

Chin J Plant Ecol ›› 2025, Vol. 49 ›› Issue (7): 1082-1095.DOI: 10.17521/cjpe.2024.0434 cstr: 32100.14.cjpe.2024.0434
• Research Articles • Previous Articles Next Articles
ZHANG Bin, ZHANG Hao-Cheng, QIAO Tian, LÜ Zhi-Bing, XU Ya-Nan, LI Xue-Qin, YUAN Xiang-Yang, FENG Mei-Chen, ZHANG Mei-Jun*( )
)
Received:2024-12-04
Accepted:2025-04-08
Online:2025-07-20
Published:2025-07-28
Contact:
ZHANG Mei-Jun
Supported by:ZHANG Bin, ZHANG Hao-Cheng, QIAO Tian, LÜ Zhi-Bing, XU Ya-Nan, LI Xue-Qin, YUAN Xiang-Yang, FENG Mei-Chen, ZHANG Mei-Jun. Effect of arbuscular mycorrhizal fungi inoculation on non-structural carbohydrates and C, N and P stoichiometry in oat plants under drought stress[J]. Chin J Plant Ecol, 2025, 49(7): 1082-1095.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2024.0434
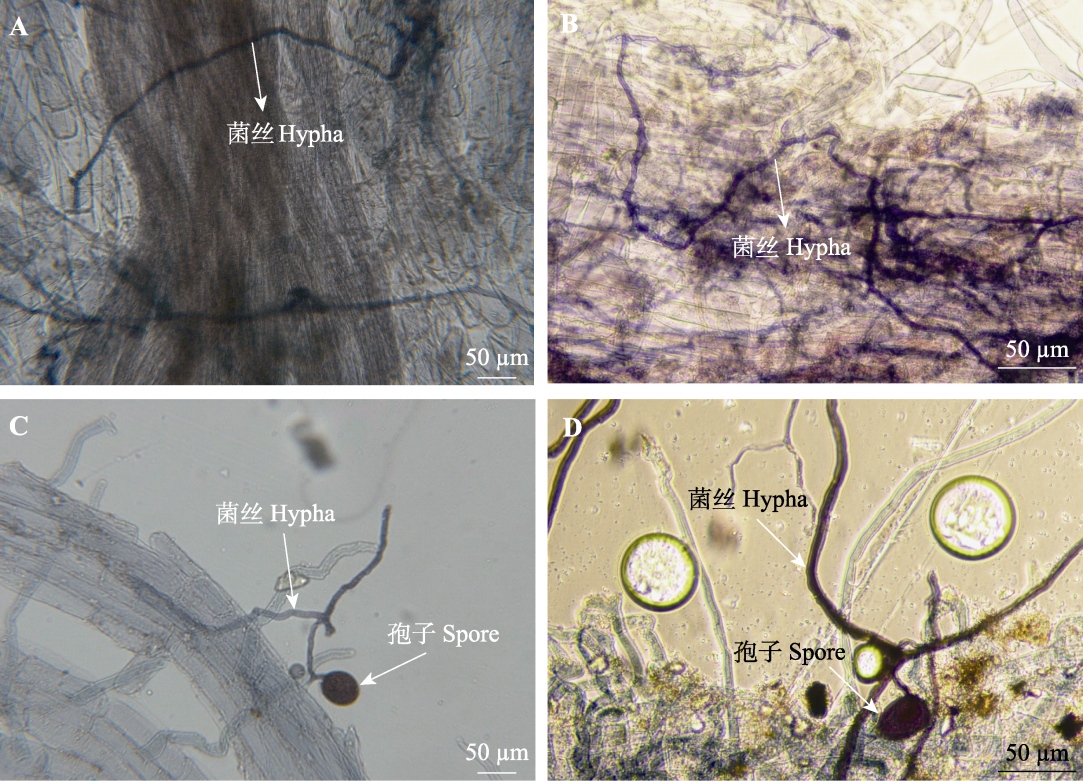
Fig. 1 Arbuscular mycorrhizal fungi (AMF) colonization to oat root. A, 75% soil relative water content without AMF inoculation. B, 75% soil relative water content with AMF inoculation. C, 55% soil relative water content without AMF inoculation. D, 55% soil relative water content with AMF inoculation.
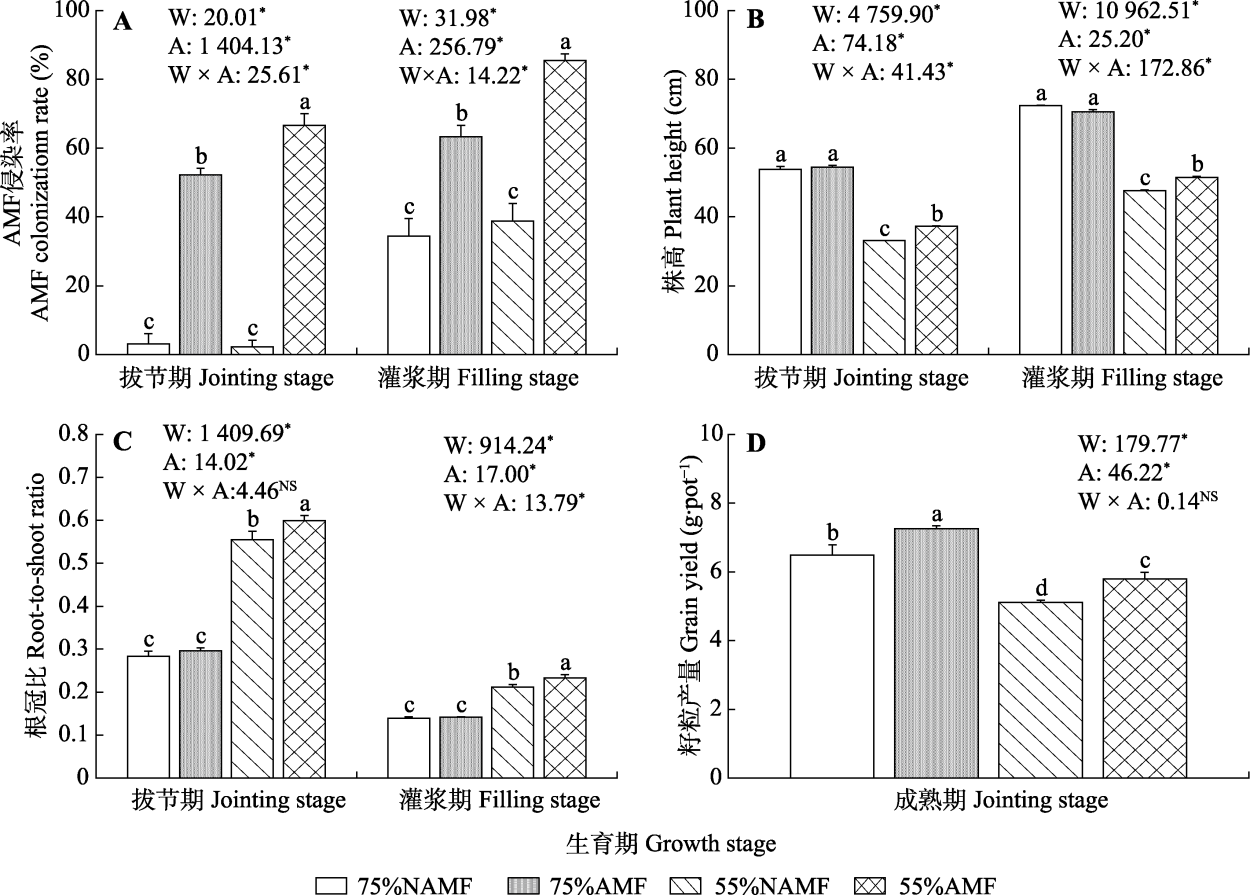
Fig. 2 Effects of arbuscular mycorrhizal fungi (AMF) inoculation on AMF colonization rate, plant height, root-to-shoot ratio and grain yield of oat under drought stress (mean ± SE). W and A represented water treatment and AMF treatment, and the following values are F values. * indicated that soil water, AMF and their interactions had significant influence (p < 0.05), NS indicated no significance. Different lowercase letters at same growth stage indicated significant difference among treatments (p < 0.05). 75%NAMF, 75% soil relative water content without AMF inoculation; 75%AMF, 75% soil relative water content with AMF inoculation; 55%NAMF, 55% soil relative water content without AMF inoculation; 55%AMF, 55% soil relative water content with AMF inoculation.
