

Chinese Journal of Plant Ecology >
Effects of growing position on leaflet trait variations and its correlations in Fraxinus mandshurica
Received date: 2021-11-18
Accepted date: 2022-01-17
Online published: 2022-06-09
Supported by
National Natural Science Foundation of China(31971636);Fundamental Research Funds for the Central Universities(2572022DS11);Undergraduate Training Programs for Innovations by Heilongjiang Province(202010225086)
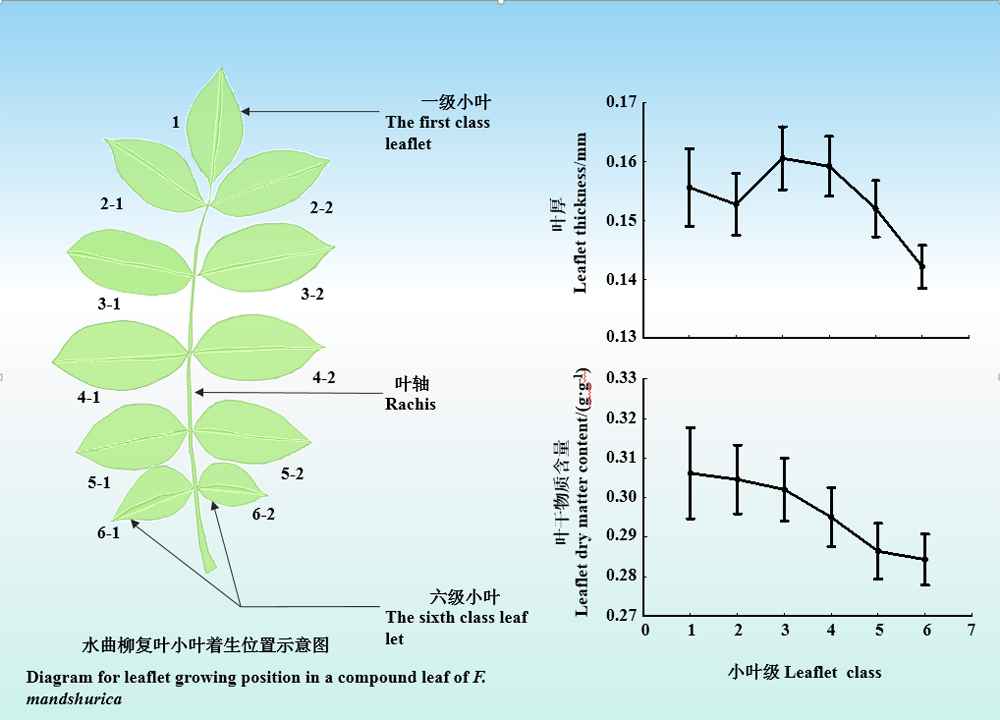
Aims Compound-leaved plants commonly grow better than simple-leaved plants, but it is unknown about how leaflet growing position influences leaflet trait variations and its correlations in compound leaves. Our aim was to address this question with a model tree species Fraxinus mandshurica.
Methods Fraxinus mandshurica, a typical compound-leaved tree in northeastern China, was selected as a focal plant species. We measured leaflet thickness (LT), leaflet area (LA), leaflet dry matter content (LDMC), specific leaflet area (SLA), leaflet nitrogen content (LNC), and leaflet phosphorus content (LPC) across leaflet growing position in the compound leaves of F. mandshurica.We analyzed its leaflet trait variations with leaflet growing position and examined if leaflet growing position significantly affected leaflet traits using the least significant difference (LSD) method. Similarly, we analyzed the relationships among leaflet traits and examined if leaflet growing position significantly affected these relationships using the standardized major axis (SMA) method.
