

菌根真菌在陆地生态系统碳循环中的作用
收稿日期: 2023-03-15
录用日期: 2023-10-09
网络出版日期: 2024-01-25
基金资助
政府间国际科技创新合作项目(2022YFE0114000);国家自然科学基金(42177277);中国博士后科学基金(2022M723322)
Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling
Received date: 2023-03-15
Accepted date: 2023-10-09
Online published: 2024-01-25
Supported by
Inter-government International Science and Technology Innovation Cooperation Project(2022YFE0114000);National Natural Science Foundation of China(42177277);China Postdoctoral Science Foundation(2022M723322)
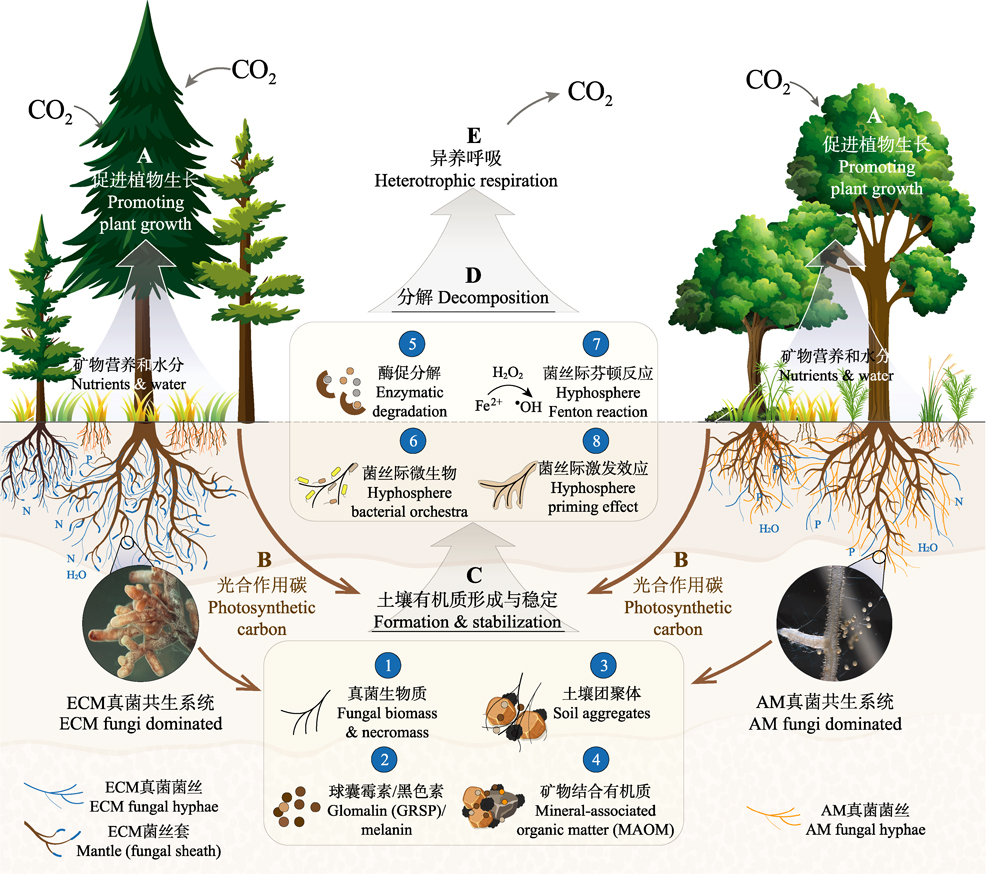
在陆地生态系统中, 土壤、植被与大气之间有着可观的碳交换通量, 陆地生态系统碳循环也和全球气候变化密切关联。菌根真菌可与绝大多数陆地植物建立菌根共生关系, 通过矿质养分-碳交换连接起生态系统地上与地下部分, 深度参与和影响陆地生态系统的碳循环过程。该文从碳的输入, 土壤有机质的形成、稳定和分解等4个关键环节分别论述了菌根真菌在陆地生态系统碳循环中的作用。研究表明, 菌根真菌在陆地生态系统碳的输入过程中扮演关键角色, 其通过改善植物矿质营养, 参与植物逆境响应, 影响植物的光合作用强度, 以及调控植物多样性与生产力之间的关系等多种途径, 维持或提高植被初级生产力; 大气中的CO2被植物固定后, 一部分碳经由菌丝网络输送到土壤中, 随后经微生物的分解和转化, 与矿物结合或被团聚体包裹而被稳定在土壤中; 同时, 菌根真菌通过影响根际激发效应和菌丝际生物化学过程, 如分泌特定胞外酶, 与菌丝际微生物互作, 驱动芬顿反应, 以及与腐生微生物竞争等, 调控土壤有机质的分解和转化过程。考虑到菌根真菌对环境和气候变化的敏感性, 该文还探讨了全球变化因子对菌根真菌介导的碳循环过程的影响。最后, 该文对未来研究方向进行了展望, 并提出以下建议: 依托联网研究, 全面解析菌根真菌参与陆地生态系统碳循环的机理过程及其环境依赖性; 加强定量研究, 将菌根真菌的作用纳入生态系统碳循环模型; 构建菌根应用技术体系, 推进菌根真菌的生态和农业应用, 提升陆地生态系统“碳汇”功能, 为实现国家碳中和目标和应对气候变化提供可选择的技术方案。
陈保冬 , 付伟 , 伍松林 , 朱永官 . 菌根真菌在陆地生态系统碳循环中的作用[J]. 植物生态学报, 2024 , 48(1) : 1 -20 . DOI: 10.17521/cjpe.2023.0075
There are substantial carbon exchange fluxes among soil, vegetation and atmosphere in the terrestrial ecosystems, which are highly relevant to global climate changes. Mycorrhizal fungi can form symbiotic associations with most terrestrial plants, linking the above- and below-ground ecosystems through mineral nutrient-carbon exchange; thus, mycorrhizal fungi play crucial roles in terrestrial carbon cycling. This review summarized the involvements of mycorrhizal fungi in the terrestrial carbon cycling processes, including the carbon input, and formation, stabilization, and decomposition of soil organic matter. Studies have demonstrated that mycorrhizal fungi markedly influence the terrestrial carbon input processes by alleviating plant nutrient deficiencies, improving plant stress resistance, influencing plant photosynthesis, and regulating plant diversity-productivity relationships, subsequently sustaining or improving primary productivity of terrestrial vegetation. A considerable proportion of photosynthetic carbon is channeled directly into the soil matrix via the fungal mycelial network, where it is partly converted into microbial-derived organic carbon, further changes the composition of soil organic carbon, and be stabilized through association with minerals and/or forming soil aggregates. Mycorrhizal fungi can affect the decomposition and transformation of soil organic matter mainly through two mechanisms: the rhizosphere priming effects and/or hyphosphere biogeochemical processes. These mechanisms involve the secretion of specific extracellular enzymes, shaping hyphosphere microbial communities, induction of chemical oxidation, and competition for limited resources (e.g., nutrients and water) with free-living saprotrophs. Considering the sensitivity of mycorrhizal fungi to environmental and climate changes, we also discuss the impact of global change factors on soil carbon cycling mediated by mycorrhizal fungi. Finally, we proposed future research directions, emphasizing a need for in-depth studies on the role of mycorrhizal fungi in terrestrial carbon cycling and their environmental dependence based on network experiments in typical ecosystems. Quantitative studies should be strengthened to integrate mycorrhizal fungi into ecosystem carbon cycling models, and mycorrhizal technologies should be developed and practiced in ecological restoration and agriculture to facilitate terrestrial carbon sequestration for achieving the national carbon neutrality goals and combating climate changes.

| [1] | Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008). Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biology, 14, 2636-2660. |
| [2] | Allen MF, Kitajima K (2014). Net primary production of ectomycorrhizas in a California forest. Fungal Ecology, 10, 81-90. |
| [3] | Angst G, Mueller KE, Nierop KGJ, Simpson MJ (2021). Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biology & Biochemistry, 156, 108189. DOI: 10.1016/j.soilbio.2021.108189. |