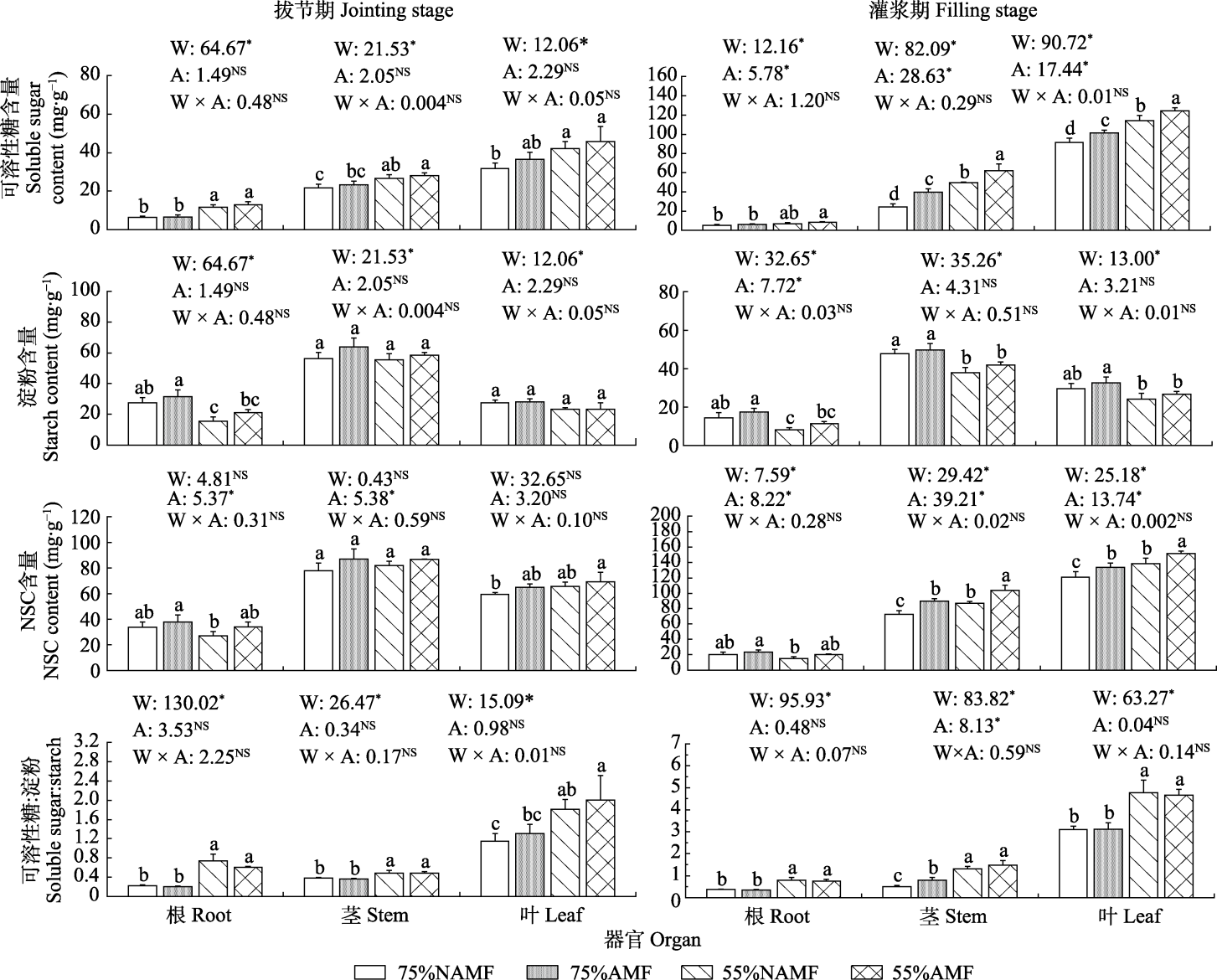
Fig. 3 Effects of arbuscular mycorrhizal fungi (AMF) inoculation on non-structural carbohydrates (NSC) of oat organs under drought stress (mean ± SE). W and A represented water treatment and AMF treatment, and the following values are F values. * indicated that soil water, AMF and their interactions had significant influence (p < 0.05), NS indicated no significance. Different lowercase letters of the same organ at the same growth stage indicated significant difference among treatments (p < 0.05). 75%NAMF, 75% soil relative water content without AMF inoculation; 75%AMF, 75% soil relative water content with AMF inoculation; 55%NAMF, 55% soil relative water content without AMF inoculation; 55%AMF, 55% soil relative water content with AMF inoculation.
| 生育时期 Growth stage | 器官 Organ | 处理 Treatment | C | N | P | C:N | N:P | C:P |
|---|---|---|---|---|---|---|---|---|
| 拔节期 Jointing stage | 根 Root | W | 529.24* | 85.09* | 85.16* | 57.00* | 2.48 NS | 69.60* |
| A | 104.57* | 123.01* | 47.47* | 2.63NS | 30.53* | 4.55 NS | ||
| W × A | 2.21NS | 14.23* | 0.002 | 5.31* | 12.32* | 0.09 NS | ||
| 茎 Stem | W | 391.94* | 161.40* | 1 364.34* | 21.98* | 0.22 NS | 28.39* | |
| A | 154.92* | 139.41* | 323.21* | 0.88NS | 28.31* | 28.56* | ||
| W × A | 2.61NS | 0.11NS | 5.49* | 4.27NS | 0.72 NS | 8.76* | ||
| 叶 Leaf | W | 534.48* | 150.29* | 239.67* | 0.002NS | 71.94* | 45.39* | |
| A | 344.69* | 37.92* | 138.98* | 9.29NS | 72.44* | 21.58* | ||
| W × A | 24.08* | 3.62NS | 6.28* | 14.14* | 2.73 | 2.51 NS | ||
| 灌浆期 Filling stage | 根 Root | W | 807.34* | 64.28* | 146.04* | 49.02* | 0.01 NS | 72.57* |
| A | 261.64* | 37.47* | 44.13* | 5.61* | 1.76NS | 23.50* | ||
| W × A | 26.66* | 1.62NS | 2.17NS | 0.51NS | 2.80 NS | 12.02* | ||
| 茎 Stem | W | 833.29* | 60.87* | 94.90* | 23.91* | 0.50 NS | 27.93* | |
| A | 355.78* | 49.97* | 31.31* | 0.93NS | 7.71* | 14.93* | ||
| W × A | 70.14* | 8.21* | 0.92NS | 0.17NS | 10.58* | 14.20* | ||
| 叶 Leaf | W | 611.60* | 700.33* | 667.78* | 1.09NS | 74.46* | 47.59* | |
| A | 227.67* | 95.68* | 46.38* | 17.63* | 18.06* | 39.50* | ||
| W × A | 21.66* | 38.60* | 317.30* | 0.82NS | 182.00* | 131.77* |
Table 1 The main effects and interactions of water and arbuscular mycorrhizal fungi (AMF) inoculation on carbon (C), nitrogen (N), phosphorus (P) contents and its stoichiometric ratio of oat organs
| 生育时期 Growth stage | 器官 Organ | 处理 Treatment | C | N | P | C:N | N:P | C:P |
|---|---|---|---|---|---|---|---|---|
| 拔节期 Jointing stage | 根 Root | W | 529.24* | 85.09* | 85.16* | 57.00* | 2.48 NS | 69.60* |
| A | 104.57* | 123.01* | 47.47* | 2.63NS | 30.53* | 4.55 NS | ||
| W × A | 2.21NS | 14.23* | 0.002 | 5.31* | 12.32* | 0.09 NS | ||
| 茎 Stem | W | 391.94* | 161.40* | 1 364.34* | 21.98* | 0.22 NS | 28.39* | |
| A | 154.92* | 139.41* | 323.21* | 0.88NS | 28.31* | 28.56* | ||
| W × A | 2.61NS | 0.11NS | 5.49* | 4.27NS | 0.72 NS | 8.76* | ||
| 叶 Leaf | W | 534.48* | 150.29* | 239.67* | 0.002NS | 71.94* | 45.39* | |
