

植物生态学报 ›› 2024, Vol. 48 ›› Issue (1): 1-20.DOI: 10.17521/cjpe.2023.0075 cstr: 32100.14.cjpe.2023.0075
所属专题: 全球变化与生态系统; 生态系统碳水能量通量; 碳循环
• 侯学煜评述 • 下一篇
陈保冬1,2,*( )(
)( ), 付伟1,2, 伍松林1, 朱永官1,2
), 付伟1,2, 伍松林1, 朱永官1,2
收稿日期:2023-03-15
接受日期:2023-10-09
出版日期:2024-01-20
发布日期:2024-01-25
作者简介:陈保冬, ORCID: 0000-0002-1790-7800中国科学院生态环境研究中心研究员, 博士生导师; 中国科学院大学岗位教授。日本学术振兴会(JSPS)博士后、德国“洪堡学者”。主要研究方向为土壤生态学, 目前已在国内外学术期刊发表研究论文200余篇。作为主要完成人分别于2009年和2014年获得国家自然科学二等奖和国家科学技术进步二等奖基金资助:
CHEN Bao-Dong1,2,*( )(
)( ), FU Wei1,2, WU Song-Lin1, ZHU Yong-Guan1,2
), FU Wei1,2, WU Song-Lin1, ZHU Yong-Guan1,2
Received:2023-03-15
Accepted:2023-10-09
Online:2024-01-20
Published:2024-01-25
Contact:
E-mail: Supported by:摘要:
在陆地生态系统中, 土壤、植被与大气之间有着可观的碳交换通量, 陆地生态系统碳循环也和全球气候变化密切关联。菌根真菌可与绝大多数陆地植物建立菌根共生关系, 通过矿质养分-碳交换连接起生态系统地上与地下部分, 深度参与和影响陆地生态系统的碳循环过程。该文从碳的输入, 土壤有机质的形成、稳定和分解等4个关键环节分别论述了菌根真菌在陆地生态系统碳循环中的作用。研究表明, 菌根真菌在陆地生态系统碳的输入过程中扮演关键角色, 其通过改善植物矿质营养, 参与植物逆境响应, 影响植物的光合作用强度, 以及调控植物多样性与生产力之间的关系等多种途径, 维持或提高植被初级生产力; 大气中的CO2被植物固定后, 一部分碳经由菌丝网络输送到土壤中, 随后经微生物的分解和转化, 与矿物结合或被团聚体包裹而被稳定在土壤中; 同时, 菌根真菌通过影响根际激发效应和菌丝际生物化学过程, 如分泌特定胞外酶, 与菌丝际微生物互作, 驱动芬顿反应, 以及与腐生微生物竞争等, 调控土壤有机质的分解和转化过程。考虑到菌根真菌对环境和气候变化的敏感性, 该文还探讨了全球变化因子对菌根真菌介导的碳循环过程的影响。最后, 该文对未来研究方向进行了展望, 并提出以下建议: 依托联网研究, 全面解析菌根真菌参与陆地生态系统碳循环的机理过程及其环境依赖性; 加强定量研究, 将菌根真菌的作用纳入生态系统碳循环模型; 构建菌根应用技术体系, 推进菌根真菌的生态和农业应用, 提升陆地生态系统“碳汇”功能, 为实现国家碳中和目标和应对气候变化提供可选择的技术方案。
陈保冬, 付伟, 伍松林, 朱永官. 菌根真菌在陆地生态系统碳循环中的作用. 植物生态学报, 2024, 48(1): 1-20. DOI: 10.17521/cjpe.2023.0075
CHEN Bao-Dong, FU Wei, WU Song-Lin, ZHU Yong-Guan. Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling. Chinese Journal of Plant Ecology, 2024, 48(1): 1-20. DOI: 10.17521/cjpe.2023.0075
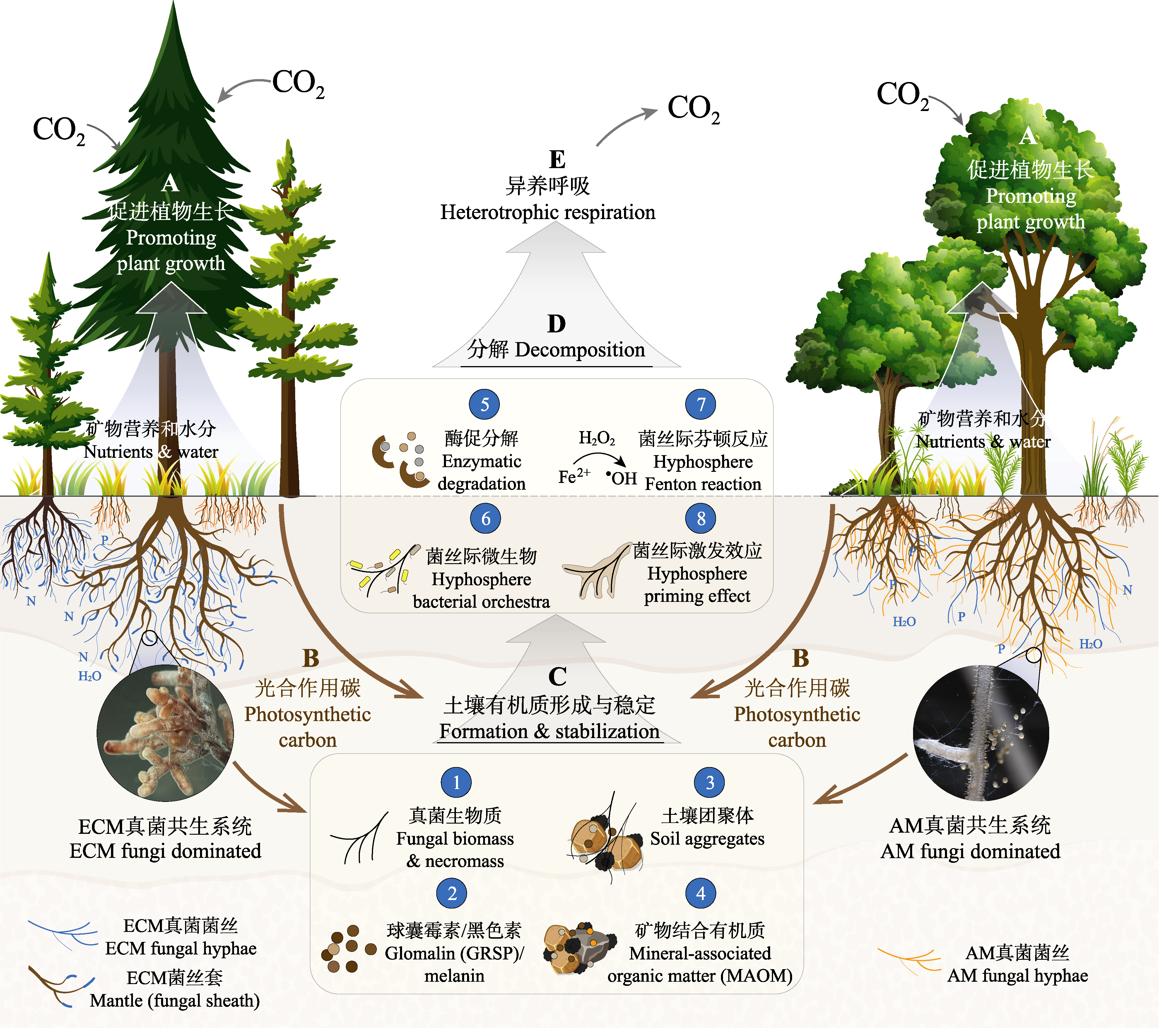
图1 菌根真菌在陆地生态系统碳循环中的作用。菌根真菌促进植物养分和水分的吸收, 间接促进植物生长和光合作用(A), 同时植物将一部分光合产物通过根系直接输送给菌根真菌(B), 供其增殖生成①真菌生物质和菌丝际分泌物等(如②稳定的土壤糖蛋白球囊霉素和黑色素等), 广泛参与土壤有机质的形成和稳定过程(C); 菌根真菌参与形成的有机质可以被③土壤团聚体包裹(物理保护作用)或与④土壤矿物结合(化学保护作用)被稳定在土壤系统中; 菌根真菌通过⑤分泌胞外酶、与⑥菌丝际微生物互作、驱动菌丝际⑦芬顿反应和⑧激发效应等对土壤有机质进行分解和转化(D), 释放有机质中的氮(N)、磷(P)营养并通过菌丝输送给植物, 缓解植物营养限制(A); 此外, 菌根真菌也会通过自身以及其菌丝际微生物的异养呼吸作用消耗植物提供的光合碳(E), 并最终以CO2的形式释放到大气中。
Fig. 1 Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling. Mycorrhizal fungi promote plant acquisition of mineral nutrients and water and thus facilitate plant growth and photosynthetic carbon sequestration (A), while plants transfer a significant portion of photoassimilates to mycorrhizal fungi via plant roots (B). Such plant-derived carbon supply sustains mycorrhizal fungal growth ① and hyphosphere exudates (e.g., stable glycoprotein glomalin and melanin ②) that play key roles in the formation and stabilization of soil organic matter (C). The organic substances formed by mycorrhizal