Important findings (1) LT, LA, LDMC and LNC decreased with leaflet growing position (from the tip to the base of a compound leaf), but SLA and LPC increased with leaflet growing position. LT and LA were significantly variable with leaflet growing position. (2) Within a compound leaf, there was an isometric relationship between SLA and LNC or between LDMC and LT. However, LA showed an allometric relationship with LT, SLA and LPC; LDMC show an allometric relationship with SLA, LNC and LPC; LPC showed an allometric relationship with LT. (3) Leaflet growing position had significant effects on the relationships of LA with LT, SLA, and LPC. The greatest slopes between LT and LA and between SLA and LA occurred at the third leaflet growing position (the middle of a compound leaf). The smallest values of absolute slopes between LT and LA and between LPC and LA appeared at the sixth leaflet growing position (the base of a compound leaf). The results suggest that leaflet traits in the compound leaves of F. mandshurica could change with leaflet growing position, and most of trait relationships might be allometric. To a certain extent, the growth relationships among leaflet traits could vary with leaflet growing position.
WANG Guang-Ya, CHEN Bing-Hua, HUANG Yu-Chen, JIN Guang-Ze, LIU Zhi-Li . Effects of growing position on leaflet trait variations and its correlations in Fraxinus mandshurica[J]. Chinese Journal of Plant Ecology, 2022 , 46(6) : 712 -721 . DOI: 10.17521/cjpe.2021.0421
| [1] | Anderegg LDL, Berner LT, Badgley G, Sethi ML, Law BE, HilleRisLambers J (2018). Within-species patterns challenge our understanding of the leaf economics spectrum. Ecology Letters, 21, 734-744. |
| [2] | Anderegg LDL, Loy X, Markham IP, Elmer CM, Hovenden MJ, HilleRisLambers J, Mayfield MM (2021). Aridity drives coordinated trait shifts but not decreased trait variance across the geographic range of eight Australian trees. New Phytologist, 229, 1375-1387. |
| [3] | Burton JI, Perakis SS, McKenzie SC, Lawrence CE, Puettmann KJ (2017). Intraspecific variability and reaction norms of forest understorey plant species traits. Functional Ecology, 31, 1881-1893. |
| [4] | Coble AP, Cavaleri MA (2017). Vertical leaf mass per area gradient of mature sugar maple reflects both height-driven increases in vascular tissue and light-driven increases in palisade layer thickness. Tree Physiology, 37, 1337-1351. |
| [5] | Cui EQ, Weng ES, Yan ER, Xia JY (2020). Robust leaf trait relationships across species under global environmental changes. Nature Communications, 11, 2999. DOI: 10.1038/s41467-020-16839-9. |
| [6] | Dang JJ, Zhao CZ, Li Y, Hou ZJ, Dong XG (2015). Relationship between leaf traits of Melica przewalskyi and slope aspects in alpine grassland of Qilian Mountains, China. Chinese Journal of Plant Ecology, 39, 23-31. |
| [6] | [党晶晶, 赵成章, 李钰, 侯兆疆, 董小刚 (2015). 祁连山高寒草地甘肃臭草叶性状与坡向间的关系. 植物生态学报, 39, 23-31.] |
| [7] | Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC, Garnier E, Bönisch G, Westoby M, Poorter H, Reich PB, et al. (2016). The global spectrum of plant form and function. Nature, 529, 167-171. |
| [8] | Freschet GT, Violle C, Bourget MY, Scherer-Lorenzen M, Fort F (2018). Allocation, morphology, physiology, architecture: the multiple facets of plant above- and below-ground responses to resource stress. New Phytologist, 219, 1338-1352. |
| [9] | Funk JL, Larson JE, Vose G (2021). Leaf traits and performance vary with plant age and water availability in Artemisia californica. Annals of Botany, 127, 495-503. |