| A | 344.69* | 37.92* | 138.98* | 9.29NS | 72.44* | 21.58* | ||
| W × A | 24.08* | 3.62NS | 6.28* | 14.14* | 2.73 | 2.51 NS | ||
| 灌浆期 Filling stage | 根 Root | W | 807.34* | 64.28* | 146.04* | 49.02* | 0.01 NS | 72.57* |
| A | 261.64* | 37.47* | 44.13* | 5.61* | 1.76NS | 23.50* | ||
| W × A | 26.66* | 1.62NS | 2.17NS | 0.51NS | 2.80 NS | 12.02* | ||
| 茎 Stem | W | 833.29* | 60.87* | 94.90* | 23.91* | 0.50 NS | 27.93* | |
| A | 355.78* | 49.97* | 31.31* | 0.93NS | 7.71* | 14.93* | ||
| W × A | 70.14* | 8.21* | 0.92NS | 0.17NS | 10.58* | 14.20* | ||
| 叶 Leaf | W | 611.60* | 700.33* | 667.78* | 1.09NS | 74.46* | 47.59* | |
| A | 227.67* | 95.68* | 46.38* | 17.63* | 18.06* | 39.50* | ||
| W × A | 21.66* | 38.60* | 317.30* | 0.82NS | 182.00* | 131.77* |
| 生育时期 Growth stage | 器官 Organ | 处理 Treatment | C (g·kg-1) | N (g·kg-1) | P (g·kg-1) | C:N | N:P | C:P |
|---|---|---|---|---|---|---|---|---|
| 拔节期 Jointing stage | 根 Root | 75%NAMF | 93.73 ± 1.67b | 8.44 ± 0.25b | 2.63 ± 0.08b | 11.12 ± 0.47a | 3.20 ± 0.03c | 35.61 ± 1.39a |
| 75%AMF | 109.10 ± 2.23a | 10.78 ± 0.38a | 2.90 ± 0.09a | 10.13 ± 0.53b | 3.72 ± 0.06a | 37.72 ± 1.96a | ||
| 55%NAMF | 65.50 ± 2.80d | 7.58 ± 0.29c | 2.29 ± 0.03c | 8.65 ± 0.35c | 3.31 ± 0.17bc | 28.65 ± 1.53b | ||
| 55%AMF | 76.97 ± 2.25c | 8.73 ± 0.10b | 2.55 ± 0.03b | 8.82 ± 0.35c | 3.43 ± 0.07b | 30.24 ± 0.94b | ||
| 茎 Stem | 75%NAMF | 87.57 ± 1.88b | 8.48 ± 0.33b | 2.58 ± 0.03b | 10.33 ± 0.18a | 3.29 ± 0.15b | 33.99 ± 0.98a | |
| 75%AMF | 97.73 ± 1.61a | 9.82 ± 0.09a | 2.80 ± 0.02a | 9.96 ± 0.23ab | 3.51 ± 0.05a | 34.97 ± 0.48a | ||
| 55%NAMF | 67.47 ± 2.00d | 7.11 ± 0.09c | 2.20 ± 0.01d | 9.49 ± 0.30c | 3.23 ± 0.02b | 30.60 ± 0.88b | ||
| 55%AMF | 80.67 ± 0.68c | 8.38 ± 0.15b | 2.37 ± 0.02c | 9.63 ± 0.11bc | 3.53 ± 0.06a | 34.00 ± 0.17a | ||
| 叶 Leaf | 75%NAMF | 79.17 ± 1.18b | 9.52 ± 0.45b | 0.88 ± 0.04b | 8.33 ± 0.50ab | 10.84 ± 0.27b | 90.34 ± 5.80b | |
| 75%AMF | 88.17 ± 1.58a | 10.75 ± 0.20a | 1.21 ± 0.05a | 8.20 ± 0.25bc | 8.93 ± 0.48c | 73.21 ± 1.73c | ||
| 55%NAMF | 60.70 ± 0.80d | 7.94 ± 0.19d | 0.58 ± 0.02d | 7.65 ± 0.26c | 13.67 ± 0.61a | 104.53 ± 5.11a | ||
| 55%AMF | 76.17 ± 0.81c | 8.59 ± 0.02c | 0.79 ± 0.04c | 8.87 ± 0.07a | 10.84 ± 0.51b | 96.12 ± 5.32ab | ||
| 灌浆期 Filling stage | 根 Root | 75%NAMF | 98.93 ± 2.41b | 10.70 ± 0.39b | 2.88 ± 0.06b | 9.26 ± 0.42a | 3.72 ± 0.18a | 34.40 ± 1.54b |
| 75%AMF | 123.87 ± 3.00a | 12.52 ± 0.40a | 3.09 ± 0.04a | 9.90 ± 0.42a | 4.05 ± 0.18a | 40.10 ± 1.16a | ||
| 55%NAMF | 71.77 ± 0.55d | 9.03 ± 0.17c | 2.32 ± 0.11d | 7.94 ± 0.11b | 3.89 ± 0.11a | 30.94 ± 1.24c | ||
| 55%AMF | 84.63 ± 1.12c | 10.23 ± 0.62b | 2.65 ± 0.05c | 8.29 ± 0.41b | 3.85 ± 0.27a | 31.89 ± 0.63c | ||
| 茎 Stem | 75%NAMF | 102.93 ± 1.38b | 9.64 ± 0.32b | 2.71 ± 0.03b | 10.68 ± 0.21a | 3.55 ± 0.08c | 37.92 ± 0.12b | |
| 75%AMF | 127.43 ± 1.68a | 11.65 ± 0.55a | 2.89 ± 0.07a | 10.96 ± 0.57a | 4.03 ± 0.10a | 44.09 ± 1.23a | ||
| 55%NAMF | 84.50 ± 1.10d | 8.64 ± 0.29c | 2.31 ± 0.09d | 9.79 ± 0.30b | 3.75 ± 0.22ab | 36.70 ± 1.96b | ||