| [4] | Anthony MA, Crowther TW, van der Linde S, Suz LM, Bidartondo MI, Cox F, Schaub M, Rautio P, Ferretti M, Vesterdal L, De Vos B, Dettwiler M, Eickenscheidt N, Schmitz A, Meesenburg H, et al. (2022). Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. The ISME Journal, 16, 1327-1336. |
| [5] | Averill C, Bhatnagar JM, Dietze MC, Pearse WD, Kivlin SN (2019). Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proceedings of the National Academy of Sciences of the United States of America, 116, 23163-23168. |
| [6] | Averill C, Hawkes CV (2016). Ectomycorrhizal fungi slow soil carbon cycling. Ecology Letters, 19, 937-947. |
| [7] | Averill C, Turner BL, Finzi AC (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature, 505, 543-545. |
| [8] | Barnes CJ, van der Gast CJ, McNamara NP, Rowe R, Bending GD (2018). Extreme rainfall affects assembly of the root-associated fungal community. New Phytologist, 220, 1172-1184. |
| [9] | Bender SF, Conen F, van der Heijden MGA, (2015). Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biology & Biochemistry, 80, 283-292. |
| [10] | Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017). Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science, 355, 181-184. |
| [11] | Bonfante P, Genre A (2008). Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends in Plant Science, 13, 492-498. |
| [12] | Brundrett MC, Tedersoo L (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytologist, 220, 1108-1115. |
| [13] | Bukovská P, Bonkowski M, Konvalinková T, Beskid O, Hujslová M, Püschel D, ?ezá?ová V, Gutiérrez-Nú?ez MS, Gryndler M, Jansa J (2018). Utilization of organic nitrogen by arbuscular mycorrhizal fungi―Is there a specific role for protists and ammonia oxidizers? Mycorrhiza, 28, 269-283. |
| [14] | Bunn RA, Simpson DT, Bullington LS, Lekberg Y, Janos DP (2019). Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME Journal, 13, 1891-1898. |
| [15] | Carrillo Y, Dijkstra FA, LeCain D, Pendall E (2016). Mediation of soil C decomposition by arbuscular mycorrhizal fungi in grass rhizospheres under elevated CO2. Biogeochemistry, 127, 45-55. |
| [16] | Chen L, Swenson NG, Ji NN, Mi XC, Ren HB, Guo LD, Ma KP (2019). Differential soil fungus accumulation and density dependence of trees in a subtropical forest. Science, 366, 124-128. |
| [17] | Chen YL, Xu ZW, Xu TL, Veresoglou SD, Yang GW, Chen BD (2017). Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil Biology & Biochemistry, 115, 233-242. |
| [18] | Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu SJ (2012). Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science, 337, 1084-1087. |
| [19] | Chot E, Reddy MS (2022). Role of ectomycorrhizal symbiosis behind the host plants ameliorated tolerance against heavy metal stress. Frontiers in Microbiology, 13, 855473. DOI: 10.3389/fmicb.2022.855473. |
| [20] | Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013). Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science, 339, 1615-1618. |
| [21] | Colpaert JV, Wevers JHL, Krznaric E, Adriaensen K (2011). How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Annals of Forest Science, 68, 17-24. |
| [22] | Cornelissen J, Aerts R, Cerabolini B, Werger M, van der Heijden M (2001). Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia, 129, 611-619. |
| [23] | Cotrufo MF, Ranalli MG, Haddix ML, Six J, Lugato E (2019). Soil carbon storage informed by particulate and mineral- associated organic matter. Nature Geoscience, 12, 989-994. |
| [24] | Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013). The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Global Change Biology, 19, 988-995. |
| [25] | Cotton TA (2018). Arbuscular mycorrhizal fungal communities and global change: an uncertain future. FEMS Microbiology Ecology, 94, fiy179. DOI: 10.1093/femsec/fiy179. |
| [26] | Craven D, Eisenhauer N, Pearse WD, Hautier Y, Isbell F, Roscher C, Bahn M, Beierkuhnlein C, B?nisch G, Buchmann N, Byun C, Catford JA, Cerabolini BEL, Cornelissen JHC, Craine JM, et al. (2018). Multiple facets of biodiversity drive the diversity-stability relationship. Nature Ecology & Evolution, 2, 1579-1587. |
| [27] | Davidson EA, Janssens IA (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature, 440, 165-173. |