| [10] | Gao J, Xu B, Wang JN, Zhou HY, Wang YX, Wu Y (2015). Correlations among leaf traits of typical shrubs and their responses to different light environments in shrub- grassland of southern China. Chinese Journal of Ecology, 34, 2424-2431. |
| [10] | [高景, 徐波, 王金牛, 周海燕, 王彦星, 吴彦 (2015). 南方灌草丛典型灌木不同叶片性状的相关性及其对不同光环境的响应. 生态学杂志, 34, 2424-2431.] |
| [11] | Givnish TJ, Montgomery RA, Goldstein G (2004). Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole- plant compensation points. American Journal of Botany, 91, 228-246. |
| [12] | Givnish TJ, Vermeij GJ (1976). Sizes and shapes of liane leaves. The American Naturalist, 110, 743-778. |
| [13] | Griffith DM, Quigley KM, Anderson TM (2016). Leaf thickness controls variation in leaf mass per area (LMA) among grazing-adapted grasses in Serengeti. Oecologia, 181, 1035-1040. |
| [14] | Guo YP, Yan ZB, Gheyret G, Zhou GY, Xie ZQ, Tang ZY (2020). The community-level scaling relationship between leaf nitrogen and phosphorus changes with plant growth, climate and nutrient limitation. Journal of Ecology, 108, 1276-1286. |
| [15] | Hallik L, Niinemets Ü, Kull O (2012). Photosynthetic acclimation to light in woody and herbaceous species: a comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biology, 14, 88-99. |
| [16] | He NP, Li Y, Liu CC, Xu L, Li MX, Zhang JH, He JS, Tang ZY, Han XG, Ye Q, Xiao CW, Yu Q, Liu SR, Sun W, Niu SL, Li SG, Sack L, Yu GR (2020a). Plant trait networks: improved resolution of the dimensionality of adaptation. Trends in Ecology & Evolution, 35, 908-918. |
| [17] | He LL, Liu Y, He H, Liu Y, Qi JF, Zhang XJ, Li YH, Mao YW, Zhou SL, Zheng XL, Bai QZ, Zhao BL, Wang DF, Wen JQ, Mysore KS, Tadege M, Xia YM, Chen JH (2020b). A molecular framework underlying the compound leaf pattern of Medicago truncatula. Nature Plants, 6, 511-521. |
| [18] | Hu YK, Liu XY, He NP, Pan X, Long SY, Li W, Zhang MY, Cui LJ (2021). Global patterns in leaf stoichiometry across coastal wetlands. Global Ecology and Biogeography, 30, 852-869. |
| [19] | Ji M, Jin GZ, Liu ZL (2021). Effects of ontogenetic stage and leaf age on leaf functional traits and the relationships between traits in Pinus koraiensis. Journal of Forestry Research, 32, 2459-2471. |
| [20] | Keenan TF, Niinemets Ü (2017). Global leaf trait estimates biased due to plasticity in the shade. Nature Plants, 3, 16201. DOI: 10.1038/nplants.2016.201. |
| [21] | Koch G, Rolland G, Dauzat M, Bédiée A, Baldazzi V, Bertin N, Guédon Y, Granier C (2018). Are compound leaves more complex than simple ones? A multi-scale analysis. Annals of Botany, 122, 1173-1185. |
| [22] | Li L, McCormack ML, Ma CG, Kong DL, Zhang Q, Chen XY, Zeng H, Niinemets Ü, Guo DL (2015). Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecology Letters, 18, 899-906. |
| [23] | Li SJ, Wang H, Gou W, White JF, Kingsley KL, Wu GQ, Su PX (2021). Leaf functional traits of dominant desert plants in the Hexi Corridor, Northwestern China: trade-off relationships and adversity strategies. Global Ecology and Conservation, 28, e01666. DOI: 10.1016/j.gecco.2021.e01666. |
| [24] | Lusk CH, Grierson ERP, Laughlin DC (2019). Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency. New Phytologist, 223, 1319-1327. |
| [25] | Nardini A, Pedá G, Salleo S (2012). Alternative methods for scaling leaf hydraulic conductance offer new insights into the structure-function relationships of sun and shade leaves. Functional Plant Biology, 39, 394-401. |
| [26] | Niinemets Ü (1998). Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs. Plant Ecology, 134, 1-11. |