| 55%AMF | 93.93 ± 1.94c | 9.49 ± 0.04b | 2.56 ± 0.06c | 9.90 ± 0.17b | 3.71 ± 0.10c | 36.77 ± 1.57b | ||
| 叶 Leaf | 75%NAMF | 99.17 ± 1.10b | 10.33 ± 0.16b | 0.95 ± 0.03b | 9.60 ± 0.26ab | 10.87 ± 0.21b | 104.39 ± 0.50b | |
| 75%AMF | 114.93 ± 0.96a | 11.47 ± 0.17a | 1.31 ± 0.02a | 10.02 ± 0.18a | 8.76 ± 0.39c | 87.73 ± 2.46c | ||
| 55%NAMF | 83.13 ± 1.91d | 8.89 ± 0.02c | 0.68 ± 0.03d | 9.35 ± 0.21b | 13.03 ± 0.55a | 121.96 ± 7.66a | ||
| 55%AMF | 91.47 ± 1.37c | 9.14 ± 0.07b | 0.84 ± 0.03c | 10.00 ± 0.23a | 10.89 ± 0.38c | 108.94 ± 3.24b |
Table 2 Effects of arbuscular mycorrhizal fungi (AMF) inoculation on carbon (C), nitrogen (N), phosphorus (P) and stoichiometric ratio of oat organs under drought stress (mean ± SE)
| 生育时期 Growth stage | 器官 Organ | 处理 Treatment | C (g·kg-1) | N (g·kg-1) | P (g·kg-1) | C:N | N:P | C:P |
|---|---|---|---|---|---|---|---|---|
| 拔节期 Jointing stage | 根 Root | 75%NAMF | 93.73 ± 1.67b | 8.44 ± 0.25b | 2.63 ± 0.08b | 11.12 ± 0.47a | 3.20 ± 0.03c | 35.61 ± 1.39a |
| 75%AMF | 109.10 ± 2.23a | 10.78 ± 0.38a | 2.90 ± 0.09a | 10.13 ± 0.53b | 3.72 ± 0.06a | 37.72 ± 1.96a | ||
| 55%NAMF | 65.50 ± 2.80d | 7.58 ± 0.29c | 2.29 ± 0.03c | 8.65 ± 0.35c | 3.31 ± 0.17bc | 28.65 ± 1.53b | ||
| 55%AMF | 76.97 ± 2.25c | 8.73 ± 0.10b | 2.55 ± 0.03b | 8.82 ± 0.35c | 3.43 ± 0.07b | 30.24 ± 0.94b | ||
| 茎 Stem | 75%NAMF | 87.57 ± 1.88b | 8.48 ± 0.33b | 2.58 ± 0.03b | 10.33 ± 0.18a | 3.29 ± 0.15b | 33.99 ± 0.98a | |
| 75%AMF | 97.73 ± 1.61a | 9.82 ± 0.09a | 2.80 ± 0.02a | 9.96 ± 0.23ab | 3.51 ± 0.05a | 34.97 ± 0.48a | ||
| 55%NAMF | 67.47 ± 2.00d | 7.11 ± 0.09c | 2.20 ± 0.01d | 9.49 ± 0.30c | 3.23 ± 0.02b | 30.60 ± 0.88b | ||
| 55%AMF | 80.67 ± 0.68c | 8.38 ± 0.15b | 2.37 ± 0.02c | 9.63 ± 0.11bc | 3.53 ± 0.06a | 34.00 ± 0.17a | ||
| 叶 Leaf | 75%NAMF | 79.17 ± 1.18b | 9.52 ± 0.45b | 0.88 ± 0.04b | 8.33 ± 0.50ab | 10.84 ± 0.27b | 90.34 ± 5.80b | |
| 75%AMF | 88.17 ± 1.58a | 10.75 ± 0.20a | 1.21 ± 0.05a | 8.20 ± 0.25bc | 8.93 ± 0.48c | 73.21 ± 1.73c | ||
| 55%NAMF | 60.70 ± 0.80d | 7.94 ± 0.19d | 0.58 ± 0.02d | 7.65 ± 0.26c | 13.67 ± 0.61a | 104.53 ± 5.11a | ||
| 55%AMF | 76.17 ± 0.81c | 8.59 ± 0.02c | 0.79 ± 0.04c | 8.87 ± 0.07a | 10.84 ± 0.51b | 96.12 ± 5.32ab | ||
| 灌浆期 Filling stage | 根 Root | 75%NAMF | 98.93 ± 2.41b | 10.70 ± 0.39b | 2.88 ± 0.06b | 9.26 ± 0.42a | 3.72 ± 0.18a | 34.40 ± 1.54b |
| 75%AMF | 123.87 ± 3.00a | 12.52 ± 0.40a | 3.09 ± 0.04a | 9.90 ± 0.42a | 4.05 ± 0.18a | 40.10 ± 1.16a | ||
| 55%NAMF | 71.77 ± 0.55d | 9.03 ± 0.17c | 2.32 ± 0.11d | 7.94 ± 0.11b | 3.89 ± 0.11a | 30.94 ± 1.24c | ||
| 55%AMF | 84.63 ± 1.12c | 10.23 ± 0.62b | 2.65 ± 0.05c | 8.29 ± 0.41b | 3.85 ± 0.27a | 31.89 ± 0.63c | ||
| 茎 Stem | 75%NAMF | 102.93 ± 1.38b | 9.64 ± 0.32b | 2.71 ± 0.03b | 10.68 ± 0.21a | 3.55 ± 0.08c | 37.92 ± 0.12b | |
| 75%AMF | 127.43 ± 1.68a | 11.65 ± 0.55a | 2.89 ± 0.07a | 10.96 ± 0.57a | 4.03 ± 0.10a | 44.09 ± 1.23a | ||
| 55%NAMF | 84.50 ± 1.10d | 8.64 ± 0.29c | 2.31 ± 0.09d | 9.79 ± 0.30b | 3.75 ± 0.22ab | 36.70 ± 1.96b | ||
| 55%AMF | 93.93 ± 1.94c | 9.49 ± 0.04b | 2.56 ± 0.06c | 9.90 ± 0.17b | 3.71 ± 0.10c | 36.77 ± 1.57b | ||