| [28] | Delavaux CS, Smith-Ramesh LM, Kuebbing SE (2017). Beyond nutrients: a meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology, 98, 2111-2119. |
| [29] | Deng MF, Hu SJ, Guo LL, Jiang L, Huang YY, Schmid B, Liu C, Chang P, Li S, Liu XJ, Ma KP, Liu LL (2023). Tree mycorrhizal association types control biodiversity- productivity relationship in a subtropical forest. Science Advances, 9, eadd4468. DOI: 10.1126/sciadv.add4468. |
| [30] | Dietrich P, Ferlian O, Huang Y, Luo S, Quosh J, Eisenhauer N (2023). Tree diversity effects on productivity depend on mycorrhizae and life strategies in a temperate forest experiment. Ecology, 104, e3896. DOI: 10.1002/ecy.3896. |
| [31] | Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, et al. (2011). Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecology Letters, 14, 349-357. |
| [32] | Dreischhoff S, Das IS, Jakobi M, Kasper K, Polle A (2020). Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Frontiers in Plant Science, 11, 590063. DOI: 10.3389/fpls.2020.590063. |
| [33] | Drigo B, Pijl AS, Duyts H, Kielak AM, Gamper HA, Houtekamer MJ, Boschker HTS, Bodelier PLE, Whiteley AS, van Veen JA, Kowalchuk GA (2010). Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proceedings of the National Academy of Sciences of the United States of America, 107, 10938-10942. |
| [34] | D?afi? E, Pongrac P, Likar M, Regvar M, Vogel-Miku? K (2013). The arbuscular mycorrhizal fungus Glomus mosseae alleviates autotoxic effects in maize (Zea mays L.). European Journal of Soil Biology, 58, 59-65. |
| [35] | Emmett BD, Lévesque-Tremblay V, Harrison MJ (2021). Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. The ISME Journal, 15, 2276-2288. |
| [36] | Evelin H, Kapoor R, Giri B (2009). Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of Botany, 104, 1263-1280. |
| [37] | Fang JY (2021). Ecological perspectives of carbon neutrality. Chinese Journal of Plant Ecology, 45, 1173-1176. |
| [方精云 (2021). 碳中和的生态学透视. 植物生态学报, 45, 1173-1176.] | |
| [38] | Fernandez CW, Heckman K, Kolka R, Kennedy PG (2019). Melanin mitigates the accelerated decay of mycorrhizal necromass with peatland warming. Ecology Letters, 22, 498-505. |
| [39] | Fernandez CW, Kennedy PG (2016). Revisiting the ‘Gadgil effect’: Do interguild fungal interactions control carbon cycling in forest soils? New Phytologist, 209, 1382-1394. |
| [40] | Fernandez CW, Langley JA, Chapman S, McCormack ML, Koide RT (2016). The decomposition of ectomycorrhizal fungal necromass. Soil Biology & Biochemistry, 93, 38-49. |
| [41] | Field KJ, Pressel S, Duckett JG, Rimington WR, Bidartondo MI (2015). Symbiotic options for the conquest of land. Trends in Ecology & Evolution, 30, 477-486. |
| [42] | Fochi V, Chitarra W, Kohler A, Voyron S, Singan VR, Lindquist EA, Barry KW, Girlanda M, Grigoriev IV, Martin F, Balestrini R, Perotto S (2017). Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytologist, 213, 365-379. |
| [43] | Frey SD (2019). Mycorrhizal fungi as mediators of soil organic matter dynamics. Annual Review of Ecology, Evolution, and Systematics, 50, 237-259. |
| [44] | Fu W, Chen BD, Rillig MC, Jansa J, Ma W, Xu C, Luo WT, Wu HH, Hao ZP, Wu H, Zhao AH, Yu Q, Han XG (2022). Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. New Phytologist, 234, 2003-2017. |
| [45] | Fu W, Wu H, Zhao AH, Hao ZP, Chen BD (2020). Ecological impacts of nitrogen deposition on terrestrial ecosystems: research progresses and prospects. Chinese Journal of Plant Ecology, 44, 475-493. |
| [付伟, 武慧, 赵爱花, 郝志鹏, 陈保冬 (2020). 陆地生态系统氮沉降的生态效应: 研究进展与展望. 植物生态学报, 44, 475-493.] | |
| [46] | Gadgil RL, Gadgil PD (1971). Mycorrhiza and litter decomposition. Nature, 233, 133. |
| [47] | Gavito ME, Jakobsen I, Mikkelsen TN, Mora F (2019). Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytologist, 223, 896-907. |
| [48] | Guerrero-Galán C, Calvo-Polanco M, Zimmermann SD (2019). Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza, 29, 291-301. |
| [49] | Han MG, Feng JG, Chen Y, Sun LJ, Fu LC, Zhu B (2021). Mycorrhizal mycelial respiration: a substantial component of soil respired CO2. Soil Biology & Biochemistry, 163, 108454. DOI: 10.1016/j.soilbio.2021.108454. |
| [50] | Hasselquist NJ, Metcalfe DB, H?gberg P (2012). Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Global Change Biology, 18, 3596-3605. |