fungi can be stabilized in soil by wrapping into soil aggregates (i.e., physical protection) ③ or by binding to soil minerals (i.e., chemical protection) ④. Mycorrhizal fungi decompose and transform soil organic matter (D) via enzymatic breakdown ⑤, stimulation of hyphosphere microbial communities ⑥, Fenton oxidation ⑦ and hyphosphere priming effect ⑧, and transfer nutrients, particularly nitrogen (N) and phosphorus (P), to plants (A). In addition, mycorrhizal fungi and hyphosphere microbial communities also consume plant-derived photosynthetic carbon through heterotrophic respiration (E) and release CO2 into the atmosphere.
| [1] |
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008). Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biology, 14, 2636-2660.
DOI URL |
| [2] |
Allen MF, Kitajima K (2014). Net primary production of ectomycorrhizas in a California forest. Fungal Ecology, 10, 81-90.
DOI URL |
| [3] | Angst G, Mueller KE, Nierop KGJ, Simpson MJ (2021). Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biology & Biochemistry, 156, 108189. DOI: 10.1016/j.soilbio.2021.108189. |
| [4] |
Anthony MA, Crowther TW, van der Linde S, Suz LM, Bidartondo MI, Cox F, Schaub M, Rautio P, Ferretti M, Vesterdal L, De Vos B, Dettwiler M, Eickenscheidt N, Schmitz A, Meesenburg H, et al. (2022). Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. The ISME Journal, 16, 1327-1336.
DOI URL |
| [5] |
Averill C, Bhatnagar JM, Dietze MC, Pearse WD, Kivlin SN (2019). Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proceedings of the National Academy of Sciences of the United States of America, 116, 23163-23168.
DOI PMID |
| [6] |
Averill C, Hawkes CV (2016). Ectomycorrhizal fungi slow soil carbon cycling. Ecology Letters, 19, 937-947.
DOI PMID |
| [7] |
Averill C, Turner BL, Finzi AC (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature, 505, 543-545.
DOI |
| [8] |
Barnes CJ, van der Gast CJ, McNamara NP, Rowe R, Bending GD (2018). Extreme rainfall affects assembly of the root-associated fungal community. New Phytologist, 220, 1172-1184.
DOI PMID |
| [9] |
Bender SF, Conen F, van der Heijden MGA, (2015). Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biology & Biochemistry, 80, 283-292.
DOI URL |
| [10] |
Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017). Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science, 355, 181-184.
DOI PMID |
| [11] |
Bonfante P, Genre A (2008). Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends in Plant Science, 13, 492-498.
DOI PMID |
| [12] |
Brundrett MC, Tedersoo L (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytologist, 220, 1108-1115.