| 叶 Leaf | 75%NAMF | 99.17 ± 1.10b | 10.33 ± 0.16b | 0.95 ± 0.03b | 9.60 ± 0.26ab | 10.87 ± 0.21b | 104.39 ± 0.50b | |
| 75%AMF | 114.93 ± 0.96a | 11.47 ± 0.17a | 1.31 ± 0.02a | 10.02 ± 0.18a | 8.76 ± 0.39c | 87.73 ± 2.46c | ||
| 55%NAMF | 83.13 ± 1.91d | 8.89 ± 0.02c | 0.68 ± 0.03d | 9.35 ± 0.21b | 13.03 ± 0.55a | 121.96 ± 7.66a | ||
| 55%AMF | 91.47 ± 1.37c | 9.14 ± 0.07b | 0.84 ± 0.03c | 10.00 ± 0.23a | 10.89 ± 0.38c | 108.94 ± 3.24b |
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | X12 | X13 | X14 | X15 | X16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.60 | 0.54 | 0.68 | 0.54 | 0.51 | 0.48 | 0.46 | 0.53 | 0.57 | 0.57 | 0.62 | 0.58 | 0.66 | 0.60 | 0.68 | 0.51 |
Table 3 Correlation coefficient between arbuscular mycorrhizal fungi (AMF) colonization rate and each indicators (p < 0.05)
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | X12 | X13 | X14 | X15 | X16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.60 | 0.54 | 0.68 | 0.54 | 0.51 | 0.48 | 0.46 | 0.53 | 0.57 | 0.57 | 0.62 | 0.58 | 0.66 | 0.60 | 0.68 | 0.51 |
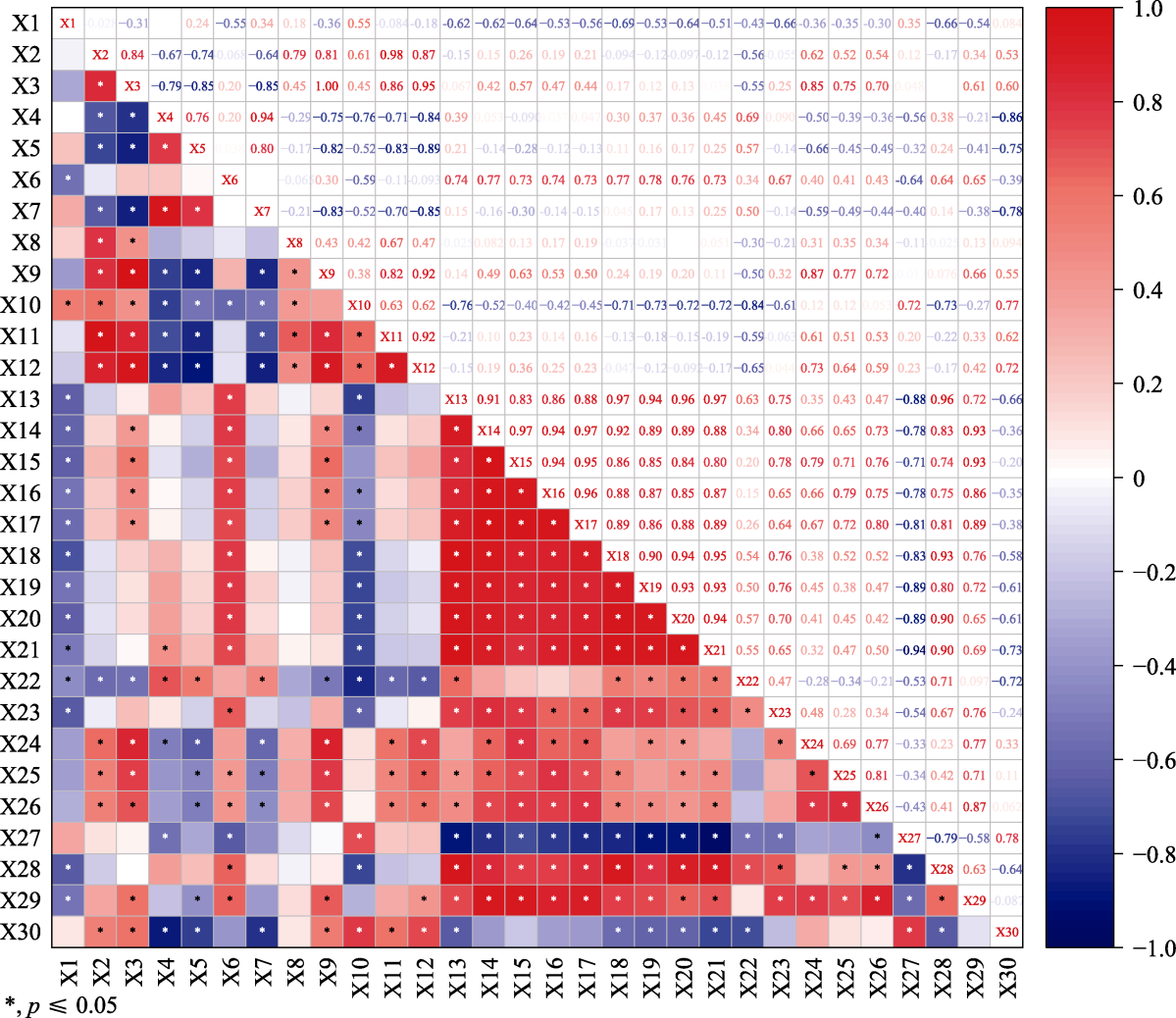
Fig. 4 Correlation between non-structural carbohydrates (NSC) and carbon (C), nitrogen (N), phosphorus (P) contents and its stoichiometric ratio. X1-X30 represented root soluble sugar content, stem soluble sugar content, leaf soluble sugar content, root starch content, stem starch content, leaf starch content, root NSC content, stem NSC content, leaf NSC content, root soluble sugar:starch, stem soluble sugar:starch, leaf soluble sugar:starch, root C content, stem C content, leaf C content, root N content, stem N content, leaf N content, root P content, stem P content, leaf P content, root C:N, stem C:N, leaf C:N, root N:P, stem N:P, leaf N:P, root C:P, stem C:P, leaf C:P, respectively.
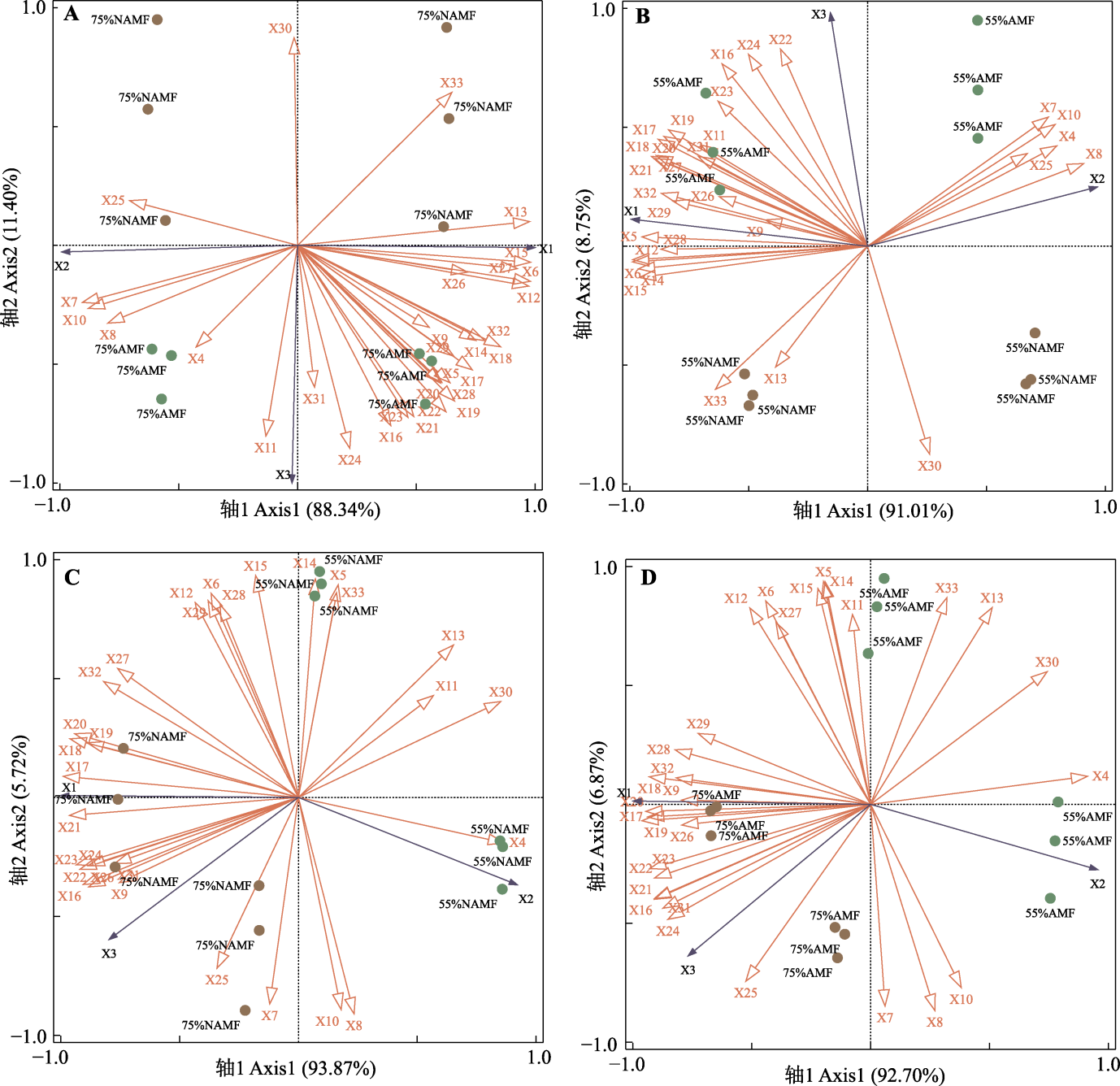
Fig. 5 Redundancy analysis of the effects of non-structural carbohydrates (NSC) content, carbon (C), nitrogen (N), phosphorus (P) contents and its stoichiometric ratio on growth indicators and grain yield of oat organs under water and inoculation treatments. A, 75% water supply. B, 55% water supply. C, AMF-free. D, AMF inoculation. X1-X33 represented plant height, root-to-shoot ratio, grain yield, root sucrose content, stem sucrose content, leaf sucrose content, root starch content, stem starch content, leaf starch content, root NSC content, stem NSC content, leaf NSC content, root soluble sugar:starch, stem soluble sugar:starch, leaf soluble sugar:starch, root C content, stem C content, leaf C content, root N content, stem N content, leaf N content, root P content, stem P content, leaf P content, root C:N, stem C:N, leaf C:N, root N:P, stem N:P, leaf N:P, root C:P, stem C:P, leaf C:P, respectively. 75%NAMF, 75% soil relative water content without AMF inoculation; 75%AMF, 75% soil relative water content with AMF inoculation; 55%NAMF, 55% soil relative water content without AMF inoculation; 55%AMF, 55% soil relative water content with AMF inoculation.
