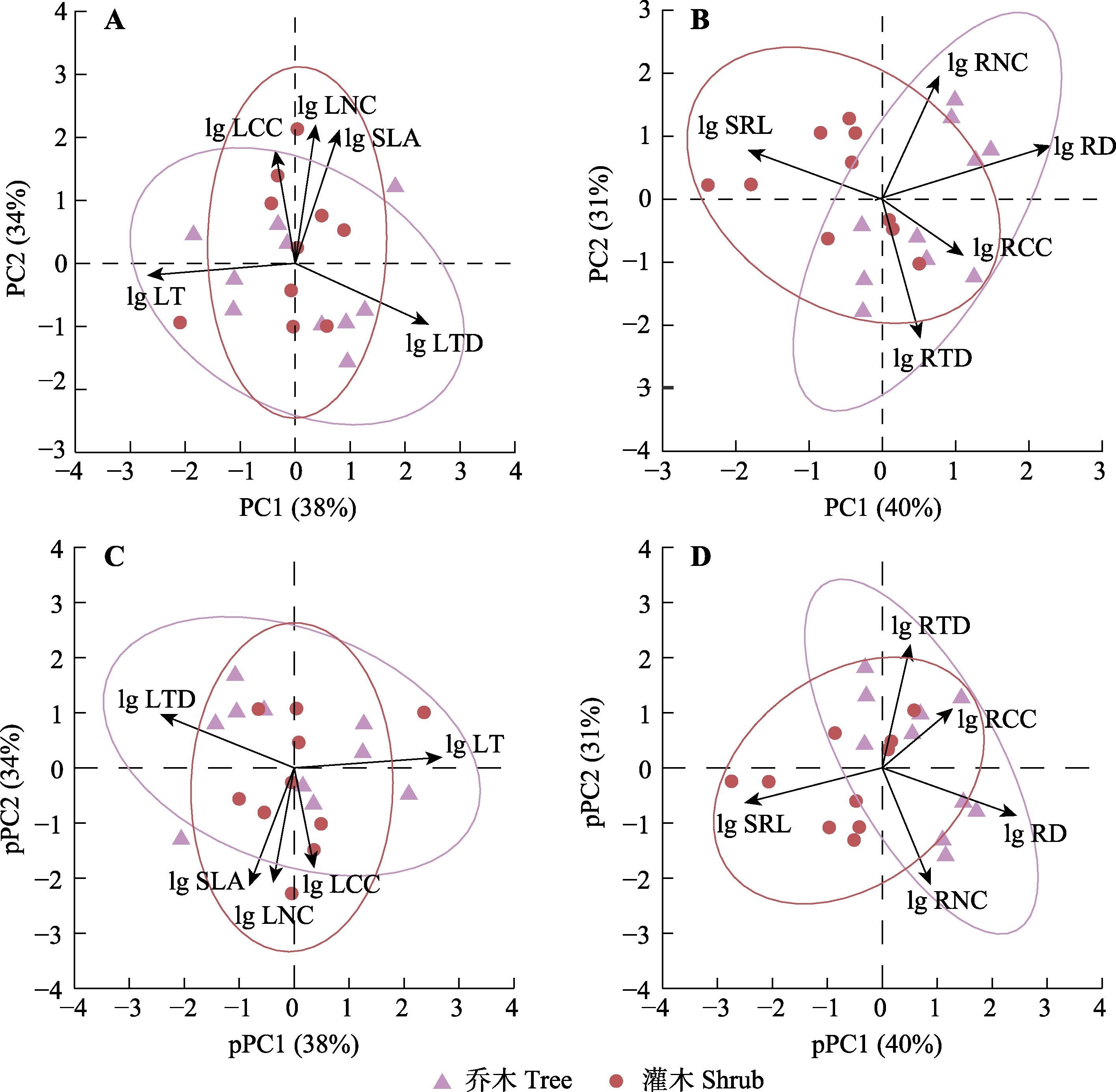
研究叶片和细根功能性状的协调性及差异, 有助于从植物整体的角度更好地认识植物的生态策略。为此, 该研究通过对福建省万木林自然保护区天然常绿阔叶林内20种木本植物(10种乔木、10种灌木)的叶片与根性状进行测定与分析, 探讨亚热带天然常绿阔叶林乔木与林下灌木树种根叶功能性状协调性及生存策略的差异。研究发现, 叶片与1级根相似性状间, 仅叶氮浓度和根氮浓度间存在较强的相关性, 且不受系统发育影响。群落内叶性状存在一个叶经济轴和叶组织密度-叶厚度变异轴, 1级根性状存在一个合作轴(由负相关的根直径-比根长表示)和根经济轴(由负相关的根氮浓度-根组织密度表示)。根叶经济轴之间无显著相关性。乔木和灌木间仅在根系合作轴上存在显著差异, 乔木具有较大的根直径, 而灌木具有较高的比根长。除此之外, 灌木的比叶面积显著大于乔木。研究结果表明, 亚热带天然常绿阔叶林群落内叶性状与根性状呈现复杂的整合关系, 乔木和灌木间采取不同的地上和地下策略来适应群落内的生境异质性。该研究结果扩充了对局部尺度上根叶性状间协调性的认识, 有助于深入理解群落内的生态过程和物种共存机制。
杜英杰 , 范爱连 , 王雪 , 闫晓俊 , 陈廷廷 , 贾林巧 , 姜琦 , 陈光水 . 亚热带天然常绿阔叶林乔木树种与林下灌木树种根-叶功能性状协调性及差异[J]. 植物生态学报, 2025 , 49(4) : 585 -595 . DOI: 10.17521/cjpe.2024.0140
Aims Studying the coordination and differences in the functional traits of leaves and fine roots can help better understand the ecological strategies of plants from a whole-plant perspective.
Methods In this study, we measured and analyzed the leaf and root traits of 20 woody species (10 trees and 10 shrubs) from the natural evergreen broadleaf forest in Wanmulin Nature Reserve, Fujian Province. We explored the coordination of root and leaf functional traits and differences in survival strategies between tree and understory shrub species in this subtropical natural evergreen broadleaf forest.
Important findings We found a strong correlation between the leaf nitrogen concentration and root nitrogen concentration, but this was observed only for similar traits of leaf and first-order root, irrespective of phylogeny. In the studied forest, there was a leaf economics spectrum and a leaf tissue density-leaf thickness variance axis, shaped by the measured leaf traits. For first-order root, we observed a cooperative axis (represented by the negative correlation between root diameter and specific root length) and a root economics spectrum (represented by the negative correlation between root nitrogen concentration and root tissue density). There was no significant correlation between root and leaf economic spectra. Significant differences were found between tree and shrub species only along the root collaboration axis, with trees having larger root diameters and shrubs having higher specific root lengths. In addition, the specific leaf area of shrub species was significantly larger than that of tree species. The results indicated that leaf and root traits are integrated into a complex relationship, with tree and shrub species adopting different aboveground and belowground strategies to adapt to the habitat heterogeneity in the studied area. Our results expand the understanding of the coordination between root and leaf traits at a local scale, and provide deeper insights into the ecological processes and species coexistence mechanisms within the community.

| [1] | Ackerly D, Knight C, Weiss S, Barton K, Starmer K (2002). Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia, 130, 449-457. |
| [2] | Bardgett RD, Mommer L, de Vries FT (2014). Going underground: root traits as drivers of ecosystem processes. Trends in Ecology & Evolution, 29, 692-699. |
| [3] | Bergmann J, Weigelt A, van Der Plas F, Laughlin DC, Kuyper TW, Guerrero-Ramirez NR, Valverde-Barrantes OJ, Bruelheide H, Freschet GT, Iversen CM, Kattge J, McCormack ML, Meier IC, Rillig MC, Roumet C, et al. (2020). The fungal collaboration gradient dominates the root economics space in plants. Science Advances, 6, eaba3756. DOI: 10.1126/sciadv.aba3756. |
| [4] | Blomberg SP, Garland Jr T, Ives AR (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57, 717-745. |
| [5] | Chen WL, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016). Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proceedings of the National Academy of Sciences of the United States of America, 113, 8741-8746. |
| [6] | Chen YT, Xu ZZ (2014). Review on research of leaf economics spectrum. Chinese Journal of Plant Ecology, 38, 1135-1153. |
| [陈莹婷, 许振柱 (2014). 植物叶经济谱的研究进展. 植物生态学报, 38, 1135-1153.] | |
| [7] | Cheng JH, Chu PF, Chen DM, Bai YF (2016). Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Functional Ecology, 30, 985-997. |
| [8] | Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC (2005). Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology, 86, 12-19. |
| [9] | Erktan A, Roumet C, Munoz F (2023). Dissecting fine root diameter distribution at the community level captures root morphological diversity. Oikos, 2023, e08907. DOI: 10.1111/oik.08907. |
| [10] | Evans JR, Poorter H (2001). Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell & Environment, 24, 755-767. |
| [11] | Freschet GT, Cornelissen JHC, Aerts R (2010). Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology, 98, 362-373. |
| [12] | Freschet GT, Violle C, Bourget MY, Scherer-Lorenzen M, Fort F (2018). Allocation, morphology, physiology, architecture: the multiple facets of plant above- and below-ground responses to resource stress. New Phytologist, 219, 1338-1352. |
| [13] | Geng Y, Wang L, Jin DM, Liu HY, He JS (2014). Alpine climate alters the relationships between leaf and root morphological traits but not chemical traits. Oecologia, 175, 445-455. |
| [14] | Guo DL, Xia MX, Wei X, Chang WJ, Liu Y, Wang ZQ (2008). Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist, 180, 673-683. |
| [15] | Holdaway RJ, Richardson SJ, Dickie IA, Peltzer DA, Coomes DA (2011). Species- and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. Journal of Ecology, 99, 954-963. |
| [16] | Hu YK, Pan X, Yang XJ, Liu GF, Liu XY, Song YB, Zhang MY, Cui LJ, Dong M (2019). Is there coordination of leaf and fine root traits at local scales? A test in temperate forest swamps. Ecology and Evolution, 9, 8714-8723. |
| [17] | Jin N, Yu XC, Dong JL, Duan MC, Mo YX, Feng LY, Bai R, Zhao JL, Song J, Dossa GGO, Lu HZ (2024). Vertical variation in leaf functional traits of Parashorea chinensis with different canopy layers. Frontiers in Plant Science, 15, 1335524. DOI: 10.3389/fpls.2024.1335524. |
| [18] | Joswig JS, Wirth C, Schuman MC, Kattge J, Reu B, Wright IJ, Sippel SD, Rüger N, Richter R, Schaepman ME, van Bodegom PM, Cornelissen JHC, Díaz S, Hattingh WN, Kramer K, et al. (2022). Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nature Ecology & Evolution, 6, 36-50. |
| [19] | Kandlikar GS, Kleinhesselink AR, Kraft NJB (2022). Functional traits predict species responses to environmental variation in a California grassland annual plant community. Journal of Ecology, 110, 833-844. |
| [20] | Kitajima K, Poorter L (2010). Tissue-level leaf toughness, but not Lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytologist, 186, 708-721. |
| [21] | Kleyer M, Trinogga J, Cebrián-Piqueras MA, Trenkamp A, Fl?jgaard C, Ejrn?s R, Bouma TJ, Minden V, Maier M, Mantilla-Contreras J, Albach DC, Blasius B (2019). Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. Journal of Ecology, 107, 829-842. |
| [22] | Kong DL, Ma CG, Zhang Q, Li L, Chen XY, Zeng H, Guo DL (2014). Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytologist, 203, 863-872. |
| [23] | Kong DL, Wang JJ, Kardol P, Wu HF, Zeng H, Deng XB, Deng Y (2016). Economic strategies of plant absorptive roots vary with root diameter. Biogeosciences, 13, 415-424. |
| [24] | Kramer-Walter KR, Bellingham PJ, Millar TR, Smissen RD, Richardson SJ, Laughlin DC (2016). Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. Journal of Ecology, 104, 1299-1310. |
| [25] | Li YP, Ni YL, Xu H, Lian JY, Ye WH (2021). Relationship between variation of plant functional traits and individual growth at different vertical layers in a subtropical evergreen broad-leaved forest of Dinghushan. Biodiversity Science, 29, 1186-1197. |
| [李艳朋, 倪云龙, 许涵, 练琚愉, 叶万辉 (2021). 鼎湖山南亚热带常绿阔叶林植物功能性状变异与不同垂直层次个体生长的关联. 生物多样性, 29, 1186-1197.] | |
| [26] | Liu GF, Freschet GT, Pan X, Cornelissen JHC, Li Y, Dong M (2010). Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytologist, 188, 543-553. |
| [27] | Luo XZ, Keenan TF, Chen JM, Croft H, Colin Prentice I, Smith NG, Walker AP, Wang H, Wang R, Xu C, Zhang Y (2021). Global variation in the fraction of leaf nitrogen allocated to photosynthesis. Nature Communications, 12, 4866. DOI: 10.1038/s41467-021-25163-9. |
| [28] | Ma ZQ, Guo DL, Xu XL, Lu MZ, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO (2018). Evolutionary history resolves global organization of root functional traits. Nature, 555, 94-97. |
| [29] | Makita N, Hirano Y, Yamanaka T, Yoshimura K, Kosugi Y (2012). Ectomycorrhizal-fungal colonization induces physio-morphological changes in Quercus serrata leaves and roots. Journal of Plant Nutrition and Soil Science, 175, 900-906. |
| [30] | McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo DL, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Lepp?lammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, et al. (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytologist, 207, 505-518. |
| [31] | Niinemets ü (2001). Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology, 82, 453-469. |
| [32] | Niinemets ü, Ellsworth DS, Lukjanova A, Tobias M (2001). Site fertility and the morphological and photosynthetic acclimation of Pinus sylvestris needles to light. Tree Physiology, 21, 1231-1244. |
| [33] | Ntawuhiganayo EB, Uwizeye FK, Zibera E, Dusenge ME, Ziegler C, Ntirugulirwa B, Nsabimana D, Wallin G, Uddling J (2020). Traits controlling shade tolerance in tropical montane trees. Tree Physiology, 40, 183-197. |
| [34] | Pagel M (1999). Inferring the historical patterns of biological evolution. Nature, 401, 877-884. |
| [35] | Poorter H, Lambers H, Evans JR (2014). Trait correlation networks: a whole-plant perspective on the recently criticized leaf economic spectrum. New Phytologist, 201, 378-382. |
| [36] | Poorter H, Niinemets ü, Poorter L, Wright IJ, Villar R (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist, 182, 565-588. |
| [37] | Silva JLA, Souza AF, Caliman A, Voigt EL, Lichston JE (2018). Weak whole-plant trait coordination in a seasonally dry South American stressful environment. Ecology and Evolution, 8, 4-12. |
| [38] | Silvertown J (2004). Plant coexistence and the niche. Trends in Ecology & Evolution, 19, 605-611. |
| [39] | Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005). Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist, 167, 493-508. |
| [40] | Valladares F, Martinez-ferri E, Balaguer L, Perez-corona E, Manrique E (2000). Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy? New Phytologist, 148, 79-91. |
| [41] | Valverde-Barrantes OJ, Freschet GT, Roumet C, Blackwood CB (2017). A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytologist, 215, 1562-1573. |
| [42] | Valverde-Barrantes OJ, Horning AL, Smemo KA, Blackwood CB (2016). Phylogenetically structured traits in root systems influence arbuscular mycorrhizal colonization in woody angiosperms. Plant and Soil, 404, 1-12. |
| [43] | Valverde-Barrantes OJ, Smemo KA, Blackwood CB (2015). Fine root morphology is phylogenetically structured, but nitrogen is related to the plant economics spectrum in temperate trees. Functional Ecology, 29, 796-807. |
| [44] | Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J (2012). The return of the variance: intraspecific variability in community ecology. Trends in Ecology & Evolution, 27, 244-252. |
| [45] | Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007). Let the concept of trait be functional! Oikos, 116, 882-892. |
| [46] | Wang RL, Wang QF, Zhao N, Yu GR, He NP (2017). Complex trait relationships between leaves and absorptive roots: coordination in tissue N concentration but divergence in morphology. Ecology and Evolution, 7, 2697-2705. |
| [47] | Wang ZY, Chen XP, Cheng Y, Wang MT, Zhong QL, Li M, Cheng DL (2021). Leaf and fine root economics spectrum across 49 woody plant species in Wuyi Mountains. Chinese Journal of Plant Ecology, 45, 242-252. |
| [王钊颖, 陈晓萍, 程英, 王满堂, 钟全林, 李曼, 程栋梁 (2021). 武夷山49种木本植物叶片与细根经济谱. 植物生态学报, 45, 242-252.] | |
| [48] | Weemstra M, Freschet GT, Stokes A, Roumet C (2020). Patterns in intraspecific variation in root traits are species-specific along an elevation gradient. Functional Ecology, 35, 342-356. |
| [49] | Weemstra M, Mommer L, Visser EJW, van Ruijven J, Kuyper TW, Mohren GMJ, Sterck FJ (2016). Towards a multidimensional root trait framework: a tree root review. New Phytologist, 211, 1159-1169. |
| [50] | Weigelt A, Mommer L, Andraczek K, Iversen CM, Bergmann J, Bruelheide H, Fan Y, Freschet GT, Guerrero-Ramírez NR, Kattge J, Kuyper TW, Laughlin DC, Meier IC, van der Plas F, Poorter H, et al. (2021). An integrated framework of plant form and function: the belowground perspective. New Phytologist, 232, 42-59. |
| [51] | Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, et al. (2004). The worldwide leaf economics spectrum. Nature, 428, 821-827. |
| [52] | Yang YY, Xiao CC, Wu XM, Long WX, Feng G, Liu GY (2021). Differing trade-off patterns of tree vegetative organs in a tropical cloud forest. Frontiers in Plant Science, 12, 680379. DOI: 10.3389/fpls.2021.680379. |
| [53] | Zhou T, Cui YC, Ye YY, Zhao WJ, Hou YJ, Wu P, Ding FJ (2022). Leaf functional traits of typical karst forest plants under different niches. Journal of Central South University of Forestry & Technology, 42(10), 129-140. |
| [周汀, 崔迎春, 叶雨艳, 赵文君, 侯贻菊, 吴鹏, 丁访军 (2022). 不同小生境下典型喀斯特森林植物叶片功能性状特征. 中南林业科技大学学报, 42(10), 129-140.] |
/
| 〈 |
|
〉 |