DOI PMID |
| [13] |
Bukovská P, Bonkowski M, Konvalinková T, Beskid O, Hujslová M, Püschel D, Řezáčová V, Gutiérrez-Núñez MS, Gryndler M, Jansa J (2018). Utilization of organic nitrogen by arbuscular mycorrhizal fungi―Is there a specific role for protists and ammonia oxidizers? Mycorrhiza, 28, 269-283.
DOI PMID |
| [14] |
Bunn RA, Simpson DT, Bullington LS, Lekberg Y, Janos DP (2019). Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME Journal, 13, 1891-1898.
DOI URL |
| [15] |
Carrillo Y, Dijkstra FA, LeCain D, Pendall E (2016). Mediation of soil C decomposition by arbuscular mycorrhizal fungi in grass rhizospheres under elevated CO2. Biogeochemistry, 127, 45-55.
DOI URL |
| [16] |
Chen L, Swenson NG, Ji NN, Mi XC, Ren HB, Guo LD, Ma KP (2019). Differential soil fungus accumulation and density dependence of trees in a subtropical forest. Science, 366, 124-128.
DOI PMID |
| [17] |
Chen YL, Xu ZW, Xu TL, Veresoglou SD, Yang GW, Chen BD (2017). Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil Biology & Biochemistry, 115, 233-242.
DOI URL |
| [18] |
Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu SJ (2012). Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science, 337, 1084-1087.
DOI PMID |
| [19] | Chot E, Reddy MS (2022). Role of ectomycorrhizal symbiosis behind the host plants ameliorated tolerance against heavy metal stress. Frontiers in Microbiology, 13, 855473. DOI: 10.3389/fmicb.2022.855473. |
| [20] |
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013). Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science, 339, 1615-1618.
DOI PMID |
| [21] |
Colpaert JV, Wevers JHL, Krznaric E, Adriaensen K (2011). How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Annals of Forest Science, 68, 17-24.
DOI URL |
| [22] |
Cornelissen J, Aerts R, Cerabolini B, Werger M, van der Heijden M (2001). Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia, 129, 611-619.
DOI PMID |
| [23] |
Cotrufo MF, Ranalli MG, Haddix ML, Six J, Lugato E (2019). Soil carbon storage informed by particulate and mineral- associated organic matter. Nature Geoscience, 12, 989-994.
DOI |
| [24] |
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013). The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Global Change Biology, 19, 988-995.
DOI PMID |
| [25] | Cotton TA (2018). Arbuscular mycorrhizal fungal communities and global change: an uncertain future. FEMS Microbiology Ecology, 94, fiy179. DOI: 10.1093/femsec/fiy179. |
| [26] | Craven D, Eisenhauer N, Pearse WD, Hautier Y, Isbell F, Roscher C, Bahn M, Beierkuhnlein C, Bönisch G, Buchmann N, Byun C, Catford JA, Cerabolini BEL, Cornelissen JHC, Craine JM, et al. (2018). Multiple facets of biodiversity drive the diversity-stability relationship. Nature Ecology & Evolution, 2, 1579-1587. |
| [27] |
Davidson EA, Janssens IA (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature, 440, 165-173.
DOI |
| [28] |
Delavaux CS, Smith-Ramesh LM, Kuebbing SE (2017). Beyond nutrients: a meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology, 98, 2111-2119.
DOI PMID |
| [29] | Deng MF, Hu SJ, Guo LL, Jiang L, Huang YY, Schmid B, Liu C, Chang P, Li S, Liu XJ, Ma KP, Liu LL (2023). Tree mycorrhizal association types control biodiversity- productivity relationship in a subtropical forest. Science Advances, 9, eadd4468. DOI: 10.1126/sciadv.add4468. |
| [30] | Dietrich P, Ferlian O, Huang Y, Luo S, Quosh J, Eisenhauer N (2023). Tree diversity effects on productivity depend on mycorrhizae and life strategies in a temperate forest experiment. Ecology, 104, e3896. DOI: 10.1002/ecy.3896. |
| [31] |
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, et al. (2011). Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecology Letters, 14, 349-357.
DOI PMID |
| [32] | Dreischhoff S, Das IS, Jakobi M, Kasper K, Polle A (2020). Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Frontiers in Plant Science, 11, 590063. DOI: 10.3389/fpls.2020.590063. |
| [33] |
Drigo B, Pijl AS, Duyts H, Kielak AM, Gamper HA, Houtekamer MJ, Boschker HTS, Bodelier PLE, Whiteley AS, van Veen JA, Kowalchuk GA (2010). Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proceedings of the National Academy of Sciences of the United States of America, 107, 10938-10942.
DOI PMID |
| [34] |
Džafić E, Pongrac P, Likar M, Regvar M, Vogel-Mikuš K (2013). The arbuscular mycorrhizal fungus Glomus mosseae alleviates autotoxic effects in maize (Zea mays L.). European Journal of Soil Biology, 58, 59-65.
DOI URL |
| [35] |
Emmett BD, Lévesque-Tremblay V, Harrison MJ (2021). Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. The ISME Journal, 15, 2276-2288.
DOI URL |
| [36] |
Evelin H, Kapoor R, Giri B (2009). Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of Botany, 104, 1263-1280.
DOI PMID |
| [37] |
Fang JY (2021). Ecological perspectives of carbon neutrality. Chinese Journal of Plant Ecology, 45, 1173-1176.
DOI URL |
|
[方精云 (2021). 碳中和的生态学透视. 植物生态学报, 45, 1173-1176.]
DOI |
|
| [38] |
Fernandez CW, Heckman K, Kolka R, Kennedy PG (2019). Melanin mitigates the accelerated decay of mycorrhizal necromass with peatland warming. Ecology Letters, 22, 498-505.
DOI PMID |
| [39] |
Fernandez CW, Kennedy PG (2016). Revisiting the ‘Gadgil effect’: Do interguild fungal interactions control carbon cycling in forest soils? New Phytologist, 209, 1382-1394.
DOI URL |
| [40] |
Fernandez CW, Langley JA, Chapman S, McCormack ML, Koide RT (2016). The decomposition of ectomycorrhizal fungal necromass. Soil Biology & Biochemistry, 93, 38-49.