| [1] |
Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA (2012). Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. Journal of Plant Physiology, 169, 704-709.
DOI PMID |
| [2] |
Attarzadeh M, Balouchi H, Rajaie M, Dehnavi MM, Salehi A (2019). Improvement of Echinacea purpurea performance by integration of phosphorus with soil microorganisms under different irrigation regimes. Agricultural Water Management, 221, 238-247.
DOI |
| [3] | Azizi S, Kouchaksaraei MT, Hadian J, Abad ARFN, Sanavi SAMM, Ammer C, Bader MKF (2021). Dual inoculations of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria boost drought resistance and essential oil yield of common myrtle. Forest Ecology and Management, 497, 119478. DOI: 10.1016/j.foreco.2021.119478. |
| [4] |
Bago B, Pfeffer PE, Shachar-Hill Y (2000). Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiology, 124, 949-958.
PMID |
| [5] | Bao SD (2000). Soil Agrochemical Analysis. 3rd ed. China Agriculture Press, Beijing. |
| [鲍士旦 (2000). 土壤农化分析. 3版. 中国农业出版社, 北京.] | |
| [6] | Bitterlich M, Jansa J, Graefe J, Pauwels R, Sudová R, Rydlová J, Püschel D (2024). Drought accentuates the role of mycorrhiza in phosphorus uptake, part II—The intraradical enzymatic response. Soil Biology & Biochemistry, 193, 109414. DOI: 10.1016/j.soilbio.2024.109414. |
| [7] | Cai HX, Wu FZ, Yang WQ (2011). Effects of drought stress on the photosynthesis of Salix paraqplesia and Hippophae rhamnoides seedlings. Acta Ecologica Sinica, 31, 2430-2436. |
| [蔡海霞, 吴福忠, 杨万勤 (2011). 干旱胁迫对高山柳和沙棘幼苗光合生理特征的影响. 生态学报, 31, 2430-2436.] | |
| [8] |
Chen BD, Wang ET (2025). Research prospects on ecology, physiology and application technology of arbuscular mycorrhizal fungi. Bulletin of Botanical Research, 45, 329-332.
DOI |
|
[陈保冬, 王二涛 (2025). 丛枝菌根生态生理与应用技术研究展望. 植物研究, 45, 329-332.]
DOI |
|
| [9] | Cheng HQ, Zou YN, Wu QS, Kuča K (2021). Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Frontiers in Plant Science, 12, 659694. DOI: 10.3389/fpls.2021.659694. |
| [10] |
Chitarra W, Pagliarani C, Maserti B, Lumini E, Siciliano I, Cascone P, Schubert A, Gambino G, Balestrini R, Guerrieri E (2016). Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant physiology, 171, 1009-1023.
DOI PMID |
| [11] | Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S (2020). Roles of arbuscular mycorrhizal fungi on plant growth and performance: importance in biotic and abiotic stressed regulation. Diversity, 12, 370. DOI: 10.3390/d12100370. |
| [12] |
Ditmarová L, Kurjak D, Palmroth S, Kmeť J, Střelcová K (2010). Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiology, 30, 205-213.
DOI PMID |
| [13] | Doubková P, Vlasáková E, Sudová R (2013). Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant and Soil, 370, 149-161. |
| [14] | Du JH, Shao JY, Li SF, Qin J (2020). Non-structural carbohydrate content of trees and its influencing factors at multiple spatial-temporal scales: a review. Chinese Journal of Applied Ecology, 31, 1378-1388. |
|
[杜建会, 邵佳怡, 李升发, 秦晶 (2020). 树木非结构性碳水化合物含量多时空尺度变化特征及其影响因素研究进展. 应用生态学报, 31, 1378-1388.]
DOI |
|
| [15] | Duan HX, Luo CL, Zhu Y, Zhao L, Wang J, Wang W, Xiong YC (2024). Arbuscular mycorrhizal fungus activates wheat physiology for higher reproductive allocation under drought stress in primitive and modern wheat. European Journal of Agronomy, 161, 127376. DOI: 10.1016/j.eja.2024.127376. |
| [16] | Genre A, Lanfranco L, Perotto S, Bonfante P (2020). Unique and common traits in mycorrhizal symbioses. Nature Reviews Microbiology, 18, 649-660. |
| [17] |
Gupta A, Rico-Medina A, Caño-Delgado AI (2020). The physiology of plant responses to drought. Science, 368, 266-269.
DOI PMID |
| [18] | Jiang WT, Gong L, Yang LH, He SP, Liu XH (2021). Dynamics in C, N, and P stoichiometry and microbial biomass following soil depth and vegetation types in low mountain and hill region of China. Scientific Reports, 11, 19631. DOI: 10.1038/s41598-021-99075-5. |
| [19] | Li XX, Li JZ (2013). Determination of the content of soluble sugar in sweet corn with optimized anthrone colorimetric method. Storage and Process, 13(4), 24-27. |
| [20] | Li YL, Jin ZX, Luo GY, Chen C, Sun ZS, Wang XY (2022). Effects of arbuscular mycorrhizal fungi inoculation on non-structural carbohydrate contents and C:N:P stoichiometry of Heptacodium miconioides under drought stress. Chinese Journal of Applied Ecology, 33, 963-971. |
|
[李月灵, 金则新, 罗光宇, 陈超, 孙中帅, 王晓燕 (2022). 干旱胁迫下接种丛枝菌根真菌对七子花非结构性碳水化合物积累及C、N、P化学计量特征的影响. 应用生态学报, 33, 963-971.]
DOI |
|
| [21] | Li Z, Tan XF, Lu K, Zhang L, Long HX, Lü JB, Lin Q (2017). Influence of drought stress on the growth, leaf gas exchange, and chlorophyll fluorescence in two varieties of tung tree seedlings. Acta Ecologica Sinica, 37, 1515-1524. |
| [李泽, 谭晓风, 卢锟, 张琳, 龙洪旭, 吕佳斌, 林青 (2017). 干旱胁迫对两种油桐幼苗生长、气体交换及叶绿素荧光参数的影响. 生态学报, 37, 1515-1524.] | |
| [22] | Liu KL, Han TF, Hu HW, Huang QH, Yu XC, Li DM, Ye HC, Hu ZH (2018). Response of soil enzyme activity in flowering stages of maize to long-term fertilization in red soil. Journal of Plant Nutrition and Fertilizers, 24, 1610-1618. |
| [柳开楼, 韩天富, 胡惠文, 黄庆海, 余喜初, 李大明, 叶会财, 胡志华 (2018). 红壤旱地玉米开花期土壤酶活性对长期施肥的响应. 植物营养与肥料学报, 24, 1610-1618.] | |
| [23] |
Liu N, Zhao ZY, Jiang XL, Xing XK (2021). Review and prospect of researches on the mechanisms of mycorrhizal fungi in improving plant drought resistance. Mycosystema, 40, 851-872.