| [27] | Osnas JLD, Lichstein JW, Reich PB, Pacala SW (2013). Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science, 340, 741-744. |
| [28] | Pan YJ, Cieraad E, Armstrong J, Armstrong W, Clarkson BR, Colmer TD, Pedersen O, Visser EJW, Voesenek LACJ, van Bodegom PM, (2020). Global patterns of the leaf economics spectrum in wetlands. Nature Communications, 11, 4519. DOI: 10.1038/s41467-020-18354-3. |
| [29] | Poorter H, Niinemets Ü, Ntagkas N, Siebenkäs A, Mäenpää M, Matsubara S, Pons T (2019). A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytologist, 223, 1073-1105. |
| [30] | Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist, 182, 565-588. |
| [31] | Reich PB (2014). The world-wide “fast-slow” plant economics spectrum: a traits manifesto. Journal of Ecology, 102, 275-301. |
| [32] | Reich PB, Flores-Moreno H (2017). Peeking beneath the hood of the leaf economics spectrum. New Phytologist, 214, 1395-1397. |
| [33] | Roderick ML, Berry SL, Noble IR (2000). A framework for understanding the relationship between environment and vegetation based on the surface area to volume ratio of leaves. Functional Ecology, 14, 423-437. |
| [34] | Runions A, Tsiantis M, Prusinkiewicz P (2017). A common developmental program can produce diverse leaf shapes. New Phytologist, 216, 401-418. |
| [35] | Schulze ED, Kelliher FM, Körner C, Lloyd J, Leuning R (1994). Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: a global ecology scaling exercise. Annual Review of Ecology and Systematics, 25, 629-662. |
| [36] | Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006). Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. Journal of Experimental Botany, 57, 343-354. |
| [37] | Walker AP, McCormack ML, Messier J, Myers-Smith IH, Wullschleger SD (2017). Trait covariance: the functional warp of plant diversity? New Phytologist, 216, 976-980. |
| [38] | Warton DI, Wright IJ, Falster DS, Westoby M (2006). Bivariate line-fitting methods for allometry. Biological Reviews, 81, 259-291. |
| [39] | Wilson PJ, Thompson K, Hodgson JG (1999). Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist, 143, 155-162. |
| [40] | Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Díaz S, Gallagher RV, Jacobs BF, Kooyman R, Law EA, Leishman MR, Niinemets Ü, Reich PB, Sack L, Villar R, Wang H, Wilf P (2017). Global climatic drivers of leaf size. Science, 357, 917-921. |
| [41] | Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee TL, et al. (2004). The worldwide leaf economics spectrum. Nature, 428, 821-827. |
| [42] | Wu BD, Liu J, Jiang K, Zhou JW, Wang CY (2019). Differences in leaf functional traits between simple and compound leaves of Canavalia maritime. Polish Journal of Environmental Studies, 28, 1425-1432. |
| [43] | Yang D, Zhang YJ, Song J, Niu CY, Hao GY (2019). Compound leaves are associated with high hydraulic conductance and photosynthetic capacity: evidence from trees in Northeast China. Tree Physiology, 39, 729-739. |
| [44] | You WJ, Zhang QF, Xia L (2008). Responses of leaf structure of urban greening plants to different light conditions. Journal of Northwest Forestry University, 23, 22-25. |
| [44] | [游文娟, 张庆费, 夏檑 (2008). 城市绿化植物叶片结构对光强的响应. 西北林学院学报, 23, 22-25.] |
| [45] | Zhang L, Luo TX (2004). Advances in ecological studies on leaf lifespan and associated leaf traits. Acta Phytoecologica Sinica, 28, 844-852. |
| [45] | [张林, 罗天祥 (2004). 植物叶寿命及其相关叶性状的生态学研究进展. 植物生态学报, 28, 844-852.] |
| [46] | Zhao YT, Ali A, Yan ER (2017). The plant economics spectrum is structured by leaf habits and growth forms across subtropical species. Tree Physiology, 37, 173-185. |
/
| 〈 |
|
〉 |