DOI URL |
| [41] |
Field KJ, Pressel S, Duckett JG, Rimington WR, Bidartondo MI (2015). Symbiotic options for the conquest of land. Trends in Ecology & Evolution, 30, 477-486.
DOI URL |
| [42] |
Fochi V, Chitarra W, Kohler A, Voyron S, Singan VR, Lindquist EA, Barry KW, Girlanda M, Grigoriev IV, Martin F, Balestrini R, Perotto S (2017). Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytologist, 213, 365-379.
DOI URL |
| [43] | Frey SD (2019). Mycorrhizal fungi as mediators of soil organic matter dynamics. Annual Review of Ecology, Evolution, and Systematics, 50, 237-259. |
| [44] |
Fu W, Chen BD, Rillig MC, Jansa J, Ma W, Xu C, Luo WT, Wu HH, Hao ZP, Wu H, Zhao AH, Yu Q, Han XG (2022). Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. New Phytologist, 234, 2003-2017.
DOI URL |
| [45] |
Fu W, Wu H, Zhao AH, Hao ZP, Chen BD (2020). Ecological impacts of nitrogen deposition on terrestrial ecosystems: research progresses and prospects. Chinese Journal of Plant Ecology, 44, 475-493.
DOI URL |
|
[付伟, 武慧, 赵爱花, 郝志鹏, 陈保冬 (2020). 陆地生态系统氮沉降的生态效应: 研究进展与展望. 植物生态学报, 44, 475-493.]
DOI |
|
| [46] | Gadgil RL, Gadgil PD (1971). Mycorrhiza and litter decomposition. Nature, 233, 133. |
| [47] |
Gavito ME, Jakobsen I, Mikkelsen TN, Mora F (2019). Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytologist, 223, 896-907.
DOI PMID |
| [48] |
Guerrero-Galán C, Calvo-Polanco M, Zimmermann SD (2019). Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza, 29, 291-301.
DOI PMID |
| [49] | Han MG, Feng JG, Chen Y, Sun LJ, Fu LC, Zhu B (2021). Mycorrhizal mycelial respiration: a substantial component of soil respired CO2. Soil Biology & Biochemistry, 163, 108454. DOI: 10.1016/j.soilbio.2021.108454. |
| [50] | Hasselquist NJ, Metcalfe DB, Högberg P (2012). Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Global Change Biology, 18, 3596-3605. |
| [51] |
Hawkes CV, Hartley IP, Ineson P, Fitter AH (2008). Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Global Change Biology, 14, 1181-1190.
DOI URL |
| [52] | Hawkins HJ, Cargill RIM, Van Nuland ME, Hagen SC, Field KJ, Sheldrake M, Soudzilovskaia NA, Kiers ET (2023). Mycorrhizal mycelium as a global carbon pool. Current Biology, 33, R560-R573. |
| [53] |
Hiiesalu I, Pärtel M, Davison J, Gerhold P, Metsis M, Moora M, Öpik M, Vasar M, Zobel M, Wilson SD (2014). Species richness of arbuscular mycorrhizal fungi: associations with grassland plant richness and biomass. New Phytologist, 203, 233-244.
DOI PMID |
| [54] |
Hobbie EA (2006). Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology, 87, 563-569.
PMID |
| [55] |
Hobbie JE, Hobbie EA (2006). 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology, 87, 816-822.
PMID |
| [56] |
Hodge A, Campbell CD, Fitter AH (2001). An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature, 413, 297-299.
DOI |
| [57] | Högberg MN, Briones MJI, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, Näsholm T, Högberg P (2010). Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytologist, 187, 485-493. |
| [58] |
Holden SR, Berhe AA, Treseder KK (2015). Decreases in soil moisture and organic matter quality suppress microbial decomposition following a boreal forest fire. Soil Biology & Biochemistry, 87, 1-9.
DOI URL |
| [59] |
Hopkins F, Gonzalez-Meler MA, Flower CE, Lynch DJ, Czimczik C, Tang JW, Subke JA (2013). Ecosystem-level controls on root-rhizosphere respiration. New Phytologist, 199, 339-351.
DOI PMID |
| [60] |
Irving TB, Alptekin B, Kleven B, Ané JM (2021). A critical review of 25 years of glomalin research: a better mechanical understanding and robust quantification techniques are required. New Phytologist, 232, 1572-1581.
DOI PMID |
| [61] |
Isbell F, Craven D, Connolly J, Loreau M, Schmid B, Beierkuhnlein C, Bezemer TM, Bonin C, Bruelheide H, de Luca E, Ebeling A, Griffin JN, Guo QF, Hautier Y, Hector A, et al. (2015). Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature, 526, 574-577.
DOI |
| [62] | Jeewani PH, Luo Y, Yu GH, Fu YY, He XH, Van Zwieten L, Liang C, Kumar A, He Y, Kuzyakov Y, Qin H, Guggenberger G, Xu JM (2021). Arbuscular mycorrhizal fungi and goethite promote carbon sequestration via hyphal-aggregate mineral interactions. Soil Biology & Biochemistry, 162, 108417. DOI: 10.1016/j.soilbio.2021.108417. |
| [63] | Jevon FV, Lang AK (2022). Tree biomass allocation differs by mycorrhizal association. Ecology, 103, e3688. DOI: 10.1002/ecy.3688. |
| [64] |
Jiang FY, Zhang L, Zhou JC, George TS, Feng G (2021). Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytologist, 230, 304-315.
DOI PMID |
| [65] |
Jin HR, Liu J, Liu J, Huang XW (2012). Forms of nitrogen uptake, translocation, and transfer via arbuscular mycorrhizal fungi: a review. Science China Life Sciences, 55, 474-482.
DOI URL |
| [66] |
Johnson NC, Graham JH, Smith FA (1997). Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytologist, 135, 575-585.
DOI URL |
| [67] |
Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, Solaiman ZM, Murphy DV (2015). Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytologist, 205, 1537-1551.
DOI PMID |
| [68] |
Kakouridis A, Hagen JA, Kan MP, Mambelli S, Feldman LJ, Herman DJ, Weber PK, Pett-Ridge J, Firestone MK (2022). Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytologist, 236, 210-221.
DOI URL |
| [69] | Kallenbach CM, Frey SD, Grandy AS (2016). Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nature Communications, 7, 13630. DOI: 10.1038/ncomms13630. |
| [70] |
Kamel L, Keller-Pearson M, Roux C, Ané JM (2017). Biology and evolution of arbuscular mycorrhizal symbiosis in the light of genomics. New Phytologist, 213, 531-536.
DOI PMID |
| [71] |
Karlowsky S, Augusti A, Ingrisch J, Hasibeder R, Lange M, Lavorel S, Bahn M, Gleixner G (2018). Land use in mountain grasslands alters drought response and recovery of carbon allocation and plant-microbial interactions. Journal of Ecology, 106, 1230-1243.
DOI PMID |
| [72] |
Karst J, Marczak L, Jones MD, Turkington R (2008). The mutualism-parasitism continuum in ectomycorrhizas: a quantitative assessment using meta-analysis. Ecology, 89, 1032-1042.
PMID |
| [73] |
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009). Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biology & Biochemistry, 41, 1233-1244.
DOI URL |
| [74] |
Keller AB, Brzostek ER, Craig ME, Fisher JB, Phillips RP (2021). Root-derived inputs are major contributors to soil carbon in temperate forests, but vary by mycorrhizal type. Ecology Letters, 24, 626-635.
DOI PMID |
| [75] |
Kjøller R, Nilsson LO, Hansen K, Schmidt IK, Vesterdal L, Gundersen P (2012). Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytologist, 194, 278-286.
DOI PMID |
| [76] |
Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa MD, Doré J, Floudas D, et al. (2015). Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics, 47, 410-415.
DOI PMID |
| [77] |
Koide RT, Wu T (2003). Ectomycorrhizas and retarded decomposition in a Pinus resinosa plantation. New Phytologist, 158, 401-407.
DOI URL |
| [78] | Konvalinková T, Jansa J (2016). Lights off for arbuscular mycorrhiza: on its symbiotic functioning under light deprivation. Frontiers in Plant Science, 7, 782. DOI: 10.3389/fpls.2016.00782. |
| [79] |
Lavallee JM, Soong JL, Cotrufo MF (2020). Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Global Change Biology, 26, 261-273.
DOI PMID |
| [80] |
LeBauer DS, Treseder KK (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89, 371-379.
PMID |
| [81] | Lehmann A, Leifheit EF, Rillig MC (2017). Mycorrhizas and soil aggregation//Johnson NC, Gehring C, Jansa J. Mycorrhizal Mediation of Soil. Elsevier, Amsterdam. 241-262. |
| [82] |
Lehmann J, Kleber M (2015). The contentious nature of soil organic matter. Nature, 528, 60-68.
DOI |
| [83] |
Leigh J, Hodge A, Fitter AH (2009). Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist, 181, 199-207.
DOI PMID |
| [84] |
Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD (2013). First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist, 197, 617-630.
DOI URL |
| [85] | Li Z, Wu SL, Liu YJ, Yi Q, Nguyen TAH, Ma YY, You F, Hall M, Chan TS, Huang YF, Huang LB (2022). Plant biomass amendment regulates arbuscular mycorrhizal role in organic carbon and nitrogen sequestration in eco-engineered iron ore tailings. Geoderma, 428, 116178. DOI: 10.1016/j.geoderma.2022.116178. |
| [86] |
Liang C, Amelung W, Lehmann J, Kästner M (2019). Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biology, 25, 3578-3590.
DOI PMID |
| [87] |
Lilleskov EA, Kuyper TW, Bidartondo MI, Hobbie EA (2019). Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environmental Pollution, 246, 148-162.
DOI PMID |
| [88] |
Lindahl BD, Tunlid A (2015). Ectomycorrhizal fungi-potential organic matter decomposers, yet not saprotrophs. New Phytologist, 205, 1443-1447.
DOI PMID |
| [89] |
Lin G, Craig ME, Jo I, Wang X, Zeng DH, Phillips RP (2022). Mycorrhizal associations of tree species influence soil nitrogen dynamics via effects on soil acid-base chemistry. Global Ecology and Biogeography, 31, 168-182.
DOI URL |
| [90] |
Lin GG, McCormack ML, Ma CG, Guo DL (2017). Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytologist, 213, 1440-1451.
DOI PMID |
| [91] | Liu XJA, Pold G, Domeignoz-Horta LA, Geyer KM, Caris H, Nicolson H, Kemner KM, Frey SD, Melillo JM, DeAngelis KM (2021). Soil aggregate-mediated microbial responses to long-term warming. Soil Biology & Biochemistry, 152, 108055. DOI: 10.1016/j.soilbio.2020.108055. |
| [92] | Luo S, Phillips RP, Jo I, Fei SL, Liang JJ, Schmid B, Eisenhauer N (2023). Higher productivity in forests with mixed mycorrhizal strategies. Nature Communications, 14, 1377. DOI: 10.1038/s41467-023-36888-0. |
| [93] |
Maherali H, Klironomos JN (2007). Influence of phylogeny on fungal community assembly and ecosystem functioning. Science, 316, 1746-1748.
DOI PMID |
| [94] |
Maillard F, Fernandez CW, Mundra S, Heckman KA, Kolka RK, Kauserud H, Kennedy PG (2022). Warming drives a ‘hummockification’ of microbial communities associated with decomposing mycorrhizal fungal necromass in peatlands. New Phytologist, 234, 2032-2043.
DOI URL |
| [95] |
Maillard F, Kennedy PG, Adamczyk B, Heinonsalo J, Buée M (2021). Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbial communities in a boreal forest. Molecular Ecology, 30, 1921-1935.
DOI PMID |
| [96] | Mao ZK, van der Plas F, Corrales A, Anderson-Teixeira KJ, Bourg NA, Chu CJ, Hao ZQ, Jin GZ, Lian JY, Lin F, Li BH, Luo WQ, McShea WJ, Myers JA, Shen GC, et al. (2023). Scale-dependent diversity-biomass relationships can be driven by tree mycorrhizal association and soil fertility. Ecological Monographs, 93, e1568. DOI: 10.1002/ecm.1568. |
| [97] |
Martin F, Kohler A, Murat C, Veneault-Fourrey C, Hibbett DS (2016). Unearthing the roots of ectomycorrhizal symbioses. Nature Reviews Microbiology, 14, 760-773.
DOI PMID |
| [98] |
Melillo JM, Aber JD, Muratore JF (1982). Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology, 63, 621-626.
DOI URL |
| [99] |
Mohan JE, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, Taylor M, Witt CA (2014). Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecology, 10, 3-19.
DOI URL |
| [100] |
Morin E, Miyauchi S, San Clemente H, Chen ECH, Pelin A, de la Providencia I, Ndikumana S, Beaudet D, Hainaut M, Drula E, Kuo A, Tang N, Roy S, Viala J, Henrissat B, et al. (2019). Comparative genomics of Rhizophagus irregularis, R. cerebriforme, R. diaphanus and Gigaspora rosea highlights specific genetic features in Glomeromycotina, New Phytologist, 222, 1584-1598.
DOI URL |
| [101] |
Morris EK, Morris DJP, Vogt S, Gleber SC, Bigalke M, Wilcke W, Rillig MC (2019). Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. The ISME Journal, 13, 1639-1646.
DOI URL |
| [102] |
Morrison EW, Frey SD, Sadowsky JJ, Thomas WK, Pringle A (2016). Chronic nitrogen additions fundamentally restructure the soil fungal community in a temperate forest. Fungal Ecology, 23, 48-57.
DOI URL |
| [103] |
Nottingham AT, Turner BL, Winter K, van der Heijden MGA, Tanner EVJ (2010). Arbuscular mycorrhizal mycelial respiration in a moist tropical forest. New Phytologist, 186, 957-967.
DOI PMID |
| [104] |
Op De Beeck M, Troein C, Peterson C, Persson P, Tunlid A (2018). Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus. New Phytologist, 218, 335-343.
DOI PMID |
| [105] |
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA (2011). Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecology Letters, 14, 493-502.
DOI PMID |
| [106] |
Paterson E, Sim A, Davidson J, Daniell TJ (2016). Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation. Plant and Soil, 408, 243-254.
DOI URL |
| [107] | Pellitier PT, Ibáñez I, Zak DR, Argiroff WA, Acharya K (2021). Ectomycorrhizal access to organic nitrogen mediates CO2 fertilization response in a dominant temperate tree. Nature Communications, 12, 5403. DOI: 10.1038/s41467-021-25652-x. |
| [108] |
Pellitier PT, Zak DR (2018). Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. New Phytologist, 217, 68-73.
DOI PMID |
| [109] |
Phillips RP, Brzostek E, Midgley MG (2013). The mycorrhizal- associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytologist, 199, 41-51.
DOI PMID |
| [110] |
Pozo MJ, Azcón-Aguilar C (2007). Unraveling mycorrhiza- induced resistance. Current Opinion in Plant Biology, 10, 393-398.
DOI URL |
| [111] |
Remy W, Taylor TN, Hass H, Kerp H (1994). Four hundred- million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences of the United States of America, 91, 11841-11843.
PMID |
| [112] |
Rillig MC (2004). Arbuscular mycorrhizae, glomalin, and soil aggregation. Canadian Journal of Soil Science, 84, 355-363.
DOI URL |
| [113] |
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2015). Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytologist, 205, 1385-1388.
DOI PMID |
| [114] |
Rillig MC, Caldwell BA, Wösten HAB, Sollins P (2007). Role of proteins in soil carbon and nitrogen storage: controls on persistence. Biogeochemistry, 85, 25-44.
DOI URL |
| [115] |
Rillig MC, Mummey DL (2006). Mycorrhizas and soil structure. New Phytologist, 171, 41-53.
PMID |
| [116] |
Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang GW (2019). The role of multiple global change factors in driving soil functions and microbial biodiversity. Science, 366, 886-890.
DOI PMID |
| [117] |
Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS (2001). Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant and Soil, 233, 167-177.
DOI URL |
| [118] |
Rineau F, Shah F, Smits MM, Persson P, Johansson T, Carleer R, Troein C, Tunlid A (2013). Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. The ISME Journal, 7, 2010-2022.
DOI URL |
| [119] |
Rosier CL, Hoye AT, Rillig MC (2006). Glomalin-related soil protein: assessment of current detection and quantification tools. Soil Biology & Biochemistry, 38, 2205-2211.
DOI URL |
| [120] |
Rozmoš M, Bukovská P, Hršelová H, Kotianová M, Dudáš M, Gančarčíková K, Jansa J (2022). Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. The ISME Journal, 16, 676-685.
DOI URL |
| [121] | Ruiz-Lozano JM, Aroca R, Zamarreño ÁM, Molina S, Andreo-Jiménez B, Porcel R, García-Mina JM, Ruyter-Spira C, López-Ráez JA (2016). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant, Cell & Environment, 39, 441-452. |
| [122] |
Rumpel C, Amiraslani F, Koutika LS, Smith P, Whitehead D, Wollenberg E (2018). Put more carbon in soils to meet Paris climate pledges. Nature, 564, 32-34.
DOI |
| [123] | Ryan ME, Schreiner KM, Swenson JT, Gagne J, Kennedy PG (2020). Rapid changes in the chemical composition of degrading ectomycorrhizal fungal necromass. Fungal Ecology, 45, 100922. DOI: 10.1016/j.funeco.2020.100922. |
| [124] |
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011). Persistence of soil organic matter as an ecosystem property. Nature, 478, 49-56.
DOI |
| [125] |
Schweigert M, Herrmann S, Miltner A, Fester T, Kästner M (2015). Fate of ectomycorrhizal fungal biomass in a soil bioreactor system and its contribution to soil organic matter formation. Soil Biology & Biochemistry, 88, 120-127.
DOI URL |
| [126] |
Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S (2015). Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biology & Biochemistry, 80, 146-155.
DOI URL |
| [127] |
Six J, Conant RT, Paul EA, Paustian K (2002). Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant and Soil, 241, 155-176.
DOI URL |
| [128] |
Smith SE, Facelli E, Pope S, Andrew Smith FS (2010). Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant and Soil, 326, 3-20.
DOI URL |
| [129] | Smith SE, Read DJ (2010). Mycorrhizal Symbiosis. 3rd ed. Academic Press, London. |
| [130] |
Sokol NW, Slessarev E, Marschmann GL, Nicolas A, Blazewicz SJ, Brodie EL, Firestone MK, Foley MM, Hestrin R, Hungate BA, Koch BJ, Stone BW, Sullivan MB, Zablocki O, LLNL Soil Microbiome Consortium, Pett-Ridge J (2022). Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nature Reviews Microbiology, 20, 415-430.
DOI PMID |
| [131] | Soudzilovskaia NA, van Bodegom PM, Terrer C, van’t Zelfde M, McCallum I, Luke McCormack M, Fisher JB, Brundrett MC, Tedersoo L (2019). Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nature Communications, 10, 5077. DOI: 10.1038/s41467-019-13019-2. |
| [132] |
Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH (2003). Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science, 300, 1138-1140.
DOI PMID |
| [133] |
Steidinger BS, Crowther TW, Liang J, Van Nuland ME, Werner GDA, Reich PB, Nabuurs GJ, de-Miguel S, Zhou M, Picard N, Herault B, Zhao X, Zhang C, Routh D, Peay KG (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature, 569, 404-408.
DOI |
| [134] |
Sun YF, Li YW, Lu XM, Wang Y, Bai YF (2022). Contrasting effects of arbuscular mycorrhizal fungi on nitrogen uptake in Leymus chinensis and Cleistogenes squarrosa grasses, dominants of the Inner Mongolian steppe. Plant and Soil, 475, 395-410.
DOI |
| [135] | Tang NW, San Clemente H, Roy S, Bécard G, Zhao B, Roux C (2016). A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Frontiers in Microbiology, 7, 233. DOI: 10.3389/fmicb.2016.00233. |
| [136] | Tedersoo L, Bahram M, Zobel M (2020). How mycorrhizal associations drive plant population and community biology. Science, 367, eaba1223. DOI: 10.1126/science.aba1223. |
| [137] |
Tedersoo L, Smith ME (2013). Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biology Reviews, 27, 83-99.
DOI URL |
| [138] |
Terrer C, Vicca S, Hungate BA, Phillips RP, Prentice IC (2016). Mycorrhizal association as a primary control of the CO2 fertilization effect. Science, 353, 72-74.
DOI PMID |
| [139] |
Thirkell TJ, Pastok D, Field KJ (2020). Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varies according to cultivar and changes in atmospheric carbon dioxide concentration. Global Change Biology, 26, 1725-1738.
DOI PMID |
| [140] |
Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frei dit Frey N, Gianinazzi-Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 110, 20117-20122.
DOI PMID |
| [141] |
Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD (2007). Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiology Ecology, 61, 295-304.
DOI PMID |
| [142] |
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature, 396, 69-72.
DOI |
| [143] |
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist, 205, 1406-1423.
DOI PMID |
| [144] |
Veresoglou SD, Chen BD, Rillig MC (2012). Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biology & Biochemistry, 46, 53-62.
DOI URL |
| [145] |
Verbruggen E, Jansa J, Hammer EC, Rillig MC (2016). Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil? Journal of Ecology, 104, 261-269.
DOI URL |
| [146] |
Wang F, Zhang L, Zhou J, Rengel Z, George TS, Feng G (2022). Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes and ecological functions. Plant and Soil, 481, 1-22.
DOI |
| [147] | Wang S, Guan Y, Wang Q, Zhao J, Sun G, Hu X, Running MP, Sun H, Huang J (2020a). A mycorrhizae-like gene regulates stem cell and gametophore development in mosses. Nature Communications, 11, 2030. DOI: 10.1038/s41467-020-15967-6. |
| [148] |
Wang SH, Zhang YG, Ju WM, Chen JM, Ciais P, Cescatti A, Sardans J, Janssens IA, Wu MS, Berry JA, Campbell E, Fernández-Martínez M, Alkama R, Sitch S, Friedlingstein P, et al. (2020b). Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science, 370, 1295-1300.
DOI URL |
| [149] |
Wang T, Tian Z, Bengtson P, Tunlid A, Persson P (2017). Mineral surface-reactive metabolites secreted during fungal decomposition contribute to the formation of soil organic matter. Environmental Microbiology, 19, 5117-5129.
DOI PMID |
| [150] | Ward EB, Duguid MC, Kuebbing SE, Lendemer JC, Bradford MA (2022). The functional role of ericoid mycorrhizal plants and fungi on carbon and nitrogen dynamics in forests. New Phytologist, 235, 1701-1718. |
| [151] | Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009). Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi:results from long-term field experiments. Ecology Letters, 12, 452-461. |
| [152] |
Wright SF, Franke-Snyder M, Morton JB, Upadhyaya A (1996). Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant and Soil, 181, 193-203.
DOI URL |
| [153] |
Wu H, Yang JJ, Fu W, Rillig MC, Cao ZJ, Zhao AH, Hao ZP, Zhang X, Chen BD, Han XG (2023a). Identifying thresholds of nitrogen enrichment for substantial shifts in arbuscular mycorrhizal fungal community metrics in a temperate grassland of northern China. New Phytologist, 237, 279-294.
DOI URL |
| [154] | Wu SL, Fu W, Rillig MC, Chen BD, Zhu YG, Huang LB (2023b). Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi—An updated conceptual framework. New Phytologist, DOI: 10.1111/nph.19178. |
| [155] |
Wu SL, Zhang X, Huang LB, Chen BD (2019). Arbuscular mycorrhiza and plant chromium tolerance. Soil Ecology Letters, 1, 94-104.