DOI |
|
[刘娜, 赵泽宇, 姜喜铃, 邢晓科 (2021). 菌根真菌提高植物抗旱性机制的研究回顾与展望. 菌物学报, 40, 851-872.]
DOI |
|
| [24] |
Liu YX, Lu JH, Cui L, Tang ZH, Ci DW, Zou XX, Zhang XJ, Yu XN, Wang YF, Si T (2023). The multifaceted roles of arbuscular mycorrhizal fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biology, 23, 36. DOI: 10.1186/s12870-023-04053-w.
PMID |
| [25] |
Luginbuehl LH, Menard GN, Kurup S, van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GE, Eastmond PJ (2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science, 356, 1175-1178.
DOI PMID |
| [26] | Ma Y, Su BL, Han YG, Wu XH, Zhou WM, Wang QW, Zhou L, Yu DP (2021). Response of photosynthetic characteristics and non-structural carbohydrate accumulation of Betula ermanii seedlings to drought stress. Chinese Journal of Applied Ecology, 32, 513-520. |
|
[马玥, 苏宝玲, 韩艳刚, 吴星慧, 周旺明, 王庆伟, 周莉, 于大炮 (2021). 岳桦幼苗光合特性和非结构性碳水化合物积累对干旱胁迫的响应. 应用生态学报, 32, 513-520.]
DOI |
|
| [27] |
Mathur S, Tomar RS, Jajoo A (2019). Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynthesis Research, 139, 227-238.
DOI PMID |
| [28] | Meena VS, Meena SK, Verma JP, Kumar A, Aeron A, Mishra PK, Bisht JK, Pattanayak A, Naveed M, Dotaniya M (2017). Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Ecological Engineering, 107, 8-32. |
| [29] |
Millard P, Sommerkorn M, Grelet GA (2007). Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytologist, 175, 11-28.
DOI PMID |
| [30] | Niklas KJ, Owens T, Reich PB, Cobb ED (2005). Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecology Letters, 8, 636-642. |
| [31] | O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014). Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nature Climate Change, 4, 710-714. |
| [32] | Pu ZT, Zhang L, Zhang C, Wang H, Wang XX (2022). Research progress of arbuscular mycorrhizal fungi and plant symbiosis affecting plant water regime. Soils, 54, 882-889. |
| [蒲子天, 张林, 张弛, 王红, 王鑫鑫 (2022). 丛枝菌根真菌与植物共生影响植物水分状态的研究进展. 土壤, 54, 882-889.] | |
| [33] | Püschel D, Bitterlich M, Rydlová J, Bukovská P, Sudová R, Jansa J (2023). Benefits in plant N uptake via the mycorrhizal pathway in ample soil moisture persist under severe drought. Soil Biology & Biochemistry, 187, 109220. DOI: 10.1016/j.soilbio.2023.109220. |
| [34] | Püschel D, Bitterlich M, Rydlová J, Jansa J (2021). Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biology & Biochemistry, 157, 108243. DOI: 10.1016/j.soilbio.2021.108243. |
| [35] | Rahimzadeh S, Pirzad A (2017). Microorganisms (AMF and PSB) interaction on linseed productivity under water-deficit condition. International Journal of Plant Production, 11, 259-274. |
| [36] | Rillig MC, Mardatin NF, Leifheit EF, Antunes PM (2010). Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biology & Biochemistry, 42, 1189-1191. |
| [37] | Shi JC, Wang XL, Wang ET (2023). Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annual Review of Plant Biology, 74, 569-607. |
| [38] | Subramanian KS, Santhanakrishnan P, Balasubramanian P (2006). Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Scientia Horticulturae, 107, 245-253. |
| [39] | Sun XM, He MZ, Yang RZ, Li JX, Chen NL (2021). Correlation of non-structural carbohydrates with C:N:P stoichiometry among the organs of Nitraria tangutorum. Acta Ecologica Sinica, 41, 1081-1091. |
| [孙小妹, 何明珠, 杨睿哲, 李金霞, 陈年来 (2021). 白刺器官间非结构性碳水化合与C:N:P计量比的关联性. 生态学报, 41, 1081-1091.] | |
| [40] | Tereucán G, Ruiz A, Nahuelcura J, Oyarzún P, Santander C, Winterhalter P, Ademar Avelar Ferreira P, Cornejo P (2022). Shifts in biochemical and physiological responses by the inoculation of arbuscular mycorrhizal fungi in Triticum aestivum growing under drought conditions. Journal of the Science of Food and Agriculture, 102, 1927-1938. |
| [41] | Thioub M, Ewusi-Mensah N, Sarkodie-Addo J, Adjei-Gyapong T (2019). Arbuscular mycorrhizal fungi inoculation enhances phosphorus use efficiency and soybean productivity on a haplic acrisol. Soil and Tillage Research, 192, 174-186. |
| [42] |
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998). Ink and vinegar, a simple staining technique for arbuscular- mycorrhizal fungi. Applied and Environmental Microbiology, 64, 5004-5007.
DOI PMID |
| [43] | Wang GW, Jin ZX, George TS, Feng G, Zhang L (2023a). Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytologist, 238, 2578-2593. |
| [44] | Wang J, Tang Z (2014). The regulation of soluble sugars in the growth and development of plants. Botanical Research, 3, 71-76. |
| [45] | Wang K, Shen C, Sun B, Wang XN, Wei D, Lv LY (2018). Effects of drought stress on C, N and P stoichiometry of Ulmus pumila seedlings in Horqin sandy land, China. Chinese Journal of Applied Ecology, 29, 2286-2294. |
|
[王凯, 沈潮, 孙冰, 王潇楠, 魏东, 吕林有 (2018). 干旱胁迫对科尔沁沙地榆树幼苗C、N、P化学计量特征的影响. 应用生态学报, 29, 2286-2294.]
DOI |
|
| [46] | Wang Q, Liu MM, Wang ZF, Li JR, Liu K, Huang D (2024). The role of arbuscular mycorrhizal symbiosis in plant abiotic stress. Frontiers in Microbiology, 14, 1323881. DOI: 10.3389/fmicb.2023.1323881. |
| [47] | Wang Y, Han XY, Ai W, Zhan H, Ma SJ, Lu XJ (2023b). Non-structural carbohydrates and growth adaptation strategies of Quercus mongolica Fisch. ex Ledeb. seedlings under drought stress. Forests, 14, 404. DOI: 10.3390/f14020404. |
| [48] | Wang YN, Lin JX, Yang F, Tao S, Yan XF, Zhou ZQ, Zhang YH (2022). Arbuscular mycorrhizal fungi improve the growth and performance in the seedlings of Leymus chinensis under alkali and drought stresses. PeerJ, 10, e12890. |
| [49] | Wang Y, Zou YN, Shu B, Wu QS (2023c). Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Scientia Horticulturae, 308, 111591. DOI: 10.3390/ijms20174199. |
| [50] | Wu QS, Xia RX, Zou YN (2008). Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. European Journal of Soil Biology, 44, 122-128. |
| [51] | Wu YJ, Chen CJ, Wang GA (2024). Inoculation with arbuscular mycorrhizal fungi improves plant biomass and nitrogen and phosphorus nutrients: a meta-analysis. BMC Plant Biology, 24, 960. DOI: 10.1186/s12870-024-05638-9. |
| [52] |
Xie H, Zhang QF, Chen TT, Zeng QX, Zhou JC, Wu Y, Lin HY, Liu YY, Yin YF, Chen YM (2022). Interaction of soil arbuscular mycorrhizal fungi and plant roots acts on maintaining soil phosphorus availability under nitrogen addition. Chinese Journal of Plant Ecology, 46, 811-822.