| [51] | Hawkes CV, Hartley IP, Ineson P, Fitter AH (2008). Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Global Change Biology, 14, 1181-1190. |
| [52] | Hawkins HJ, Cargill RIM, Van Nuland ME, Hagen SC, Field KJ, Sheldrake M, Soudzilovskaia NA, Kiers ET (2023). Mycorrhizal mycelium as a global carbon pool. Current Biology, 33, R560-R573. |
| [53] | Hiiesalu I, P?rtel M, Davison J, Gerhold P, Metsis M, Moora M, ?pik M, Vasar M, Zobel M, Wilson SD (2014). Species richness of arbuscular mycorrhizal fungi: associations with grassland plant richness and biomass. New Phytologist, 203, 233-244. |
| [54] | Hobbie EA (2006). Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology, 87, 563-569. |
| [55] | Hobbie JE, Hobbie EA (2006). 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology, 87, 816-822. |
| [56] | Hodge A, Campbell CD, Fitter AH (2001). An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature, 413, 297-299. |
| [57] | H?gberg MN, Briones MJI, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, N?sholm T, H?gberg P (2010). Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytologist, 187, 485-493. |
| [58] | Holden SR, Berhe AA, Treseder KK (2015). Decreases in soil moisture and organic matter quality suppress microbial decomposition following a boreal forest fire. Soil Biology & Biochemistry, 87, 1-9. |
| [59] | Hopkins F, Gonzalez-Meler MA, Flower CE, Lynch DJ, Czimczik C, Tang JW, Subke JA (2013). Ecosystem-level controls on root-rhizosphere respiration. New Phytologist, 199, 339-351. |
| [60] | Irving TB, Alptekin B, Kleven B, Ané JM (2021). A critical review of 25 years of glomalin research: a better mechanical understanding and robust quantification techniques are required. New Phytologist, 232, 1572-1581. |
| [61] | Isbell F, Craven D, Connolly J, Loreau M, Schmid B, Beierkuhnlein C, Bezemer TM, Bonin C, Bruelheide H, de Luca E, Ebeling A, Griffin JN, Guo QF, Hautier Y, Hector A, et al. (2015). Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature, 526, 574-577. |
| [62] | Jeewani PH, Luo Y, Yu GH, Fu YY, He XH, Van Zwieten L, Liang C, Kumar A, He Y, Kuzyakov Y, Qin H, Guggenberger G, Xu JM (2021). Arbuscular mycorrhizal fungi and goethite promote carbon sequestration via hyphal-aggregate mineral interactions. Soil Biology & Biochemistry, 162, 108417. DOI: 10.1016/j.soilbio.2021.108417. |
| [63] | Jevon FV, Lang AK (2022). Tree biomass allocation differs by mycorrhizal association. Ecology, 103, e3688. DOI: 10.1002/ecy.3688. |
| [64] | Jiang FY, Zhang L, Zhou JC, George TS, Feng G (2021). Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytologist, 230, 304-315. |
| [65] | Jin HR, Liu J, Liu J, Huang XW (2012). Forms of nitrogen uptake, translocation, and transfer via arbuscular mycorrhizal fungi: a review. Science China Life Sciences, 55, 474-482. |
| [66] | Johnson NC, Graham JH, Smith FA (1997). Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytologist, 135, 575-585. |
| [67] | Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, Solaiman ZM, Murphy DV (2015). Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytologist, 205, 1537-1551. |
| [68] | Kakouridis A, Hagen JA, Kan MP, Mambelli S, Feldman LJ, Herman DJ, Weber PK, Pett-Ridge J, Firestone MK (2022). Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytologist, 236, 210-221. |
| [69] | Kallenbach CM, Frey SD, Grandy AS (2016). Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nature Communications, 7, 13630. DOI: 10.1038/ncomms13630. |
| [70] | Kamel L, Keller-Pearson M, Roux C, Ané JM (2017). Biology and evolution of arbuscular mycorrhizal symbiosis in the light of genomics. New Phytologist, 213, 531-536. |
| [71] | Karlowsky S, Augusti A, Ingrisch J, Hasibeder R, Lange M, Lavorel S, Bahn M, Gleixner G (2018). Land use in mountain grasslands alters drought response and recovery of carbon allocation and plant-microbial interactions. Journal of Ecology, 106, 1230-1243. |
| [72] | Karst J, Marczak L, Jones MD, Turkington R (2008). The mutualism-parasitism continuum in ectomycorrhizas: a quantitative assessment using meta-analysis. Ecology, 89, 1032-1042. |
| [73] | Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009). Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biology & Biochemistry, 41, 1233-1244. |
| [74] | Keller AB, Brzostek ER, Craig ME, Fisher JB, Phillips RP (2021). Root-derived inputs are major contributors to soil carbon in temperate forests, but vary by mycorrhizal type. Ecology Letters, 24, 626-635. |
| [75] | Kj?ller R, Nilsson LO, Hansen K, Schmidt IK, Vesterdal L, Gundersen P (2012). Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytologist, 194, 278-286. |
| [76] | Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canb?ck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa MD, Doré J, Floudas D, et al. (2015). Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics, 47, 410-415. |
| [77] | Koide RT, Wu T (2003). Ectomycorrhizas and retarded decomposition in a Pinus resinosa plantation. New Phytologist, 158, 401-407. |
| [78] | Konvalinková T, Jansa J (2016). Lights off for arbuscular mycorrhiza: on its symbiotic functioning under light deprivation. Frontiers in Plant Science, 7, 782. DOI: 10.3389/fpls.2016.00782. |
| [79] | Lavallee JM, Soong JL, Cotrufo MF (2020). Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Global Change Biology, 26, 261-273. |
| [80] | LeBauer DS, Treseder KK (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89, 371-379. |
| [81] | Lehmann A, Leifheit EF, Rillig MC (2017). Mycorrhizas and soil aggregation//Johnson NC, Gehring C, Jansa J. Mycorrhizal Mediation of Soil. Elsevier, Amsterdam. 241-262. |
| [82] | Lehmann J, Kleber M (2015). The contentious nature of soil organic matter. Nature, 528, 60-68. |
| [83] | Leigh J, Hodge A, Fitter AH (2009). Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist, 181, 199-207. |
| [84] | Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD (2013). First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist, 197, 617-630. |
| [85] | Li Z, Wu SL, Liu YJ, Yi Q, Nguyen TAH, Ma YY, You F, Hall M, Chan TS, Huang YF, Huang LB (2022). Plant biomass amendment regulates arbuscular mycorrhizal role in organic carbon and nitrogen sequestration in eco-engineered iron ore tailings. Geoderma, 428, 116178. DOI: 10.1016/j.geoderma.2022.116178. |
| [86] | Liang C, Amelung W, Lehmann J, K?stner M (2019). Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biology, 25, 3578-3590. |
| [87] | Lilleskov EA, Kuyper TW, Bidartondo MI, Hobbie EA (2019). Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environmental Pollution, 246, 148-162. |
| [88] | Lindahl BD, Tunlid A (2015). Ectomycorrhizal fungi-potential organic matter decomposers, yet not saprotrophs. New Phytologist, 205, 1443-1447. |
| [89] | Lin G, Craig ME, Jo I, Wang X, Zeng DH, Phillips RP (2022). Mycorrhizal associations of tree species influence soil nitrogen dynamics via effects on soil acid-base chemistry. Global Ecology and Biogeography, 31, 168-182. |
| [90] | Lin GG, McCormack ML, Ma CG, Guo DL (2017). Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytologist, 213, 1440-1451. |
| [91] | Liu XJA, Pold G, Domeignoz-Horta LA, Geyer KM, Caris H, Nicolson H, Kemner KM, Frey SD, Melillo JM, DeAngelis KM (2021). Soil aggregate-mediated microbial responses to long-term warming. Soil Biology & Biochemistry, 152, 108055. DOI: 10.1016/j.soilbio.2020.108055. |
| [92] | Luo S, Phillips RP, Jo I, Fei SL, Liang JJ, Schmid B, Eisenhauer N (2023). Higher productivity in forests with mixed mycorrhizal strategies. Nature Communications, 14, 1377. DOI: 10.1038/s41467-023-36888-0. |
| [93] | Maherali H, Klironomos JN (2007). Influence of phylogeny on fungal community assembly and ecosystem functioning. Science, 316, 1746-1748. |
| [94] | Maillard F, Fernandez CW, Mundra S, Heckman KA, Kolka RK, Kauserud H, Kennedy PG (2022). Warming drives a ‘hummockification’ of microbial communities associated with decomposing mycorrhizal fungal necromass in peatlands. New Phytologist, 234, 2032-2043. |
| [95] | Maillard F, Kennedy PG, Adamczyk B, Heinonsalo J, Buée M (2021). Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbial communities in a boreal forest. Molecular Ecology, 30, 1921-1935. |
| [96] | Mao ZK, van der Plas F, Corrales A, Anderson-Teixeira KJ, Bourg NA, Chu CJ, Hao ZQ, Jin GZ, Lian JY, Lin F, Li BH, Luo WQ, McShea WJ, Myers JA, Shen GC, et al. (2023). Scale-dependent diversity-biomass relationships can be driven by tree mycorrhizal association and soil fertility. Ecological Monographs, 93, e1568. DOI: 10.1002/ecm.1568. |
| [97] | Martin F, Kohler A, Murat C, Veneault-Fourrey C, Hibbett DS (2016). Unearthing the roots of ectomycorrhizal symbioses. Nature Reviews Microbiology, 14, 760-773. |
| [98] | Melillo JM, Aber JD, Muratore JF (1982). Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology, 63, 621-626. |
| [99] | Mohan JE, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, Taylor M, Witt CA (2014). Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecology, 10, 3-19. |
| [100] | Morin E, Miyauchi S, San Clemente H, Chen ECH, Pelin A, de la Providencia I, Ndikumana S, Beaudet D, Hainaut M, Drula E, Kuo A, Tang N, Roy S, Viala J, Henrissat B, et al. (2019). Comparative genomics of Rhizophagus irregularis, R. cerebriforme, R. diaphanus and Gigaspora rosea highlights specific genetic features in Glomeromycotina, New Phytologist, 222, 1584-1598. |
| [101] | Morris EK, Morris DJP, Vogt S, Gleber SC, Bigalke M, Wilcke W, Rillig MC (2019). Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. The ISME Journal, 13, 1639-1646. |
| [102] | Morrison EW, Frey SD, Sadowsky JJ, Thomas WK, Pringle A (2016). Chronic nitrogen additions fundamentally restructure the soil fungal community in a temperate forest. Fungal Ecology, 23, 48-57. |
| [103] | Nottingham AT, Turner BL, Winter K, van der Heijden MGA, Tanner EVJ (2010). Arbuscular mycorrhizal mycelial respiration in a moist tropical forest. New Phytologist, 186, 957-967. |
| [104] | Op De Beeck M, Troein C, Peterson C, Persson P, Tunlid A (2018). Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus. New Phytologist, 218, 335-343. |
| [105] | Orwin KH, Kirschbaum MUF, St John MG, Dickie IA (2011). Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecology Letters, 14, 493-502. |
| [106] | Paterson E, Sim A, Davidson J, Daniell TJ (2016). Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation. Plant and Soil, 408, 243-254. |
| [107] | Pellitier PT, Ibá?ez I, Zak DR, Argiroff WA, Acharya K (2021). Ectomycorrhizal access to organic nitrogen mediates CO2 fertilization response in a dominant temperate tree. Nature Communications, 12, 5403. DOI: 10.1038/s41467-021-25652-x. |
| [108] | Pellitier PT, Zak DR (2018). Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. New Phytologist, 217, 68-73. |
| [109] | Phillips RP, Brzostek E, Midgley MG (2013). The mycorrhizal- associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytologist, 199, 41-51. |
| [110] | Pozo MJ, Azcón-Aguilar C (2007). Unraveling mycorrhiza- induced resistance. Current Opinion in Plant Biology, 10, 393-398. |
| [111] | Remy W, Taylor TN, Hass H, Kerp H (1994). Four hundred- million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences of the United States of America, 91, 11841-11843. |
| [112] | Rillig MC (2004). Arbuscular mycorrhizae, glomalin, and soil aggregation. Canadian Journal of Soil Science, 84, 355-363. |
| [113] | Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2015). Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytologist, 205, 1385-1388. |
| [114] | Rillig MC, Caldwell BA, W?sten HAB, Sollins P (2007). Role of proteins in soil carbon and nitrogen storage: controls on persistence. Biogeochemistry, 85, 25-44. |
| [115] | Rillig MC, Mummey DL (2006). Mycorrhizas and soil structure. New Phytologist, 171, 41-53. |
| [116] | Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang GW (2019). The role of multiple global change factors in driving soil functions and microbial biodiversity. Science, 366, 886-890. |
| [117] | Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS (2001). Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant and Soil, 233, 167-177. |
| [118] | Rineau F, Shah F, Smits MM, Persson P, Johansson T, Carleer R, Troein C, Tunlid A (2013). Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. The ISME Journal, 7, 2010-2022. |
| [119] | Rosier CL, Hoye AT, Rillig MC (2006). Glomalin-related soil protein: assessment of current detection and quantification tools. Soil Biology & Biochemistry, 38, 2205-2211. |
| [120] | Rozmo? M, Bukovská P, Hr?elová H, Kotianová M, Dudá? M, Gan?ar?íková K, Jansa J (2022). Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. The ISME Journal, 16, 676-685. |
| [121] | Ruiz-Lozano JM, Aroca R, Zamarre?o áM, Molina S, Andreo-Jiménez B, Porcel R, García-Mina JM, Ruyter-Spira C, López-Ráez JA (2016). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant, Cell & Environment, 39, 441-452. |
| [122] | Rumpel C, Amiraslani F, Koutika LS, Smith P, Whitehead D, Wollenberg E (2018). Put more carbon in soils to meet Paris climate pledges. Nature, 564, 32-34. |
| [123] | Ryan ME, Schreiner KM, Swenson JT, Gagne J, Kennedy PG (2020). Rapid changes in the chemical composition of degrading ectomycorrhizal fungal necromass. Fungal Ecology, 45, 100922. DOI: 10.1016/j.funeco.2020.100922. |
| [124] | Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, K?gel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011). Persistence of soil organic matter as an ecosystem property. Nature, 478, 49-56. |
| [125] | Schweigert M, Herrmann S, Miltner A, Fester T, K?stner M (2015). Fate of ectomycorrhizal fungal biomass in a soil bioreactor system and its contribution to soil organic matter formation. Soil Biology & Biochemistry, 88, 120-127. |
| [126] | Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S (2015). Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biology & Biochemistry, 80, 146-155. |
| [127] | Six J, Conant RT, Paul EA, Paustian K (2002). Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant and Soil, 241, 155-176. |
| [128] | Smith SE, Facelli E, Pope S, Andrew Smith FS (2010). Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant and Soil, 326, 3-20. |
| [129] | Smith SE, Read DJ (2010). Mycorrhizal Symbiosis. 3rd ed. Academic Press, London. |
| [130] | Sokol NW, Slessarev E, Marschmann GL, Nicolas A, Blazewicz SJ, Brodie EL, Firestone MK, Foley MM, Hestrin R, Hungate BA, Koch BJ, Stone BW, Sullivan MB, Zablocki O, LLNL Soil Microbiome Consortium, Pett-Ridge J (2022). Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nature Reviews Microbiology, 20, 415-430. |
| [131] | Soudzilovskaia NA, van Bodegom PM, Terrer C, van’t Zelfde M, McCallum I, Luke McCormack M, Fisher JB, Brundrett MC, Tedersoo L (2019). Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nature Communications, 10, 5077. DOI: 10.1038/s41467-019-13019-2. |
| [132] | Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH (2003). Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science, 300, 1138-1140. |
| [133] | Steidinger BS, Crowther TW, Liang J, Van Nuland ME, Werner GDA, Reich PB, Nabuurs GJ, de-Miguel S, Zhou M, Picard N, Herault B, Zhao X, Zhang C, Routh D, Peay KG (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature, 569, 404-408. |
| [134] | Sun YF, Li YW, Lu XM, Wang Y, Bai YF (2022). Contrasting effects of arbuscular mycorrhizal fungi on nitrogen uptake in Leymus chinensis and Cleistogenes squarrosa grasses, dominants of the Inner Mongolian steppe. Plant and Soil, 475, 395-410. |
| [135] | Tang NW, San Clemente H, Roy S, Bécard G, Zhao B, Roux C (2016). A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Frontiers in Microbiology, 7, 233. DOI: 10.3389/fmicb.2016.00233. |
| [136] | Tedersoo L, Bahram M, Zobel M (2020). How mycorrhizal associations drive plant population and community biology. Science, 367, eaba1223. DOI: 10.1126/science.aba1223. |
| [137] | Tedersoo L, Smith ME (2013). Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biology Reviews, 27, 83-99. |
| [138] | Terrer C, Vicca S, Hungate BA, Phillips RP, Prentice IC (2016). Mycorrhizal association as a primary control of the CO2 fertilization effect. Science, 353, 72-74. |
| [139] | Thirkell TJ, Pastok D, Field KJ (2020). Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varies according to cultivar and changes in atmospheric carbon dioxide concentration. Global Change Biology, 26, 1725-1738. |
| [140] | Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frei dit Frey N, Gianinazzi-Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 110, 20117-20122. |
| [141] | Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD (2007). Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiology Ecology, 61, 295-304. |
| [142] | van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature, 396, 69-72. |
| [143] | van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist, 205, 1406-1423. |
| [144] | Veresoglou SD, Chen BD, Rillig MC (2012). Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biology & Biochemistry, 46, 53-62. |
| [145] | Verbruggen E, Jansa J, Hammer EC, Rillig MC (2016). Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil? Journal of Ecology, 104, 261-269. |
| [146] | Wang F, Zhang L, Zhou J, Rengel Z, George TS, Feng G (2022). Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant and Soil, 481, 1-22. |
| [147] | Wang S, Guan Y, Wang Q, Zhao J, Sun G, Hu X, Running MP, Sun H, Huang J (2020a). A mycorrhizae-like gene regulates stem cell and gametophore development in mosses. Nature Communications, 11, 2030. DOI: 10.1038/s41467-020-15967-6. |
| [148] | Wang SH, Zhang YG, Ju WM, Chen JM, Ciais P, Cescatti A, Sardans J, Janssens IA, Wu MS, Berry JA, Campbell E, Fernández-Martínez M, Alkama R, Sitch S, Friedlingstein P, et al. (2020b). Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science, 370, 1295-1300. |
| [149] | Wang T, Tian Z, Bengtson P, Tunlid A, Persson P (2017). Mineral surface-reactive metabolites secreted during fungal decomposition contribute to the formation of soil organic matter. Environmental Microbiology, 19, 5117-5129. |
| [150] | Ward EB, Duguid MC, Kuebbing SE, Lendemer JC, Bradford MA (2022). The functional role of ericoid mycorrhizal plants and fungi on carbon and nitrogen dynamics in forests. New Phytologist, 235, 1701-1718. |
| [151] | Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009). Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi:results from long-term field experiments. Ecology Letters, 12, 452-461. |
| [152] | Wright SF, Franke-Snyder M, Morton JB, Upadhyaya A (1996). Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant and Soil, 181, 193-203. |
| [153] | Wu H, Yang JJ, Fu W, Rillig MC, Cao ZJ, Zhao AH, Hao ZP, Zhang X, Chen BD, Han XG (2023a). Identifying thresholds of nitrogen enrichment for substantial shifts in arbuscular mycorrhizal fungal community metrics in a temperate grassland of northern China. New Phytologist, 237, 279-294. |
| [154] | Wu SL, Fu W, Rillig MC, Chen BD, Zhu YG, Huang LB (2023b). Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi—An updated conceptual framework. New Phytologist, DOI: 10.1111/nph.19178. |
| [155] | Wu SL, Zhang X, Huang LB, Chen BD (2019). Arbuscular mycorrhiza and plant chromium tolerance. Soil Ecology Letters, 1, 94-104. |
| [156] | Wu SL, Zhang X, Sun YQ, Wu ZX, Li T, Hu YJ, Su D, Lv JT, Li G, Zhang ZS, Zheng LR, Zhang J, Chen BD (2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environmental Science & Technology, 49, 14036-14047. |
| [157] | Wurzburger N, Brookshire ENJ (2017). Experimental evidence that mycorrhizal nitrogen strategies affect soil carbon. Ecology, 98, 1491-1497. |
| [158] | Yang HS, Zhang Q, Koide RT, Hoeksema JD, Tang JJ, Bian X, Hu SJ, Chen X (2017). Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. Journal of Ecology, 105, 219-228. |
| [159] | Yin LM, Dijkstra FA, Phillips RP, Zhu B, Wang P, Cheng WX (2021). Arbuscular mycorrhizal trees cause a higher carbon to nitrogen ratio of soil organic matter decomposition via rhizosphere priming than ectomycorrhizal trees. Soil Biology & Biochemistry, 157, 108246. DOI: 10.1016/j.soilbio.2021.108246. |
| [160] | Zak DR, Pellitier PT, Argiroff W, Castillo B, James TY, Nave LE, Averill C, Beidler KV, Bhatnagar J, Blesh J, Classen AT, Craig M, Fernandez CW, Gundersen P, Johansen R, et al. (2019). Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytologist, 223, 33-39. |
| [161] | Zhang L, Zhou JC, George TS, Limpens E, Feng G (2022). Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends in Plant Science, 27, 402-411. |
| [162] | Zhang Z, Xiao J, Yuan Y, Zhao C, Liu Q, Yin H (2018). Mycelium- and root-derived C inputs differ in their impacts on soil organic C pools and decomposition in forests. Soil Biology & Biochemistry, 123, 257-265. |
| [163] | Zhou J, Zang H, Loeppmann S, Gube M, Kuzyakov Y, Pausch J (2020). Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biology & Biochemistry, 140, 107641. DOI: 10.1016/j.soilbio.2019.107641. |
| [164] | Zhu YG, Chen BD, Fu W (2022). Research frontiers in soil ecology. Science & Technology Review, 40, 25-31. |
| [朱永官, 陈保冬, 付伟 (2022). 土壤生态学研究前沿. 科技导报, 40, 25-31.] | |
| [165] | Zhu YG, Miller RM (2003). Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends in Plant Science, 8, 407-409. |
/
| 〈 |
|
〉 |