DOI |
| [156] |
Wu SL, Zhang X, Sun YQ, Wu ZX, Li T, Hu YJ, Su D, Lv JT, Li G, Zhang ZS, Zheng LR, Zhang J, Chen BD (2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environmental Science & Technology, 49, 14036-14047.
DOI URL |
| [157] |
Wurzburger N, Brookshire ENJ (2017). Experimental evidence that mycorrhizal nitrogen strategies affect soil carbon. Ecology, 98, 1491-1497.
DOI PMID |
| [158] |
Yang HS, Zhang Q, Koide RT, Hoeksema JD, Tang JJ, Bian X, Hu SJ, Chen X (2017). Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. Journal of Ecology, 105, 219-228.
DOI URL |
| [159] | Yin LM, Dijkstra FA, Phillips RP, Zhu B, Wang P, Cheng WX (2021). Arbuscular mycorrhizal trees cause a higher carbon to nitrogen ratio of soil organic matter decomposition via rhizosphere priming than ectomycorrhizal trees. Soil Biology & Biochemistry, 157, 108246. DOI: 10.1016/j.soilbio.2021.108246. |
| [160] |
Zak DR, Pellitier PT, Argiroff W, Castillo B, James TY, Nave LE, Averill C, Beidler KV, Bhatnagar J, Blesh J, Classen AT, Craig M, Fernandez CW, Gundersen P, Johansen R, et al. (2019). Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytologist, 223, 33-39.
DOI PMID |
| [161] |
Zhang L, Zhou JC, George TS, Limpens E, Feng G (2022). Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends in Plant Science, 27, 402-411.
DOI URL |
| [162] |
Zhang Z, Xiao J, Yuan Y, Zhao C, Liu Q, Yin H (2018). Mycelium- and root-derived C inputs differ in their impacts on soil organic C pools and decomposition in forests. Soil Biology & Biochemistry, 123, 257-265.
DOI URL |
| [163] | Zhou J, Zang H, Loeppmann S, Gube M, Kuzyakov Y, Pausch J (2020). Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biology & Biochemistry, 140, 107641. DOI: 10.1016/j.soilbio.2019.107641. |
| [164] | Zhu YG, Chen BD, Fu W (2022). Research frontiers in soil ecology. Science & Technology Review, 40, 25-31. |
| [朱永官, 陈保冬, 付伟 (2022). 土壤生态学研究前沿. 科技导报, 40, 25-31.] | |
| [165] |
Zhu YG, Miller RM (2003). Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends in Plant Science, 8, 407-409.
DOI URL |
| [1] | 陈科宇, 邢森, 唐玉, 孙佳慧, 任世杰, 张静, 纪宝明. 不同草地类型土壤丛枝菌根真菌群落特征及其驱动因素[J]. 植物生态学报, 2024, 48(5): 660-674. |
| [2] | 胡蝶, 蒋欣琪, 戴志聪, 陈戴一, 张雨, 祁珊珊, 杜道林. 丛枝菌根真菌提高入侵杂草南美蟛蜞菊对除草剂的耐受性[J]. 植物生态学报, 2024, 48(5): 651-659. |
| [3] | 任悦, 高广磊, 丁国栋, 张英, 赵珮杉, 柳叶. 不同生长期樟子松外生菌根真菌群落物种组成及其驱动因素[J]. 植物生态学报, 2023, 47(9): 1298-1309. |
| [4] | 胡同欣, 李蓓, 李光新, 任玥霄, 丁海磊, 孙龙. 火烧黑碳对生长季兴安落叶松林外生菌根真菌群落物种组成的影响[J]. 植物生态学报, 2023, 47(6): 792-803. |
| [5] | 杨佳绒, 戴冬, 陈俊芳, 吴宪, 刘啸林, 刘宇. 丛枝菌根真菌多样性对植物群落构建和稀有种维持的研究进展[J]. 植物生态学报, 2023, 47(6): 745-755. |
| [6] | 何斐, 李川, Faisal SHAH, 卢谢敏, 王莹, 王梦, 阮佳, 魏梦琳, 马星光, 王卓, 姜浩. 丛枝菌根菌丝桥介导刺槐-魔芋间碳转运和磷吸收[J]. 植物生态学报, 2023, 47(6): 782-791. |
| [7] | 何敏, 许秋月, 夏允, 杨柳明, 范跃新, 杨玉盛. 植物磷获取机制及其对全球变化的响应[J]. 植物生态学报, 2023, 47(3): 291-305. |
| [8] | 单婷婷, 陈彤垚, 陈晓梅, 郭顺星, 王爱荣. 菌根真菌与兰科植物氮营养关系的研究进展[J]. 植物生态学报, 2022, 46(5): 516-528. |
| [9] | 谢伟, 郝志鹏, 张莘, 陈保冬. 丛枝菌根网络介导的植物间信号交流研究进展及展望[J]. 植物生态学报, 2022, 46(5): 493-515. |
| [10] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [11] | 马炬峰, 辛敏, 徐陈超, 祝琬莹, 毛传澡, 陈欣, 程磊. 丛枝菌根真菌与氮添加对不同根形态基因型水稻氮吸收的影响[J]. 植物生态学报, 2021, 45(7): 728-737. |
| [12] | 秦文超, 陶至彬, 王永健, 刘艳杰, 黄伟. 资源脉冲对外来植物入侵影响的研究进展和展望[J]. 植物生态学报, 2021, 45(6): 573-582. |
| [13] | 马书琴, 汪子微, 陈有超, 鲁旭阳. 藏北高寒草地土壤有机质化学组成对土壤蛋白酶和脲酶活性的影响[J]. 植物生态学报, 2021, 45(5): 516-527. |
| [14] | 吕亚香, 戚智彦, 刘伟, 孙佳美, 潘庆民. 早春和夏季氮磷添加对内蒙古典型草原退化群落碳交换的影响[J]. 植物生态学报, 2021, 45(4): 334-344. |
| [15] | 韩广轩, 李隽永, 屈文笛. 氮输入对滨海盐沼湿地碳循环关键过程的影响及机制[J]. 植物生态学报, 2021, 45(4): 321-333. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19