DOI |
|
[谢欢, 张秋芳, 陈廷廷, 曾泉鑫, 周嘉聪, 吴玥, 林惠瑛, 刘苑苑, 尹云锋, 陈岳民 (2022). 氮添加促进丛枝菌根真菌和根系协作维持土壤磷有效性. 植物生态学报, 46, 811-822.]
DOI |
|
| [53] | Zhang B, Lv YF, Li Y, Li L, Jia JQ, Feng MC, Wang C, Song XY, Yang WD, Shafiq F, Zhang MJ (2023). Inoculation with Rhizophagus intraradices confers drought stress tolerance in oat by improving nitrogen and phosphorus nutrition. Journal of Soil Science and Plant Nutrition, 23, 2039-2052. |
| [54] | Zhang ZF, Zhang JC, Huang YQ, Xu GP, Zhang DN, Yu YC (2016). Effects of mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings. Acta Ecologica Sinica, 36, 3402-3410. |
| [张中峰, 张金池, 黄玉清, 徐广平, 张德楠, 俞元春 (2016). 接种菌根真菌对青冈栎幼苗耐旱性的影响. 生态学报, 36, 3402-3410.] | |
| [55] | Zhao RX, Guo W, Bi N, Guo JY, Wang LX, Zhao J, Zhang J (2015). Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Applied Soil Ecology, 88, 41-49. |
| [56] | Zhu SG, Duan HX, Tao HY, Zhu L, Zhou R, Yang YM, Zhang XL, Wang WY, Zhu H, Zhang W (2023). Arbuscular mycorrhiza changes plant facilitation patterns and increases resource use efficiency in intercropped annual plants. Applied Soil Ecology, 191, 105030. DOI: 10.1016/j.apsoil.2023.105030. |
| [57] | Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012). Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant, Soil and Environment, 58, 186-191. |
| [1] | YANG Mi, 鲁 梦珍, 冯 志洋, 袁 旭东, Zhao Xiaoxiang, Tian Qiuxiang. Correlation relationship between soil phosphorus availability and ectomycorrhizal tree dominance in a subtropical forest [J]. , 2026, 50(菌根生态学): 0-. |
| [2] | LI Wen-Zhu, Junwei 军伟, DI yaping, Yi 一, CHEN Zhi-Cheng, LIU Shi-Rong. Effects of manipulative drought on mycorrhiza-mediated soil enzyme activities and soil organic carbon fractions in a warm temperate oak forest [J]. , 2026, 50(菌根生态学): 0-. |
| [3] | Zhang Cheng-Hang, WEI Xing, WU Chun-Ze, Wang Yu-Yao, Li Hao-Nan. Response of seedling growth to atmospheric reduced nitrogen under dry and wet conditions in mycorrhizal seedlings of Fraxinus mandshurica and Larix gmelinii [J]. Chin J Plant Ecol, 2026, 50(菌根生态学): 0-. |
| [4] | 卞 嘉琛, Rui Wang, Gao Yang Yang, Liang Wenjun, Hong Jin, xuan zhang wen, rong Zhang xiao, Hao Jie, Wang Chang-Hui, DONG Kuan-Hu, Huajie Diao. Effect of different levels of nitrogen addition on the plant aboveground and belowground net primary productivity in Leymus secalinus Grassland in Northern Shanxi [J]. Chin J Plant Ecol, 2025, 49(预发表): 1-. |
| [5] | ZHANG Jian-Hua, ZHOU Xiao-yang, DUAN Shan-shan, BAI Jia-ni, XU Long-Chao. Carbon density and distribution characteristics of two typical shrublands in Dongling Mountain, Beijing [J]. Chin J Plant Ecol, 2025, 49(预发表): 1-. |
| [6] | CHEN Cheng-Zhi, GAO Yu-Sen, Luo Li-Jia, Wang Dong. Twig and leaf litter production and decomposition in an alpline Sibiraea angustata shrubland of western Sichuan [J]. Chin J Plant Ecol, 2025, 49(预发表): 0-. |
| [7] | 王 蓉钧, WU Fu-Zhong, WU Qiu-Xia, Zhu Jingjing, NI Xiang-Yin. Differences in leaf nitrogen reabsorption efficiency among plants with different life forms [J]. Chin J Plant Ecol, 2025, 50(化学计量与功能性状): 1-. |
| [8] | CHEN Ya-Xuan, HAN Yu-Yin, LIU Qian-Yuan, CHEN Yan-Mei. Study on plant functional traits and carbon, nitrogen stoichiometry of plantations at different ages [J]. Chin J Plant Ecol, 2025, 50(化学计量与功能性状): 1-. |
| [9] | WANG Meng-Xue, HU Ming-Yan, CHU Cheng-Jin, CHEN Yang, LUO Wen-Qi, Ma Zilong. C, N, P stoichiometric characteristics of leaves and fine roots in different mycorrhizal tree species in subtropical forests [J]. Chin J Plant Ecol, 2025, 50(化学计量与功能性状): 1-. |
| [10] | Xiaoling Deng, Ai Ling, Xingzhou Huang, WU Fu-Zhong, Qiwen Xu Xu Qiwen, Zhu Jingjing, NI Xiang-Yin. Release rates and controlling factors of dissolved and hot-water extractable organic carbon during litter decomposition of 21 tree species in a subtropical forest [J]. , 2025, 50(化学计量与功能性状): 0-. |
| [11] | Rui Wang, LI JIA HUI, LIANG CHANG YU, LIN Mao, LIANG LI GUO, Wu shuaikai, Su Yuan, DONG Kuan-Hu, Wang Chang-Hui. Stoichiometric characteristics of Leymus secalinus under different levels of nitrogen addition and its effects on photosynthesis [J]. , 2025, 50(化学计量与功能性状): 0-. |
| [12] | ZHANG Fa-Wei, LI Hong-Qin, ZHU Jing-Bin, FAN bo, Zhou Zhou Hua-Kun, LI Ying-Nian, Liang Naishen. Response of the above- and below-ground carbon storage to nitrogen addition and precipitation change in an alpine meadow ecosystem [J]. Chin J Plant Ecol, 2025, 49(地上地下生态过程关联): 1-. |
| [13] | CHANG Peng-Fei, Li Ping, JALAID Nairsag, Wang Jing, WANG Zhen-Hua, Yang Sen, JIA Zhou, YANG Lu, LIU Ling-Li, Deng Meifeng. Contributions of soil organic carbon and inorganic carbon stocks to total carbon stock and their influencing factors between different types in temperate grasslands of Inner Mongolia, China [J]. Chin J Plant Ecol, 2025, 49(地上地下生态过程关联): 0-. |
| [14] | ZHOU Yu-Ting, XIAO Jiang, HUANG Xing-Rui, GONG Ding-Kang, LIU Juan-Yao, LIU Diao, LEI Ning-Fei, WANG Qi, Li Ling-Juan, Li Qi, PEI Xiang-Jun. Influence of root architecture on the organic carbon fraction of reconstituted soil from a granite spoil dump [J]. , 2025, 49(地上地下生态过程关联): 0-. |
| [15] | SHEN Hui-Tao, YU Xiao-Ya, QIN Yan-Jie, WU Ai-Bin. Ecosystem C:N:P stoichiometry and carbon storage along a chronosequence of Juglans regia plantations on the Eastern of Taihang Mountain, China [J]. Chin J Plant Ecol, 2025, 49(地上地下生态过程关联): 1-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn