图1
图1
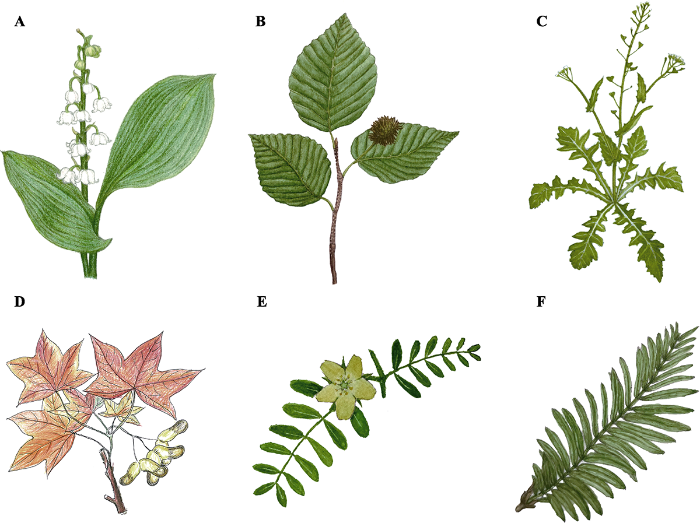
不同形态叶片的手绘图。 A, 铃兰, 单叶, 全缘, 叶椭圆形或卵状披针形。B, 水青冈, 单叶, 叶缘有锯齿, 叶卵形或长卵形。C, 荠, 基生叶丛生呈莲座状, 大头羽状分裂, 顶裂片卵形至长圆形, 侧裂片长圆形至卵形; 茎生叶抱茎, 窄披针形或披针形, 边缘有缺刻或锯齿。D, 元宝枫, 单叶, 掌状5裂。E, 蒺藜, 偶数羽状复叶, 小叶矩圆形或斜短圆形。F, 水杉, 叶条形, 呈羽状排列。A-C, F, 蔡琼绘; D, 买一慧绘; E, 罗晓图绘。描述参考中国植物志中英文版(
Fig. 1
Leaves with varied morphology. A, Convallaria majalis, single leaf with entire margin, leaf blade elliptic to ovate-lanceolate. B, Fagus longipetiolata, single leaf with serrate margin, blade ovate to ovate-oblong. C, Capsella bursa-pastoris, basal leaves rosulate, leaf blade oblong or oblanceolate; cauline leaves amplexicaul, narrowly oblong, lanceolate, or linear, margin entire or dentate. D, Acer truncatum, single leaf, usually 5-lobed. E, Tribulus terrestris, even-pinnately compound leaf, leaflet blades oblong to obliquely oblong, margin entire. F, Metasequoia glyptostroboides, linear leaves, pinnately aligned. Hand painted by CAI Qiong (A-C, F), MAI Yi-Hui (D), and LUO Xiao-Tu (E). Leaf morphology description was obtained from eflora (
叶片形态变化的生态和进化机制一直是植物学和生态学领域研究的热点问题(Givnish, 1978a; Traiser et al., 2005; Peppe et al., 2014)。叶形态与植物的生理-生物力学需求(physiological and bio- mechanical demand)联系紧密(Feild et al., 2005; Royer & Wilf, 2006; Peppe et al., 2011), 叶片形态的变化是植物在选择压力下适应不同生境的一种表现, 反映了植物适应环境变化所形成的生存策略 (Baker-Brosh & Peet, 1997; Vendramini et al., 2002; Liu et al., 2020)。例如, 大的叶片通常具有较高的光截获能力(Niinemets et al., 2005; Niklas et al., 2007; Smith et al., 2017; Lusk et al., 2019), 又因边界层较厚、边界层导度较低、叶表温度较高, 而提高了光合和暗呼吸速率(Mebrahtu et al., 1991; Okajima et al., 2012)。小叶具有较高的主脉密度和较高的水力导度, 可抵抗干旱(Scoffoni et al., 2011; Sack et al., 2012)。叶齿通常被认为是对寒冷的适应, 能通过增强液流, 促进叶片与外界的气体交换和碳固定(Royer & Wilf, 2006; Peppe et al., 2011), 还可通过吐水作用释放叶肉细胞间过多的水流(mesophyll flooding), 缓解过高的根压(Feild et al., 2005)。叶片形态发育受多种遗传因素影响, 如激素、转录因子、微核糖核酸、小分子肽和外基因标记等, 通过诱导叶原基、建立背腹性及调控叶片扁平化等过程, 进而形成不同大小和形状的叶片(Ali et al., 2020; Ren et al., 2020)。例如, 近期研究证明, TCP转录因子在植物叶形态多样化的过程中起到重要作用, 其与转录抑制因子TIE1和E3泛素连接酶TEAR1相互作用, 共同调控植物的叶形态(Tao et al., 2013; Zhang et al., 2017)。
目前叶形态的研究主要集中于两个方面。一方面是叶性状间的权衡关系, 主要研究叶片形态、结构和生理性状间的关系, 包括叶片大小与数目间的权衡(Kleiman & Aarssen, 2007; Whitman & Aarssen, 2009; Huang et al., 2016)、叶形态对叶片生理结构(如叶脉网络、栅栏组织和海绵组织)和支撑结构(如小枝、叶柄)间生物量分配的影响(Milla & Reich, 2007; Niinemets et al., 2007; Yang et al., 2010; Tian et al., 2016; He et al., 2018)以及叶片大小和排列对叶片光合作用、水分运输等生理过程的影响(Poorter & Rozendaal, 2008; Pyakurel & Wang, 2014)。另一方面是叶形态与环境间的关系及其应用。以往研究发现, 多个叶形态指标(包括叶片大小、叶片长宽比、叶缘类型、单复叶等)与环境因子(主要为年平均气温、降水量和土壤性质)间具有较强的相关性(Jacobs, 1999; Royer et al., 2005; Traiser et al., 2005; Adams et al., 2008; Xia et al., 2009; Peppe et al., 2011)。例如, 在干旱、寒冷或盐碱环境下, 群落中的植物以小叶或肉质叶为主, 而在湿热环境下大叶植物较多(McDonald et al., 2003; Peppe et al., 2011); 在寒冷气候下叶片边缘通常具有较大、较多的齿, 且叶片倾向于高度分裂(Peppe et al., 2011)。此外, 叶形态对气候变化响应敏感(Wiemann et al., 1998; Royer et al., 2009b; Guerin et al., 2012; Li et al., 2020b)。因此, 叶形态的变异能有效反映生境的变化, 常用来重建古气候(Wilf et al., 1998; Greenwood et al., 2004; Adams et al., 2008; Peppe et al., 2011; Chen et al., 2014)。定量叶形态与气候间的关系有助于理解植物对气候变化的响应(Dolph & Dilcher, 1980; Fonseca et al., 2000; Peppe et al., 2011), 亦可用来推测全球气候变化对植被组成和分布的影响(Kowalski, 2002; Yang et al., 2016, 2019b), 为植被-气候模型的构建提供新的视角。
1 叶片大小的生态功能及其地理格局
1.1 叶片大小的生态功能
叶片的大小是叶形态研究中广受关注的性状。研究叶大小最常用的指标是叶片表面积。早期研究常利用分级的方法定性估算叶面积大小, 常用的叶面积分级估算方法有Raunkiaer-Webb系统(Webb, 1959)和气候-叶相多变量综合分析项目(Climate-Leaf Analysis Multivariate Program, CLAMP) (Wolfe, 1993), 但分级定性估算方法无法准确估算某物种或地区的叶面积大小。近年来, 人们更多采用定量测量方法估算叶面积, 主要方法有: 方格法、称纸重法、打孔称重法、回归方程法和数字图像处理技术法等(热娜古丽•热西提等, 2020)。前三种方法相对比较精确, 但工作量大, 适用于样本量较少的情况。此外, 这些方法易受叶片脱水萎缩的影响, 且其中的打孔法属于破坏性测量。回归方程法一般是基于叶长、叶宽与叶面积间的回归关系估算叶面积。多个基于单物种的研究指出, 叶面积与叶长和叶宽的乘积呈显著的线性关系(Cristofori et al., 2007; Rouphael et al., 2010)。植物志中通常收录有物种叶长和叶宽的范围, 可用于批量估算叶面积。图像处理方法是目前叶面积测量的主要方法, 在野外采集新鲜叶片后先进行扫描存储, 后续再用专门的图像处理软件(例如ImageJ)根据比例尺和像元数估算叶面积。该方法需要注意保持叶片湿润平展, 避免脱水萎蔫。
叶大小对植物的生理过程具有多方面的影响。首先, 叶大小反映了植物对光的截获能力。基于种群或同一区域中多个物种的研究指出, 光获取效率随叶面积增大而增大, 大叶具有较高的光获取效率(Niinemets et al., 2005; Niklas et al., 2007; Smith et al., 2017; Lusk et al., 2019)。这可能因为以下几点: 1)大叶通常具有较长的叶柄, 且出叶强度(leafing intensity)较低(Wang et al., 2019), 叶片在空间上排列分散, 即单位小枝上叶数较少(Duursma et al., 2012; Smith et al., 2017), 因此叶片间遮蔽程度(self-shading)较低(Falster & Westoby, 2003), 光获取面积较大; 2)随着叶面积增大, 叶绿素a/b增大(Terashima & Hikosaka, 1995), 补光能力增强; 3)大叶的扁平细胞占比大, 气孔密度较低(Conesa et al., 2020), 主脉密度也较低(Scoffoni et al., 2011), 叶内遮阴可能较弱, 与小叶相比, 对到达叶绿体的光照量限制要小(Terashima & Hikosaka, 1995)。因此, 不考虑叶倾角、叶排列以及风速等外界条件, 大叶通常具有更大的光截获效率或光截获能力, 可显著影响植物的光合生产能力(Terashima & Hikosaka, 1995; Duursma et al., 2012; Lusk et al., 2019)。其次, 叶大小可通过限制叶片表面气孔的数目和叶脉分布间接影响植物的水分利用效率。有研究指出, 叶大小与一些水分利用相关的植物性状密切相关, 包括叶脉密度(Price et al., 2012; Sack et al., 2012)、气孔密度(Pyakurel & Wang, 2014; Conesa et al., 2020)、比叶面积(Fonseca et al., 2000; Ackerly et al., 2002; Milla & Reich, 2007)、树高以及木材密度(Malhado et al., 2009)等, 进而影响叶片乃至整个植株的生理活动。例如, 多个研究指出主脉密度与叶大小呈负相关关系, 而细脉密度相对独立(Scoffoni et al., 2011; Sack et al., 2012; Kawai & Okada, 2016; Schneider et al., 2017), 二者共同决定了叶片的水力导度和水分供应容量(Scoffoni et al., 2011; Schneider et al., 2017)。通过分析全球485种双子叶植物的叶脉结构与叶大小的关系, Sack等(2012)发现, 大的叶片具有较大直径的主脉, 但主脉密度较低。小叶因较高的主脉密度, 具有较高的水力导度和较低的干旱脆弱性(Scoffoni et al., 2011; Nardini et al., 2014)。又如, 气孔密度随着叶面积减小而增大(Franks & Farquhar, 2007; Peel et al., 2017; Conesa et al., 2020), 决定了叶片的水分损失和最大光合速率(Schneider et al., 2017)。最新的研究结合同质园(common garden)实验和贝叶斯多层模型指出, 叶面积与气孔密度显著负相关, 但与气孔长度不相关(Conesa et al., 2020)。这可能是因为大叶具有较大比例的扁平细胞, 因而气孔密度较低(Conesa et al., 2020), 而小叶具有较高的细胞嵌入度(Salisbury & Oliver, 1928), 高气孔密度可满足光合所需的CO2通量(Franks & Farquhar, 2007)。第三, 叶大小可显著影响叶片的能量平衡(辐射增温和蒸腾降温)。随叶片增大, 边界层厚度增大, 边界层导度降低(Schuepp, 1993; Wright et al., 2017), 叶片内CO2浓度降低, 因此叶表与空气间的温度差(ΔT)增大(Nobel, 2009; Okajima et al., 2012; Wright et al., 2017)。大叶温度较高, 可补偿叶片表面降低的CO2含量, 以提高光合和暗呼吸速率(Mebrahtu et al., 1991; Okajima et al., 2012)。当风速可忽略时, 叶表温度与叶大小呈正相关关系(Schuepp, 1993; Yates et al., 2010; Okajima et al., 2012; Wright et al., 2017), 进而影响叶片的生理、生化过程以适应外界气候的变化。
在自然条件下, 生活在不同生境的植物通常具有不同的叶大小。叶大小的变化可能受多种因素影响。首先, 叶大小的变化可能反映了植物在最大化光合生产能力和适应逆境的权衡。不同大小的叶片通过调节叶片边缘处的空气对流速度影响叶片表面温度, 以最大化碳吸收能力并提高水分利用效率(Parkhurst & Loucks, 1972; Michaletz et al., 2016)。对于大叶植物, 随着温度的升高, 光合作用所增加的碳收益可能会低于因蒸腾作用加快而造成的水分散失(Givnish & Vermeij, 1976; Givnish, 1984; Fonseca et al., 2000), 因此大叶仅在水分充足的温暖地区是有利性状。在强辐射、炎热且干旱的地区, 大叶因边界层较厚, 叶片表面与空气间温度差增大, 白天辐射增温大于蒸腾冷却, 叶片容易热损伤; 而小叶则因较薄的边界层, 不易过热损伤, 拥有更接近于光合作用的最适叶温, 光合速率加快(Scoffoni et al., 2011; Okajima et al., 2012), 同时小叶具有较低的气孔导度, 蒸腾速率降低, 可实现水分利用效率的最大化(Parkhurst & Loucks, 1972; Okajima et al., 2012)。在寒冷的夜间, 大叶又因边界层导度低而阻碍感热通量, 叶温低于空气温度, 容易受夜间冻害(Schuepp, 1993; Wright et al., 2017)。因此, 与大叶相比, 小叶因较高的水分利用效率且不易受热损伤或夜间冻害, 是对强辐射、炎热且干旱的环境或者寒冷环境的适应(Scoffoni et al., 2011; Okajima et al., 2012; Leigh et al., 2017; Wright et al., 2017)。其次, 叶大小反映了植物不同组织结构间的生物量分配。通过整合2 000余种植物的性状数据, 研究发现叶面积是冠层高度、种子质量和叶干质量的常数函数, 即叶大小与植物整株和种子大小间存在异速增长关系, 虽然在干扰或者贫瘠情况下有例外(Hodgson et al., 2017)。研究指出, 比叶面积随着叶片增大而减小, 表明大叶子虽然有较大的光截获面积, 但单位叶面积所需的干物质投入更大, 意味着叶子获取光能的代价增大(Niinemets, 2001; Milla & Reich, 2007; Niklas et al., 2007)。叶大小与比叶面积的异速增长关系可能与叶片在叶柄和中脉等支撑性结构上的投入有关(Niinemets et al., 2007)。与小叶相比, 大叶脉管和厚壁细胞增多以保证水分供应和养分运输, 也需要更长的叶柄以减少叶片间的自荫作用, 因此大叶需要分配更多的干物质用于支撑结构(Milla & Reich, 2007; Niinemets et al., 2007; Yang et al., 2010)。依据科纳法则(Corner’s rule), 小枝的大小与小枝所承受的附加物(如叶柄、叶片)大小呈正相关关系(Corner, 1949; Smith et al., 2017)。对中国四川4个阔叶林234种植物的研究发现, 叶面积与茎和小枝的质量呈异速增长的关系, 且这种关系不受植物生活型和生境类型的影响(Yang et al., 2010)。此外, 叶大小与叶片的解剖、生化性状紧密相关。例如, 叶大小影响叶脉分布和主脉密度, 一般而言, 主脉和侧脉(即一级和二级叶脉)的密度随着叶大小的增大而呈几何递减(Sack et al., 2012; Schneider et al., 2017); 大叶多具有较密的表皮毛和稀疏的气孔以减少叶片的水分损失(Pyakurel & Wang, 2014), 小叶具有较大的气孔密度和中脉厚度, 以降低呼吸速率, 改善光合潜力(Liu et al., 2020)。综上, 叶大小反映了植物的资源获取策略, 是长期进化过程中对环境梯度的适应和投入-产出的权衡, 受温度和水分共同限制, 也与不同组织器官间的生物量分配和叶片结构性状等有关。
以往研究指出, 功能性状的变异受进化历史和环境变化的共同驱动, 反映了植物资源获取策略和对环境的适应性变异(Reich et al., 2003)。宏观大尺度上, 叶大小更多地受气候而非进化历史的影响。基于中国1万余种木本植物的分析发现, 叶大小在科、属水平上均未表现出显著的谱系信号, 表明叶大小谱系不保守, 在进化上不稳定(Li et al., 2020a)。叶大小作为一种谱系不保守的性状, 其变异更多地受持续的环境筛选而非进化历史的影响(Reich et al., 2003; Schellenberger Costa et al., 2018)。对中亚干旱区典型的荒漠灌木——红砂(Reaumuria soongarica)野生类群的研究表明, 红砂倾向于改变叶长以应对环境变化; 相应地, 对比野外与同质园的类群发现, 叶宽的变化是局域环境和表型可塑性共同作用的结果(Fan et al., 2020)。基于Populus tremuloides 492个基因型的同质园实验发现, 虽然叶大小也受基因型影响, 树冠位置对叶大小变异的影响要远高于基因型(Eisenring et al., 2021)。因此, 叶大小主要受光、叶片表面温度、水分可利用性等非生物因素的影响, 也受光竞争、取食防御等生物因素的限制(Reich et al., 2003; Williams et al., 2020)。
叶大小对气候变化的响应敏感, 可作为生境气候的指示因子(Werger & Ellenbroek, 1978; Greenwood, 1992; Malhado et al., 2009; Guerin et al., 2012; Li et al., 2020b)。例如, Werger和Ellenbroek (1978)通过研究南非奥兰治河沿岸森林中木本植物的叶大小发现, 当气候从温暖变为炎热、干旱时, 叶片大小发生分化, 小型叶被更小的叶片取代, 大型叶消失。Li等(2020b)基于中国自1910年以来的6 000余份叶标本发现, 叶大小的年际变化与降水量变化呈显著正相关关系。此外, Wilf等(1998)通过整合50个森林样点发现, 植物叶面积与降水量有很强的相关性, 指出该关系可用来重建更新世降水格局。
1.2 叶大小的空间格局及其主导因子
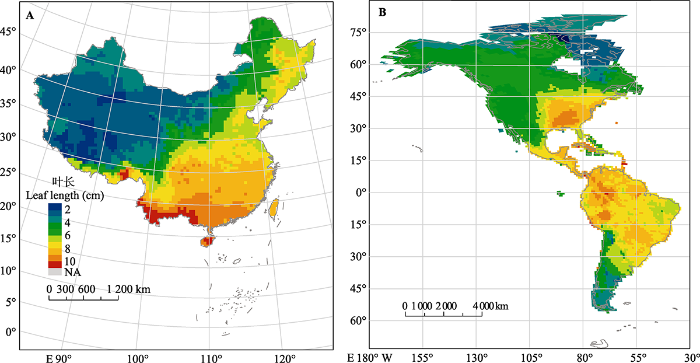
目前对叶片大小空间分布的研究多集中在某些类群沿海拔的分布(Milla & Reich, 2011; Li & Bao, 2014; Liu et al., 2020)以及多类群沿纬度的分布, 主要有Traiser等(2005)对欧洲阔叶乔木叶大小的分布研究, Li等(2020a)对中国和北美木本植物叶大小的研究(图2), Wright等(2017)对全球叶大小的研究。对欧洲阔叶乔木的研究发现, 小型叶主要分布在地中海沿岸, 大型叶在地中海沿岸和北方内陆分布均较多(Traiser et al., 2005)。对中国木本植物的研究发现, 叶片平均大小从中国东南到西北逐渐减小(Li et al., 2020a)。
图2
叶大小的空间分布主要受气候驱动, 在温暖、降水充沛且没有直接强光辐射的地区, 植物一般具有较大的叶片, 而在寒冷、光辐射较强、干旱或土壤贫瘠的地方, 植物通常具有较小的叶片(Dolph & Dilcher, 1980; Ackerly et al., 2002; Xu et al., 2009; Wright et al., 2017), 以避免叶片过热或减少水分损失(Okajima et al., 2012; Leigh et al., 2017; Wang et al., 2019)。在群落水平上, 平均叶大小随降水量增多、气温升高而变大(Li et al., 2020a)。这是因为干旱环境下, 小叶可减少蒸腾作用引起的水分、热量损失, 而当水分不是限制因素时, 增大有效光合叶面积才是对植物生长更有利的策略(Parkhurst & Loucks, 1972; Givnish, 1984; Okajima et al., 2012)。除了气温和降水量等气候因子, 辐射、光和风速对叶大小也有一定的影响(Ackerly et al., 2002; Li et al., 2020a; Williams et al., 2020)。Ackerly等(2002)通过研究地中海22种浓密常绿阔叶灌丛灌木(chaparral shrub)的叶大小沿海拔和坡向的分布发现, 大叶植物在不同辐射梯度上均有分布, 但小叶植物集中分布在辐射较强的南坡。基于同质园37个种群的分析指出, 光强解释了叶大小等性状在种内的大部分变异(Williams et al., 2020)。此外, 叶大小也受树冠高度/位置的影响, 如叶面积一般沿着树冠从低处到高处递增(Jahdi et al., 2020; Eisenring et al., 2021)。受资金、时间等成本影响, 先前研究多关注特定类群, 取样数量有限; 基于部分类群的叶大小分析是否能代表当地整体情况还需进一步探索。
目前, 叶大小的气候主导因子或者说降水量和气温对叶大小空间变异的相对作用强弱还存在一定争议(Greenwood, 1992; McDonald et al., 2003; Traiser et al., 2005; Li & Bao, 2014)。例如, 有研究指出大叶物种比例与降水量有较弱的负相关性(Werger & Ellenbroek, 1978; Malhado et al., 2009), 但基于中国木本植物群落的研究发现, 平均叶大小与降水量呈较强的正相关关系(Li et al., 2020a)。又如, 对南北美洲热带地区叶片的研究发现, 叶大小对降水量的响应比对温度更敏感(Dolph & Dilcher, 1980; Ordoñez et al., 2009), 但有整合分析表明, 包括叶大小在内的大多数植物性状与年平均气温间的关系均显著强于其与年降水量间的关系(Moles et al., 2014)。最近关于全球叶大小分布的研究指出, 叶大小在寒冷地区与年降水量间关系不显著, 但在温暖地区与年降水量呈正相关关系, 且这种关系随气温的增加而增强, 暗示了叶大小受水热交互作用的影响(Wright et al., 2017)。综上, 叶大小显著受气温、降水量及其季节性(seasonality)的影响(Dolph & Dilcher, 1980; Ackerly et al., 2002; Wright et al., 2017)。
1.3 叶大小与生态系统功能
表征叶大小的重要指标之一是叶表面积, 这在群落尺度上反映为叶面积指数(leaf area index, LAI)。在较温暖、湿润且气候季节性较低的环境下, 叶片较大, 群落通常具有较高、较大的林冠层, 具有较高的叶面积指数, 以增大光接受率。已有研究指出, 叶面积指数与森林初级生产力呈正相关关系(Gower et al., 1999; Chen et al., 2012; Reich, 2012)。叶大小、叶面积指数与气候间的联系暗示了叶大小可作为指示陆地生态系统初级生产力空间变化的潜在指标。最近的研究指出, 与叶面积指数相比, 群落内平均叶大小与生态系统初级生产力间的关系更强(Li et al., 2020a)。限于古叶面积很难估算, 叶面积指数并不适用于重建古初级生产力(Dunn et al., 2015)。通过建立叶大小与初级生产力间的关系方程, 保存完好的叶化石可作为一种潜在的方法用于重建陆生生态系统古生产力。因此, 叶大小作为重要的叶形态指标, 可根据其与环境变量间的关系重建古环境指标, 亦可联系植物个体尺度的光合作用和群落尺度上的生物量积累, 为研究生态系统生产力提供一个简单有效的功能指标。
叶大小可能与其他功能性状共变, 进而影响生态系统功能。青藏高原高寒草甸的氮添加实验发现, 生态系统总生产力和水分利用效率随着氮添加先增加, 随后略微降低, 这种非线性的响应与平均叶面积的增加以及叶磷含量的降低密切相关(Zhang et al., 2019)。此外, 叶大小可作为预测植物入侵潜力的一个关键指标。基于新西兰16种本地种和3种入侵种的研究发现, 在22个叶片结构、叶生化性状中, 唯有叶面积是入侵种与本地种显著不同的性状(Heberling & Mason, 2018)。入侵种与本地种的性状组合显著不同, 前者占据叶经济型谱快速回报的一端, 倾向于具有较大叶片、较高的资源获取和较低的防御投入, 具有较大的最大光合速率和较高的氮利用效率和能量利用效率(Heberling & Mason, 2018; Mathakutha et al., 2019)。
2 叶片形状的生态功能与研究进展
广义的叶片形状(以下简称叶形)包括叶片整体的形状以及叶尖、叶基和叶缘等部位的形态, 是表征叶形态和植物分类的重要指标。近年来, 有研究采用机器学习算法或数字化方法, 基于叶形和叶脉进行植物分类(Wilf et al., 2016; 金梦然等, 2020)。狭义的叶形通常指叶片二维表面的几何形状, 常用叶片的长宽比和叶片最宽处的位置表征。最近基于木兰科9个物种多个叶形指数的研究指出, 叶片长宽比与叶形分形维度显著强相关, 可作为衡量叶形的简易指标(Peijian et al., 2021)。叶形通常有两类划分方法, 一种是定性描述, 如将叶形分为椭圆形、卵形和倒卵形等若干类(Traiser et al., 2005; Xia et al., 2009); 另一类是依据叶长宽比分段(如, 长宽比<1、1-2、2-3、3-4、>4)(Jacobs, 1999; Traiser et al., 2005; Xia et al., 2009)。
叶形可影响叶片的光合面积, 较宽的叶子更能截获足够的光。与此同时, 植物可通过改变叶片的周长-面积比, 影响叶片边界层与外界的水气交换。有研究指出, 被子植物的叶形变异与叶片边缘的限制性生长和叶脉网络的整体分布有关(Pray, 1955; Boyce, 2005, 2009)。据此可推测, 叶片形状的空间变异可能受温度和水分可利用性的共同影响。在湿热环境下, 叶片需要尽量扁平、近圆形以促进与外界的水气交换(Hirokazu, 2005), 而在炎热、干旱的环境下, 较厚、狭窄的叶片通常有较强的支撑结构以抵抗枯萎(Werger & Ellenbroek, 1978; Abrams, 1994)。
一般认为, 叶形是基因调控和自然选择双重作用的结果(Hirokazu, 2005; Nicotra et al., 2011)。叶片二维的扩展主要取决于原基的背腹性, 也受叶片伸展后的环境条件(如光照条件和重力作用)影响(Hirokazu, 2005)。Nicotra等(2011)总结被子植物叶形的进化和功能时指出, 叶形在不同发育阶段受多个基因调控, 叶形变异反映了自然选择对叶片功能的选择作用, 以使植物能更好地生长。目前已提出多个假说解释叶形的多样性, 包括水分限制、叶片温度调控、生物力学限制、规避植食风险和优化光截获的适应以及响应花型选择等(Brown & Lawton, 1991; Nicotra et al., 2011)。
叶形的变异主要反映了叶片水分供应的权衡, 这部分是因为叶形与叶脉的分布息息相关, 共同决定了水分在叶片间的分布和运输。通过计算机模拟3种模式植物的叶发育, 发现叶脉网络的分布对叶形起决定作用, 模型参数的较小改变就可产生丰富的叶形(Runions et al., 2017)。叶形虽然也与叶片温度的调节有关, 但作用相对较弱, 叶温主要受叶大小、叶厚度和叶倾角的影响(Nicotra et al., 2011)。叶形反映了植物对生境养分的适应策略。基于富营养化湖泊中大型沉水植物的研究表明, 环境对水生植物性状的影响依赖于叶形, 扁平叶型沉水植物具有较高的与光合相关的形态性状, 其性状主要受水深影响; 针叶型沉水植物则通常具有较高的化学计量性状, 性状主要受水质量影响; 与针叶型相比, 扁平叶型植物更能改善水下的光条件和水质量(Liu et al., 2021)。此外, 叶形也与植食防御密切相关。例如,叶形会影响象甲(Apoderus praecellens)在两种香茶菜(Isodon umbrosus var. hakusanensis和I. trichocarpus)上的产卵行为, 虽然两者亲缘关系相近且营养、气味没有差别, 相比于前者叶缘深裂的叶片, 象甲更倾向于选择后者完整(未分裂)的叶片, 因此, 叶片分裂可视为香茶菜防御捕食的一种手段(Higuchi & Kawakita, 2019)。
目前关于叶形的大尺度格局研究相对较少, 比较经典的有Traiser等(2005)对欧洲当地阔叶乔木的叶形态研究, 发现狭长的叶片(长宽比>3)主要分布在地中海沿岸, 圆形叶(长宽比<1)在整个北方较多; 叶形更多地受温度影响, 且最低温的影响要显著强于最高温。与之相对的, Glukhov和Strelnikov (2014)发现, 15种榕属(Ficus)植物的叶形主要由夏季降水量决定, 与年降水量的峰值、丰度以及气温的季节性呈显著的正相关关系。Li等(2020b)在中国7种植物的叶形研究中指出, 叶形在空间上的变异主要受降水量影响, 而在时间上的变化更多地与年平均气温有关。气温和降水量对叶形在时空上变异的相对作用还有待进一步探讨。
3 叶缘的生态功能及研究进展
3.1 叶缘的生态功能
首先, 叶齿影响叶片的水分利用和运输。叶齿影响叶脉网络结构的分布(Givnish, 1978a), 齿尖通常是叶脉在叶片边缘的分布终端(Bailey & Sinnott, 1916)。解剖观察发现, 齿尖通常有开放气孔或排水孔, 这些结构降低了叶片边界层的厚度, 促进叶片与外界的水气交换, 提高水分的运输效率(Canny, 1990)。此外, 在水分充沛、地下水位浅的地方(如水滨和湖边生境), 具齿物种占有较高的比例, 即淡水-叶缘效应(Greenwood, 2005; Royer et al., 2009a)。这是因为叶齿可以通过吐水作用缓解过高的正根压, 减轻水胁迫对植物生理活动的影响(Feild et al., 2005)。
其次, 叶齿是叶片能量交换的热点区域, 能促进植物对低温的适应。在生长季早期, 叶齿能增强液流, 提高寒冷气候下叶片的光合、蒸腾速率, 促进碳的吸收(Royer & Wilf, 2006; Peppe et al., 2011), 弥补低温对植物光合作用的影响。Royer和Wilf (2006)通过测量不同生境下木本植物叶缘的蒸腾和光合作用发现, 叶缘的生理活动开始较早, 尤其是在寒冷地区, 叶缘带齿的物种在生长季早期比全缘叶物种具有更高的光合、蒸腾作用, 说明在温度受限时, 叶齿能够增加叶片在生长季早期的碳吸收。在寒冷气候下, 叶齿的存在也有助于缓解冻融栓塞, 防止早春冻害(Feild et al., 2005)。因此叶齿被认为是植物对寒冷气候的一种适应策略(Royer & Wilf, 2006; Peppe et al., 2011)。
叶片边缘锯齿的形成受遗传因素、环境和植物特性的共同调控。首先, 叶齿的发育涉及多个基因的表达调控。有研究显示, TCP转录因子在植物叶形态多样化的过程中起到重要作用, 而E3泛素连接酶TEAR1可通过降解转录抑制因子TIE1而增强TCP转录因子的活动, 因此TEAR1过度表达有利于叶片边缘完整, 破坏TEAR1及其同源基因的功能可使得叶缘锯齿增多(Zhang et al., 2017)。此外, 叶齿发育也受植物激素的影响。例如, 生长素浓度直接控制叶缘锯齿的形成(李晓屿等, 2019)。第二, 叶齿的形成受周围环境的影响。例如, Royer等(2009a)通过探讨扰动、水分可利用性和植物生长策略等对澳大利亚亚热带雨林中具齿植物的影响, 检验了淡水-叶缘假说、盐胁迫假说、生活策略假说以及澳大利亚无齿假说, 证明了区域内水分可利用性对具齿物种分布的重要影响。第三, 叶齿的形成与植物的特性有关。植物生活型(乔木、灌木和草本)、叶习性(常绿、落叶)、叶厚度以及比叶面积等均影响物种的叶缘状态(是否具齿)。多个研究发现, 落叶物种的叶片叶缘倾向于具齿, 而常绿物种倾向于叶片全缘(Jacobs, 1999; Adams et al., 2008; Royer et al., 2012)。叶片厚度也显著影响叶缘是否具齿, 且齿的尖锐程度受叶片厚度调控。这是因为较薄的叶片需要更强的支撑结构以维持其伸展, 在叶脉伸向叶缘时, 脉间的叶片组织可能因为没有足够的结构支持而缺失, 从而形成波状或齿状的叶缘(Givnish, 1978a)。相对应地, 厚叶能减小胞内水流所受的阻力, 有利于二级叶脉间的均匀生长, 从而形成光滑的叶缘(Wilf, 1997)。
环境特征和植物特性对叶缘特征的影响是综合的, 而非某一因素单独起作用。比如, 结合叶经济谱, 与常绿种相比, 落叶种的叶片寿命相对较短, 处于快速回报的一端, 倾向于在叶片上投入较少的生物量, 因此叶片相对较薄(Givnish, 1978a; Wright et al., 2004)。较薄的叶片倾向于具齿以增强叶片的物理支撑, 避免在强风中撕裂(Baker-Brosh & Peet, 1997)。再如, 落叶性和叶齿间的强相关性可用叶片气体交换假说解释(Royer & Wilf, 2006)。在寒冷的气候下, 叶齿通常能增强生长季早期的气体交换, 加强碳固定, 因此叶齿可能是落叶物种对低温的一种适应性策略(Peppe et al., 2011; Royer et al., 2012)。Li等(2016)在分析中国木本植物的叶缘特征时发现, 常绿和落叶物种的叶缘特征差异在乔木中要强于灌木, 即常绿(或落叶)乔木比常绿(或落叶)灌木具有更高比例的全缘叶(或具齿叶)物种, 这可能意味着长得高且生活周期长的植物的叶缘状态更容易受温度影响。需要注意的是, 叶习性对叶缘状态的影响与温度对叶缘状态的影响共相关, 因为常绿种通常分布在温暖的区域, 而在寒冷的区域相对较少。因此, 在探讨叶习性对叶缘的影响时需要考虑如何控制或消除气候的影响。
3.2 叶缘组成的空间格局及其与气候的关系
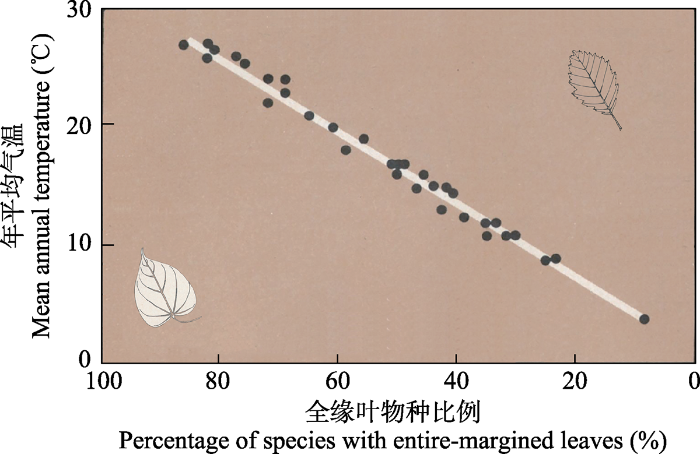
叶缘组成是指一定区域内全缘叶物种(或具齿叶物种)占区域内全部物种的比例。叶缘组成与气候间的关系一直是古生态学家研究的热点(Wolfe, 1979; Adams et al., 2008; Peppe et al., 2011)。叶缘分析通过研究现有植被的叶缘组成与年平均气温的关系, 构建转换方程, 再根据叶化石重建古温度(Bailey & Sinnott, 1915; Wolfe, 1979, 1993)。叶缘分析最早起源于Bailey和Sinnott在20世纪初对木本植物区系的观察。他们发现, 热带和亚热带地区的双子叶植物具有较高比例的全缘叶物种, 而温带地区则具有较高比例的具齿叶物种(Bailey & Sinnott, 1915, 1916)。之后, Wolfe等(1979)研究了东亚和北美植物区系的叶缘组成, 首次定量验证了叶缘组成与年平均气温间的线性关系(图3), 并将其用于重建第三纪气候。
图3
图3
东亚阔叶林中全缘叶物种比例随年平均气温升高而增大。从左到右, 全缘叶物种降低, 具齿叶物种增多。白色实线为回归拟合线, 决定系数高达0.98。改编自Wolfe等(1979),
Fig. 3
Percentage of species with entire-margined leaves increased with mean annual temperature in broad-leaved forests of east Asia. From left to right, the percentage of species with entire leaves increased while those with teethed leaves decreased. Fitted regression line was showed in white with R2 = 0.98. Modified from
此后, 叶缘组成与年平均气温间的关系被广泛研究。多个研究表明, 区域内植物的全缘叶物种比例与当地的年平均气温存在显著的线性关系, 可用于重建古温度(Gregory-Wodzicki, 2000; Royer et al., 2005, 2009a; Royer & Wilf, 2006; Peppe et al., 2011; Chen et al., 2014)。基于这一关系, 人们已在北美(Wolfe, 1979, 1995; Dolph & Dilcher, 1980; Wilf et al., 1998; Adams et al., 2008)、南美(Kowalski, 2002)、欧洲南部(Traiser et al., 2005)、非洲热带地区(Jacobs, 1999)、东亚(Wolfe, 1979; Su et al., 2010; Chen et al., 2014)、澳大利亚(Greenwood et al., 2004; Greenwood, 2005), 乃至全球(Peppe et al., 2011; Royer et al., 2012)建立多个温度重建的转换方程。然而, 不同区域构建的转换方程在模型拟合度和回归斜率上存在显著的差异(Greenwood et al., 2004; Adams et al., 2008; Peppe et al., 2011), 限制了转换模型的普适性与外推。此外, 目前基于大尺度连续分布数据的定量叶缘分析还很欠缺(Li et al., 2016), 叶缘组成对温度的响应及其潜在的生态影响因子尚需进一步研究。
以往的叶缘分析主要关注叶缘组成与温度间的关系(Gregory-Wodzicki, 2000; Royer et al., 2005, 2009a; Royer & Wilf, 2006; Peppe et al., 2011; Chen et al., 2014)。基于叶齿的生态学功能, 叶缘状态与水分运输和利用率密切相关。在水分充足的湿润地区, 叶缘可能更多地响应温度的变化(Adams et al., 2008), 因而全缘叶物种比例与温度表现出很强的相关性。与之相对的, 干旱地区的植物会采取一些适应性策略以应对缺水, 例如调整叶大小和叶型等(Parkhurst & Loucks, 1972; Givnish & Vermeij, 1976), 从而降低温度对叶缘的影响。Wolfe (1993)在分析全球样点的叶缘时指出, 相对于来自湿润地区的样点, 来自干旱、寒冷地区以及高原地区的样点更多地表现为离群值(outliers), 即这些地区的全缘叶物种比例明显不同于其他地区。已有研究探讨了叶缘组成与降水量间的关系(Wiemann et al., 1998; Wilf et al., 1998; Peppe et al., 2011; Moles et al., 2014)。定量化年平均气温和降水量对叶缘的相对影响, 有助于评估叶缘分析用于古温度重建的不确定性。
叶缘组成与气候间的关系也受植物生活型的影响。有研究指出, 木本植物、草本植物和蕨类植物在叶缘组成与温度间的关系上存在显著差异: 木本植物由于其生活周期较长, 叶缘状态受周围环境的影响较大(Traiser et al., 2005); 而草本和蕨类植物因其茎叶独特的导水方式和叶脉分布类型, 叶缘受外界气候的影响较小(Royer et al., 2012; Peppe et al., 2014)。此外, 乔灌草也具有不同的叶缘-温度关系, 即乔木的叶缘状态对温度响应最敏感, 灌木居中, 草本最弱(Royer et al., 2012)。目前大尺度的叶缘分析多以双子叶木本植物或被子植物为研究对象, 如对欧洲阔叶树的叶缘研究(Traiser et al., 2005)、对全球双子叶被子植物的研究(Royer et al., 2012)等; 部分研究以双子叶乔木为研究对象, 如对北美(Adams et al., 2008)和对中国湿润地区(Chen et al., 2014)双子叶乔木的叶缘研究, 均将大灌木同乔木一起纳入分析。值得注意的是, 以往研究较少考虑叶缘组成与气候间的关系在不同生活型(如乔木与灌木)间的差异。Li等(2016)研究指出, 在中国干旱地区, 各生活型的全缘叶物种比例与温度均具有较弱的相关性, 降水量增多并不能加强灌木的叶缘与温度间的关系, 但湿润地区的乔木叶缘与温度呈显著强相关关系。
与叶缘有关的叶性状具有很强的谱系保守性(Jordan, 1997), 说明植物叶缘是否具齿是一种谱系保守的性状, 进化历史可能影响叶缘分布的格局(Dolph & Dilcher, 1980; Ackerly, 2004; Schmerler et al., 2012)及其与温度间的关系(Jordan, 1997; Little et al., 2010; Jordan, 2011)。Little等(2010)根据北美17个样点的叶缘数据发现, 叶缘状态存在很显著的谱系信号, 进化历史影响叶缘组成与年平均气温间的关系。叶缘分析的前提假设是叶缘与气温间的关系在地质历史上保持稳定, 这一假设可能并不准确。有学者认为现代叶缘与气温间的关系可能是第四纪冰期以来环境筛选作用的结果, 并推测地质年代越久远, 叶缘分析的不确定性越大(Jordan, 1997, 2011)。Li等(2016)用中国木本植物进一步验证了宏观进化历史对叶缘-温度间关系的影响, 发现科年龄显著影响叶缘组成与年平均气温间的关系, 尤其是中国特有种, 叶缘与气温间关系随着科年龄的增加而变弱, 叶缘分析在古老科中的不确定性变大。
植物叶缘组成与气候间的关系还受其他因素的影响, 例如区系内物种组成、地形异质性、土壤性质以及系统发育历史等(Wilf, 1997; Adams et al., 2008; Peppe et al., 2011)。对北美乔木的叶缘分析发现, 全缘叶物种比例与年平均气温呈非线性关系, 且北美西海岸乔木的叶缘组成与温度并没有显著的相关性, 这可能与该区域地形复杂或者植物区系组成不完整有关(Adams et al., 2008)。对澳大利亚的叶缘分析发现, 澳大利亚与其他区域相比具有较高比例的全缘叶物种(即“澳大利亚无齿”假说), 且其叶缘组成与温度间的关系也比其他地区弱(Greenwood, 1992; Greenwood et al., 2004), 这可能是由于澳大利亚独特的地质历史和进化历史。由于叶缘与气候的关系受多因素的影响且存在区域差异, 因而, 基于不同区域建立的转换模型可能并不具有广泛适用性。这些发现为叶缘分析校准古温度带来一定的不确定性。
在叶缘分析中, 早期研究所选用的叶缘指标通常为区域内叶片具齿(或全缘)的物种比例, 齿的数目与大小、叶裂程度和叶齿形状相关的研究较少。近年来随着扫描和分析技术的进步, 关于齿的类型、数目和大小的研究增多(Jacobs, 1999; Royer et al., 2005; Peppe et al., 2011)。叶齿数目和大小均与年平均气温呈负相关关系, 在寒冷的环境下, 叶片通常具有更多、更大的齿(Royer et al., 2005; Peppe et al., 2011)。此外, 对Acer rubrum的研究发现, 叶齿数目和叶裂程度对气候变化的响应非常敏感(Royer et al., 2009b)。在未来研究中, 应进一步突破叶缘分析仅聚焦于全缘叶物种比例这一单一指标的限制, 通过结合运用叶齿类型、数目、大小等定量指标, 综合分析叶缘特征与气候间的关系, 为古气候重建提供更准确的方法。
4 叶型的生态功能及其空间分布
叶型有单叶和复叶之分。单叶只有一个叶片和叶柄, 复叶则有多个小叶沿叶轴分布, 且小叶与叶轴间通常连有小叶柄。一般认为复叶起源于单叶, 单叶的叶缘出现缺刻并增大, 先形成叶裂, 之后缺刻加大逐渐进化至叶片全裂, 当裂片与主叶轴之间出现小叶柄或者小叶与叶轴间的关节明显时, 复叶形成。在叶型的发育上, 复叶比单叶有着更复杂的形态建成过程。最近的研究通过解析豆科模式植 物——蒺藜苜蓿(Medicago truncatula)复叶的形态建立机制, 发现域蛋白PINNA1可独立也能与其他蛋白协同合作, 实现对复叶中小叶数目与排列方式的精细调控(He et al., 2020)。小叶作为复叶的一部分, 并不是独立的单元, 复叶的小叶在某些生理功能上类似于单叶的叶裂或叶齿(Xu et al., 2009)。
关于复叶的生态形成机制, 主要有两个假说, 分别是季节性干旱假说(Givnish, 1978b; Gates, 1980) 和快速生长适应假说(Niinemets et al., 2006; Malhado et al., 2010)。季节性干旱假说从叶片结构入手, 对比了单叶和复叶的水分利用效率, 发现与单叶相比, 相同面积的复叶具有较大的周长面积比, 能增强对流, 有效地散热, 从而降低叶片表面温度和蒸腾作用, 有利于保持水分(Gates, 1980)。此外, 复叶物种因能便捷地脱去小枝, 在面临干旱时可以快速地减小叶面积, 有效降低蒸腾, 因此复叶物种在温暖、干旱半干旱且强光的环境下比较常见(Givnish, 1978a; Stowe & Brown, 1981)。快速生长适应假说则考虑物质投入, 即单、复叶的生物量分配。复叶在支撑组织, 如叶轴、叶柄和叶脉上的投入较多(Niinemets et al., 2006; Wu et al., 2019), 这增加了侧向生长的投入, 但相对于永久性枝条等木质结构, 复叶的叶轴比较“廉价”, 可减少植株侧枝的生物量分配, 从而更有效地促进垂直生长(Malhado et al., 2010)。Malhado等(2010)基于亚马孙雨林137个永久样地的研究发现, 复叶物种具有较低的木材密度和较快的直径生长速率, 从而支持了快速生长假说。此外, 许多先锋种是复叶植物(Givnish, 1978b), 而先锋种通常生长较快以占领新的生境, 这一现象间接支持了快速生长假说。
目前关于叶型的研究以对比单、复叶物种的解剖结构、生理生态等过程的差异为主, 如单叶和复叶物种在叶片解剖结构上的差异(Koch et al., 2018), 在叶片和叶柄等组织结构上的生物量分配(Niinemets, 1998), 在叶片光合、导水率等生理活动上的差异(Yang et al., 2019a; 赵万里等, 2019)等。赵万里等(2019)通过对比分析豆科11个复叶树种和6个单叶树种, 指出复叶树种的正午枝条水势和气孔导度降低比例均显著高于单叶树种, 即复叶树种能在缺水状态下快速降低气孔导度, 以降低枝条气穴化风险。单、复叶与其他叶性状(如叶柄长度、叶面积、比叶面积和元素含量等)的关系也是研究的热点(Niinemets, 1998; Warman et al., 2011; Wu et al., 2019)。与单叶相比, 复叶有相对较高的水分传导率和光合速率, 因此具有较高的资源获取能力和相对生长速率(Wu et al., 2019; Yang et al., 2019a)。此外, 有研究指出, 复叶在耐阴性和规避植食上与单叶相比没有显著不同(Niinemets, 1998; Warman et al., 2011)。
5 展望
目前, 植物叶片形态数据主要通过三种途径获取, 一是野外直接采样, 二是来自野外或温室受控实验, 三是从公开数据库或者已发表的植物志和其他文献中收集。三种方法各有利弊(Wilf, 1997; Wiemann et al., 1998; Royer et al., 2005; Traiser et al., 2005; Peppe et al., 2011)。前两种方法获取的数据直接、可靠, 且能考虑性状的种间、种内差异, 但耗时费力, 仅限于小区域部分类群的研究, 难以拓展到大尺度。整合数据的方法可用于大尺度的研究, 数据获取性强, 但无法考察种内的形态变异(Wolfe et al., 1999)。此外, 整合数据受数据库中数据质量和覆盖度的影响, 需要考虑不同来源的数据在测量方法上的差异、对类群和区域的采样偏好以及数据库间重复收录等问题。
目前在线公开的植物性状数据库中, TRY植物性状数据库(TRY Plant Trait Database,
除上文提到的叶缘分析, 目前叶形态的研究方法还有CLAMP (Wolfe, 1995)、数字叶相分析(Digital Leaf Physiognomy, DLP)(Huff et al., 2003; Royer et al., 2005)等。三者的对比性研究较多(Wilf, 1997; Wiemann et al., 1998; Royer et al., 2005; Peppe et al., 2011)。CLAMP是多变量分析方法, 分析多个(一般为29或31个)叶形态分类变量与气候变量间的关系。CLAMP已被广泛用于区域尺度的叶大小和叶形态研究(Jacobs, 1999; Traiser et al., 2005; Xia et al., 2009)。DLP则是一种新电子图像方法, 更多地依赖于电脑运算, 多选择连续的变量(Peppe et al., 2011)。当下图像数字化技术的发展将推动CLAMP和DLP方法的进步。
植物叶片的形态变化和空间分布是环境过滤和生物互作共同作用的结果, 反映了植物对生境的适应。目前的研究多聚焦在局域尺度下特定的类群, 分析叶形态的空间分布及其与环境间的关系, 并推测叶性状分布的成因及其生态学意义, 探索植物如何响应环境变化, 对生态系统功能有何影响。未来主要的研究方向如下:
(1)考虑叶形态的可塑性, 获得覆盖类群和区域无偏的性状数据是重中之重。一方面, 整合现有的性状数据资源, 规范、简约化性状共享平台(Gallagher et al., 2020); 另一方面, 评估性状缺失的类群和区域分布, 进行野外采样或者用统计方法对性状进行填补(gap filling)。近年来遥感技术的发展, 尤其是高光谱遥感技术, 可大批量获得高分辨率的连续性状数据(Jetz et al., 2016)。已有研究尝试联合成像光谱和激光雷达等技术探索区域植被的性状变化(Durán et al., 2019; Ma et al., 2019)。技术的难点之一是如何从成像光谱中获取目标性状的分布。机器学习方法的发展, 包括随机森林、神经网络和贝叶斯分类器等, 为建立地面植物观测点和高光谱间的关系模型提供了方法支持。
(3)从叶片到个体再到生态系统, 探索不同尺度上叶性状对环境变化的响应及其与生态系统功能间的关系是研究热点。虽然叶形态间关系及其与环境间关系的研究很多, 但因研究对象或研究尺度的不同, 所得结论不尽相同(Messier et al., 2017)。如何减少研究尺度的影响以及尺度推绎是以后研究的关键。不同叶性状对生态系统功能的作用是否具有尺度一致性以及各叶性状在不同尺度上的相对贡献如何还有待进一步探讨。
参考文献
Genotypic and phenotypic variation as stress adaptations in temperate tree species: a review of several case studies
Species that occupy large geographic ranges or a variety of habitats within a limited area deal with contrasting environmental conditions by genotypic and phenotypic variation. My students and I have studied these forms of ecophysiological variation in temperate tree species in eastern North America by means of a series of field and greenhouse experiments, including controlled studies with Cercis canadensis L., Fraxinus pennsylvanica Marsh., Acer rubrum L., Prunus serotina Ehrh. and Quercus rubra L., in relation to drought stress. These studies have included measurements of gas exchange, tissue water relations and leaf morphology, and have identified genotypic variation at the biome and individual community levels. Xeric genotypes generally had higher net photosynthesis and leaf conductance and lower osmotic and water potentials at incipient wilting than mesic genotypes during drought. Xeric genotypes also produced leaves with greater thickness, leaf mass per area and stomatal density and smaller area than the mesic genotypes, suggesting general coordination among leaf morphology, gas exchange and tissue water relations. Leaf phenotypic plasticity to different light environments occurred in virtually every study species, which represented a wide array of ecological tolerances. In a study of interactions of genotypes with environment, shade plants, but not sun plants, exhibited osmotic adjustment during drought and shade plants had smaller reductions in photosynthesis with decreasing leaf water potential. In that study, sun, but not shade, plants had significant genotypic differences in leaf structure, but with certain variables phenotypic variation exceeded genotype variation. Thus, genotypic variation was not expressed in all phenotypes, and phenotypes responded differentially to stress. Overall, these studies indicate the importance of genotypic and phenotypic variation as stress adaptations in temperate tree species among both distant and nearby sites of contrasting environmental conditions.
Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California chaparral
Small leaves and low specific leaf area (SLA) have long been viewed as adaptations to Mediterranean-type climates in many species of evergreen woody plants. However, paleobotanical and floristic evidence suggests that in many cases these traits originated prior to the advent of the summer-drought climate regime. In this study, molecular phylogenies and ancestral state reconstructions were used to test the hypothesis of adaptive leaf evolution in 12 lineages of evergreen shrubs in the California chaparral. Across all lineages there was a small but significant shift toward lower SLA, but there were no trends in leaf size evolution. For individual lineages, adaptive changes were detected in only three cases for SLA and in one case for leaf size. Three of these cases of evolutionary change were observed in taxa derived from cool temperate ancestors (e.g., Heteromeles). In contrast, most lineages originating from subtropical ancestors exhibited relative stasis in leaf trait evolution (e.g., Ceanothus). The absence of change suggests that ancestors of chaparral taxa had already acquired appropriate traits that contributed to their success under Mediterranean-type climates. These results illustrate how biogeographic history may influence patterns of trait evolution and adaptation and highlight the contribution of ecological sorting processes to the assembly and functional ecology of regional biotas.
The evolution of plant ecophysiological traits: recent advances and future directions
DOI:10.1641/0006-3568(2000)050[0979:TEOPET]2.0.CO;2 URL [本文引用: 1]
Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses
DOI:10.1007/s004420100805
PMID:28547053
[本文引用: 5]

We examined variation in leaf size and specific leaf area (SLA) in relation to the distribution of 22 chaparral shrub species on small-scale gradients of aspect and elevation. Potential incident solar radiation (insolation) was estimated from a geographic information system to quantify microclimate affinities of these species across north- and south-facing slopes. At the community level, leaf size and SLA both declined with increasing insolation, based on average trait values for the species found in plots along the gradient. However, leaf size and SLA were not significantly correlated across species, suggesting that these two traits are decoupled and associated with different aspects of performance along this environmental gradient. For individual species, SLA was negatively correlated with species distributions along the insolation gradient, and was significantly lower in evergreen versus deciduous species. Leaf size exhibited a negative but non-significant trend in relation to insolation distribution of individual species. At the community level, variance in leaf size increased with increasing insolation. For individual species, there was a greater range of leaf size on south-facing slopes, while there was an absence of small-leaved species on north-facing slopes. These results demonstrate that analyses of plant functional traits along environmental gradients based on community level averages may obscure important aspects of trait variation and distribution among the constituent species.
Leaf margins and temperature in the North American flora: recalibrating the paleoclimatic thermometer
DOI:10.1016/j.gloplacha.2007.07.001 URL [本文引用: 10]
Molecular and hormonal regulation of leaf morphogenesis in Arabidopsis
DOI:10.3390/ijms21145132 URL [本文引用: 1]
A botanical index or cretaceous and tertiary climate
The climatic distribution of certain types of Angiosperm leaves
DOI:10.1002/j.1537-2197.1916.tb05397.x URL [本文引用: 2]
The ecological significance of lobed and toothed leaves in temperate forest trees
Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies
DOI:10.1666/0094-8373(2005)031<0117:POSACI>2.0.CO;2 URL [本文引用: 1]
Seeing the forest with the leaves-Clues to canopy placement from leaf fossil size and venation characteristics
DOI:10.1111/j.1472-4669.2008.00176.x
PMID:19207570
[本文引用: 1]

Although a variety of leaf characteristics appear to be induced by light environment during development, analysis of ontogenetic changes in living broad leaved trees has suggested that a number of other traits also lumped into the classic 'sun' versus 'shade' morphological distinctions, including leaf size, shape, and vein density, are instead controlled largely by local hydraulic environment within the tree canopy. The regularity in how these traits vary with canopy placement suggests a method for addressing a classic paleobotanical quandary: the stature of the source plant - from herb or shrub to canopy tree - is typically unknown for leaf fossils. The study of Ginkgo here complements previous work on Quercus that indicated that leaves throughout the crown are identical in size and venation at the time of bud break and that morphological adaptation to the local microenvironment takes place largely during the expansion phase after the determination of the vascular architecture is complete. Hence, variation in vein density does not reflect differential vein production so much as the distortion of similar vein networks over different final surface areas driven by variation in local hydraulic supply during expansion. Unlike the diffusely growing leaves of the angiosperm, Quercus, the marginally growing leaves of Ginkgo do show some potential for differential vein production, but expansion effects still dominate. The approach suggested here may prove useful for assessing the likelihood that two distinct fossil morphospecies actually represent leaves of the same plant and to gather information concerning canopy structure from disarticulated leaves.
Herbivory and the evolution of leaf size and shape
Fine veins of dicotyledon leaves as sites for enrichment of solutes of the xylem sap.
DOI:10.1111/j.1469-8137.1990.tb00478.x
PMID:33874287
[本文引用: 2]

The apoplastic tracer sulphorhodamine G (SR) was used as an indicator of the flumes, the sites where water left the apoplast and entered the symplast, in a selection of dicotyledon leaves. At these flumes the dye is deposited as crystals after a pulse of dye is fed to the transpiration stream, followed or not by a water chase. In contrast to wheat, the dicotyledons showed SR cystals inside the tracheary elements of the finest leaf veins. At short pulse times the crystals were in the stems of the branch-trees of the fine veins, but after longer pulses, had moved to the vein termini. The dye solution was moving very slowly in the tracheary elements as it approached the ends of the branch-trees, since the axial flow there is nearly balanced by radial leakage. These results are interpreted as evidence that most of the transpiration water enters the symplast in the vein sheaths of the fine veins, and that these veins are places where many of the natural solutes of the xylem sap will be enriched to quite high concentrations.
Evolution of suites of traits in response to environmental stress
Effects of foliage clumping on the estimation of global terrestrial gross primary productivity
DOI:10.1029/ 2010GB003996 [本文引用: 1]
Large-scale dataset from China gives new insights into leaf margin-temperature relationships
DOI:10.1016/j.palaeo.2014.03.016 URL [本文引用: 5]
Stomatal anatomy coordinates leaf size with Rubisco kinetics in the Balearic Limonium
DOI:10.1093/aobpla/plz050 [本文引用: 5]
The durian theory or the origin of the modern tree
DOI:10.1093/oxfordjournals.aob.a083225 URL [本文引用: 1]
A simple model for estimating leaf area of hazelnut from linear measurements
DOI:10.1016/j.scienta.2007.02.006 URL [本文引用: 1]
Variation in leaf size with respect to climate in the tropics of the Western Hemisphere
DOI:10.2307/2484220 URL [本文引用: 6]
Linked canopy, climate, and faunal change in the Cenozoic of Patagonia
DOI:10.1126/science.1260947 URL [本文引用: 1]
Informing trait-based ecology by assessing remotely sensed functional diversity across a broad tropical temperature gradient
DOI:10.1126/sciadv. aaw8114 URL [本文引用: 1]
Light interception efficiency explained by two simple variables: a test using a diversity of small- to medium-sized woody plants
DOI:10.1111/j.1469-8137.2011.03943.x
PMID:22066945
[本文引用: 2]

• Plant light interception efficiency is a crucial determinant of carbon uptake by individual plants and by vegetation. Our aim was to identify whole-plant variables that summarize complex crown architecture, which can be used to predict light interception efficiency. • We gathered the largest database of digitized plants to date (1831 plants of 124 species), and estimated a measure of light interception efficiency with a detailed three-dimensional model. Light interception efficiency was defined as the ratio of the hemispherically averaged displayed to total leaf area. A simple model was developed that uses only two variables, crown density (the ratio of leaf area to total crown surface area) and leaf dispersion (a measure of the degree of aggregation of leaves). • The model explained 85% of variation in the observed light interception efficiency across the digitized plants. Both whole-plant variables varied across species, with differences in leaf dispersion related to leaf size. Within species, light interception efficiency decreased with total leaf number. This was a result of changes in leaf dispersion, while crown density remained constant. • These results provide the basis for a more general understanding of the role of plant architecture in determining the efficiency of light harvesting.© 2011 The Authors. New Phytologist © 2011 New Phytologist Trust.
Spatial, genetic and biotic factors shape within-crown leaf trait variation and herbivore performance in a foundation tree species
DOI:10.1111/fec.v35.1 URL [本文引用: 2]
A general integrative model for scaling plant growth, carbon flux, and functional trait spectra
DOI:10.1038/nature06061 URL [本文引用: 1]
Leaf size and angle vary widely across species: What consequences for light interception
DOI:10.1046/j.1469-8137.2003.00765.x URL [本文引用: 1]
Leaf size variations in a dominant desert shrub, Reaumuria soongarica, adapted to heterogeneous environments
DOI:10.1002/ece3.v10.18 URL [本文引用: 1]
Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll
Shifts in trait-combinations along rainfall and phosphorus gradients
DOI:10.1046/j.1365-2745.2000.00506.x URL [本文引用: 3]
The mechanical diversity of stomata and its significance in gas-exchange control
Given that stomatal movement is ultimately a mechanical process and that stomata are morphologically and mechanically diverse, we explored the influence of stomatal mechanical diversity on leaf gas exchange and considered some of the constraints. Mechanical measurements were conducted on the guard cells of four different species exhibiting different stomatal morphologies, including three variants on the classical "kidney" form and one "dumb-bell" type; this information, together with gas-exchange measurements, was used to model and compare their respective operational characteristics. Based on evidence from scanning electron microscope images of cryo-sectioned leaves that were sampled under full sun and high humidity and from pressure probe measurements of the stomatal aperture versus guard cell turgor relationship at maximum and zero epidermal turgor, it was concluded that maximum stomatal apertures (and maximum leaf diffusive conductance) could not be obtained in at least one of the species (the grass Triticum aestivum) without a substantial reduction in subsidiary cell osmotic (and hence turgor) pressure during stomatal opening to overcome the large mechanical advantage of subsidiary cells. A mechanism for this is proposed, with a corollary being greatly accelerated stomatal opening and closure. Gas-exchange measurements on T. aestivum revealed the capability of very rapid stomatal movements, which may be explained by the unique morphology and mechanics of its dumb-bell-shaped stomata coupled with "see-sawing" of osmotic and turgor pressure between guard and subsidiary cells during stomatal opening or closure. Such properties might underlie the success of grasses.
Bibliometric analysis of the status quo of plant functional traits research based on databases across the Web of Science
基于数据库的植物功能性状研究现状文献计量学分析
Open Science principles for accelerating trait-based science across the Tree of Life
Linking above- and belowground traits to soil and climate variables: an integrated database on China’s grassland species
Ecological aspects of plant morphology: leaf form in relation to environment
Sizes and shapes of liane leaves
DOI:10.1086/283101 URL [本文引用: 2]
Lamina shape variability in species of the genus Ficus L. in different ecological conditions
DOI:10.1134/S1995425514020048 URL [本文引用: 1]
Direct and indirect estimation of leaf area index, fAPAR, and net primary production of terrestrial ecosystems
DOI:10.1016/S0034-4257(99)00056-5 URL [本文引用: 1]
Taphonomic constraints on foliar physiognomic interpretations of late Cretaceous and Tertiary Paleoclimates
DOI:10.1016/0034-6667(92)90161-9 URL [本文引用: 3]
Leaf margin analysis: taphonomic constraints
DOI:10.2110/palo.2004.P04-58 URL [本文引用: 2]
Paleotemperature estimation using leaf-margin analysis: Is Australia different
DOI:10.1669/0883-1351(2004)019<0129:PEULAI>2.0.CO;2 URL [本文引用: 4]
Relationships between leaf morphology and climate, Bolivia: implications for estimating paleoclimate from fossil floras
DOI:10.1666/0094-8373(2000)026<0668:RBLMAC>2.0.CO;2 URL [本文引用: 2]
Leaf morphology shift linked to climate change
DOI:10.1098/rsbl.2012.0458
PMID:22764114
[本文引用: 2]

Climate change is driving adaptive shifts within species, but research on plants has been focused on phenology. Leaf morphology has demonstrated links with climate and varies within species along climate gradients. We predicted that, given within-species variation along a climate gradient, a morphological shift should have occurred over time due to climate change. We tested this prediction, taking advantage of latitudinal and altitudinal variations within the Adelaide Geosyncline region, South Australia, historical herbarium specimens (n = 255) and field sampling (n = 274). Leaf width in the study taxon, Dodonaea viscosa subsp. angustissima, was negatively correlated with latitude regionally, and leaf area was negatively correlated with altitude locally. Analysis of herbarium specimens revealed a 2 mm decrease in leaf width (total range 1-9 mm) over 127 years across the region. The results are consistent with a morphological response to contemporary climate change. We conclude that leaf width is linked to maximum temperature regionally (latitude gradient) and leaf area to minimum temperature locally (altitude gradient). These data indicate a morphological shift consistent with a direct response to climate change and could inform provenance selection for restoration with further investigation of the genetic basis and adaptive significance of observed variation.
A molecular framework underlying the compound leaf pattern of Medicago truncatula
DOI:10.1038/s41477-020-0642-2 URL [本文引用: 1]
Variation in leaf anatomical traits from tropical to cold-temperate forests and linkage to ecosystem functions
DOI:10.1111/fec.2018.32.issue-1 URL [本文引用: 1]
Are endemics functionally distinct? Leaf traits of native and exotic woody species in a New Zealand forest
DOI:10.1371/journal.pone.0196746 URL [本文引用: 2]
Classification of the architecture of dicotyledonous leaves
Leaf shape deters plant processing by an herbivorous weevil
DOI:10.1038/s41477-019-0505-x
PMID:31477889
[本文引用: 1]

The shapes of plant leaves are remarkably diverse, but their ecological functions are largely unknown. Reports on the effects of leaf shape on biotic interactions such as herbivory are especially scarce, partly because herbivorous insects rarely rely on leaf shape for host selection. Here, we show that leaf shape acts as a physical deterrent against a leaf-processing herbivore. Plants in the genus Isodon (Lamiaceae) host a specialized leaf-rolling weevil (Apoderus praecellens) whose ovipositing females process an entire leaf into a leaf roll to serve as larval food and shelter. Among the species of Isodon, I. umbrosus var. hakusanensis is exceptional in that it has deeply lobed leaves. Because leaf processing follows a consistent sequence of complex behaviours, the unusual shape of I. umbrosus leaves may disrupt this process. Under both natural and laboratory conditions, female weevils preferred I. trichocarpus, a close relative with non-lobed leaves, over I. umbrosus. Nutritional properties of the leaves do not explain this preference because weevil larvae developed equally well on both hosts. Modifying the non-lobed I. trichocarpus leaves to mimic the shape of I. umbrosus leaves also discouraged leaf processing. Leaf processing often terminated because weevils failed to complete the inspection routine on I. umbrosus leaves. Leaf shape may be an important but overlooked factor that affects the interactions between plants and leaf-processing herbivores.
Leaf shape: genetic controls and environmental factors
In recent years, many genes have been identified that are involved in the developmental processes of leaf morphogenesis. Here, I review the mechanisms of leaf shape control in a model plant, Arabidopsis thaliana, focusing on genes that fulfill special roles in leaf development. The lateral, two-dimensional expansion of leaf blades is highly dependent on the determination of the dorsoventrality of the primordia, a defining characteristic of leaves. Having a determinate fate is also a characteristic feature of leaves and is controlled by many factors. Lateral expansion is not only controlled by general regulators of cell cycling, but also by the multi-level regulation of meristematic activities, e.g., specific control of cell proliferation in the leaf-length direction, in leaf margins and in parenchymatous cells. In collaboration with the polarized control of leaf cell elongation, these redundant and specialized regulating systems for cell cycling in leaf lamina may realize the elegantly smooth, flat structure of leaves. The unified, flat shape of leaves is also dependent on the fine integration of cell proliferation and cell enlargement. Interestingly, while a decrease in the number of cells in leaf primordia can trigger a cell volume increase, an increase in the number of cells does not trigger a cell volume decrease. This phenomenon is termed compensation and suggests the existence of some systems for integration between cell cycling and cell enlargement in leaf primordia via cell-cell communication. The environmental adjustment of leaf expansion to light conditions and gravity is also summarized.
Trade-offs between seed and leaf size (seed-phytomer-leaf theory): functional glue linking regenerative with life history strategies … and taxonomy with ecology
DOI:10.1093/aob/mcx084
PMID:28961937
[本文引用: 1]

While the 'worldwide leaf economics spectrum' (Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature : 821-827) defines mineral nutrient relationships in plants, no unifying functional consensus links size attributes. Here, the focus is upon leaf size, a much-studied plant trait that scales positively with habitat quality and components of plant size. The objective is to show that this wide range of relationships is explicable in terms of a seed-phytomer-leaf (SPL) theoretical model defining leaf size in terms of trade-offs involving the size, growth rate and number of the building blocks (phytomers) of which the young shoot is constructed.Functional data for 2400+ species and English and Spanish vegetation surveys were used to explore interrelationships between leaf area, leaf width, canopy height, seed mass and leaf dry matter content (LDMC).Leaf area was a consistent function of canopy height, LDMC and seed mass. Additionally, size traits are partially uncoupled. First, broad laminas help confer competitive exclusion while morphologically large leaves can, through dissection, be functionally small. Secondly, leaf size scales positively with plant size but many of the largest-leaved species are of medium height with basally supported leaves. Thirdly, photosynthetic stems may represent a functionally viable alternative to 'small seeds + large leaves' in disturbed, fertile habitats and 'large seeds + small leaves' in infertile ones.Although key elements defining the juvenile growth phase remain unmeasured, our results broadly support SPL theory in that phytometer and leaf size are a product of the size of the initial shoot meristem (≅ seed mass) and the duration and quality of juvenile growth. These allometrically constrained traits combine to confer ecological specialization on individual species. Equally, they appear conservatively expressed within major taxa. Thus, 'evolutionary canalization' sensu Stebbins (Stebbins GL. 1974. Flowering plants: evolution above the species level. Cambridge, MA: Belknap Press) is perhaps associated with both seed and leaf development, and major taxa appear routinely specialized with respect to ecologically important size-related traits.© The Author 2017. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com
The underlying basis for the trade-off between leaf size and leafing intensity
DOI:10.1111/fec.2016.30.issue-2 URL [本文引用: 1]
Digital future for paleoclimate estimation from fossil leaves? Preliminary results
DOI:10.1669/0883-1351(2003)018<0266:DFFPEF>2.0.CO;2 URL [本文引用: 1]
Estimation of rainfall variables from leaf characters in tropical Africa
DOI:10.1016/S0031-0182(98)00102-3 URL [本文引用: 6]
Effect of environmental gradients on leaf morphological traits in the Fandoghlo forest region (NW Iran)
DOI:10.3832/ifor3391-013 URL [本文引用: 1]
Monitoring plant functional diversity from space
DOI:10.1038/nplants.2016.24 URL [本文引用: 1]
Sdudy on digital classification of plants based on characteristics of leaf shape and leaf vein
基于叶形和叶脉特征的植物数字化分类研究
Uncertainty in palaeoclimatic reconstructions based on leaf physiognomy
DOI:10.1071/BT96035 URL [本文引用: 3]
A critical framework for the assessment of biological palaeoproxies: predicting past climate and levels of atmospheric CO2 from fossil leaves
DOI:10.1111/nph.2011.192.issue-1 URL [本文引用: 2]
TRY-A global database of plant traits
How are leaf mechanical properties and water-use traits coordinated by vein traits? A case study in Fagaceae
DOI:10.1111/fec.2016.30.issue-4 URL [本文引用: 1]
The leaf size/number trade-off in trees
DOI:10.1111/jec.2007.95.issue-2 URL [本文引用: 1]
Are compound leaves more complex than simple ones? A multi-scale analysis
DOI:10.1093/aob/mcy116 URL [本文引用: 1]
Mean annual temperature estimation based on leaf morphology: a test from tropical South America
The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions
Elevational trends in leaf size of Campylotropis polyantha in the arid Minjiang River valley, SW China
DOI:10.1016/j.jaridenv.2014.04.011 URL [本文引用: 2]
Development of plant leaf margin: advances in research
植物叶缘锯齿发育的研究进展
Leaf size of woody dicots predicts ecosystem primary productivity
DOI:10.1111/ele.v23.6 URL [本文引用: 9]
Leaf margin analysis of Chinese woody plants and the constraints on its application to palaeoclimatic reconstruction
DOI:10.1111/geb.2016.25.issue-12 URL [本文引用: 4]
Spatiotemporal variation in leaf size and shape in response to climate
DOI:10.1093/jpe/rtz053 URL [本文引用: 5]
Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history
DOI:10.1371/journal. pone.0015161 URL [本文引用: 2]
Functional traits of submerged macrophytes in eutrophic shallow lakes affect their ecological functions
DOI:10.1016/j.scitotenv.2020.143332 URL [本文引用: 1]
Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes
DOI:10.1002/ece3.v10.15 URL [本文引用: 3]
Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency
DOI:10.1111/nph.2019.223.issue-3 URL [本文引用: 3]
Inferring plant functional diversity from space: the potential of Sentinel-2
DOI:10.1016/j.rse.2019.111368 URL [本文引用: 1]
The bien R package: a tool to access the Botanical Information and Ecology Network (BIEN) database
Are compound leaves an adaptation to seasonal drought or to rapid growth? Evidence from the Amazon rain forest
DOI:10.1111/geb.2010.19.issue-6 URL [本文引用: 3]
Spatial trends in leaf size of Amazonian rainforest trees
DOI:10.5194/bg-6-1563-2009 URL [本文引用: 3]
Invasive species differ in key functional traits from native and non- invasive alien plant species
DOI:10.1111/jvs.12772
[本文引用: 1]

Questions Invasive species establish either by possessing traits, or trait trade-offs similar to native species, suggesting pre-adaptation to local conditions; or by having a different suite of traits and trait trade-offs, which allow them to occupy unfilled niches. The trait differences between invasives and non-invasives can inform on which traits confer invasibility. Here, we ask: (a) are invasive species functionally different or similar to native species? (b) which traits of invasives differ from traits of non-invasive aliens and thus confer invasibility? and (c) do results from the sub-Antarctic region, where this study was conducted, differ from findings from other regions? Location Sub-Antarctic Marion Island. Methods We measured 13 traits of all terrestrial native, invasive and non-invasive alien plant species. Using principal components analysis and phylogenetic generalized least-squares models, we tested for differences in traits between invasive (widespread alien species) and native species. Bivariate trait relationships between invasive and native species were compared using standardized major axis regressions to test for differences in trait trade-offs between the two groups. Second, using the same methods, we compared the traits of invasive species to non-invasive aliens (alien species that have not spread). Results Between invasive and native species, most traits differed, suggesting that the success of invasive species is mediated by being functionally different to native species. Additionally, most bivariate trait relationships differed either in terms of their y-intercept or their position on the axes, highlighting that plants are positioned differently along a spectrum of shared trait trade-offs. Compared to non-invasive aliens, invasive species had lower plant height, smaller leaf area, lower frost tolerance, and higher specific leaf area, suggesting that these traits are associated with invasiveness. The findings for the sub-Antarctic corresponded to those of other regions, except lower plant height which provides a competitive advantage to invaders in the windy sub-Antarctic context. Conclusion Our findings support the expectation that trait complexes of invasive species are predominantly different to those of coexisting native species, and that high resource acquisition and low defence investment are characteristic of invasive plant species.
Leaf-size divergence along rainfall and soil-nutrient gradients: Is the method of size reduction common among clades
DOI:10.1046/j.1365-2435.2003.00698.x URL [本文引用: 3]
Leaf temperature effects on net photosynthesis, dark respiration, and photorespiration of seedlings of black locust families with contrasting growth-rates
DOI:10.1139/x91-224 URL [本文引用: 2]
Trait variation and integration across scales: Is the leaf economic spectrum present at local scales
DOI:10.1111/ecog.02006 URL [本文引用: 1]
The energetic and carbon economic origins of leaf thermoregulation
DOI:10.1038/nplants.2016.129
PMID:27548589
[本文引用: 2]

Leaf thermoregulation has been documented in a handful of studies, but the generality and origins of this pattern are unclear. We suggest that leaf thermoregulation is widespread in both space and time, and originates from the optimization of leaf traits to maximize leaf carbon gain across and within variable environments. Here we use global data for leaf temperatures, traits and photosynthesis to evaluate predictions from a novel theory of thermoregulation that synthesizes energy budget and carbon economics theories. Our results reveal that variation in leaf temperatures and physiological performance are tightly linked to leaf traits and carbon economics. The theory, parameterized with global averaged leaf traits and microclimate, predicts a moderate level of leaf thermoregulation across a broad air temperature gradient. These predictions are supported by independent data for diverse taxa spanning a global air temperature range of ∼60 °C. Moreover, our theory predicts that net carbon assimilation can be maximized by means of a trade-off between leaf thermal stability and photosynthetic stability. This prediction is supported by globally distributed data for leaf thermal and photosynthetic traits. Our results demonstrate that the temperatures of plant tissues, and not just air, are vital to developing more accurate Earth system models.
The scaling of leaf area and mass: the cost of light interception increases with leaf size
DOI:10.1098/rspb.2007.0417 URL [本文引用: 4]
Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude
DOI:10.1093/aob/mcq261 URL [本文引用: 1]
Which is a better predictor of plant traits: temperature or precipitation
DOI:10.1111/jvs.12190 URL [本文引用: 2]
When smaller is better: leaf hydraulic conductance and drought vulnerability correlate to leaf size and venation density across four Coffea arabica genotypes
DOI:10.1071/FP13302
PMID:32481050
[本文引用: 1]

Leaf hydraulic conductance (Kleaf) and drought vulnerability in terms of leaf water potential inducing 50% loss of Kleaf (P50), were assessed in four genotypes of Coffea arabica L. We tested three hypotheses: (1) leaf P50 is lower in small leaves with higher vein densities; (2) lower P50 translates into lower Kleaf, limiting gas exchange rates and higher leaf mass per unit area (LMA); (3) P50 values are coordinated with symplastic drought tolerance. We found partial support for Hypotheses 1 and 3, but not for Hypothesis 2. Significant correlations existed among leaf size, vein network and drought resistance. Smaller leaves displayed higher major vein density, higher Kleaf and more negative P50. Kleaf was correlated with leaf gas exchange rates. A negative relationship was observed between Kleaf and LMA, whereas P50 was found to be positively correlated with LMA. Across coffee genotypes, reduced leaf surface area and increased vein density shifts P50 towards more negative values while not translating into higher LMA or lower Kleaf. Breeding crop varieties for both increased safety of the leaf hydraulic system towards drought-induced dysfunction and high gas exchange rates per unit of leaf area is probably a feasible target for future adaptation of crops to climate change scenarios.
The evolution and functional significance of leaf shape in the angiosperms
DOI:10.1071/FP11057 URL [本文引用: 4]
Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs
DOI:10.1023/A:1009773704558 URL [本文引用: 3]
Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs
DOI:10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2 URL [本文引用: 1]
Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy
Broad scaling relationships between leaf size and function do not take into account that leaves of different size may contain different fractions of support in petiole and mid-rib.The fractions of leaf biomass in petiole, mid-rib and lamina, and the differences in chemistry and structure among mid-ribs, petioles and laminas were investigated in 122 species of contrasting leaf size, life form and climatic distribution to determine the extent to which differences in support modify whole-lamina and whole-leaf structural and chemical characteristics, and the extent to which size-dependent support investments are affected by plant life form and site climate.For the entire data set, leaf fresh mass varied over five orders of magnitude. The percentage of dry mass in mid-rib increased strongly with lamina size, reaching more than 40 % in the largest laminas. The whole-leaf percentage of mid-rib and petiole increased with leaf size, and the overall support investment was more than 60 % in the largest leaves. Fractional support investments were generally larger in herbaceous than in woody species and tended to be lower in Mediterranean than in cool temperate and tropical plants. Mid-ribs and petioles had lower N and C percentages, and lower dry to fresh mass ratio, but greater density (mass per unit volume) than laminas. N percentage of lamina without mid-rib was up to 40 % higher in the largest leaves than the total-lamina (lamina and mid-rib) N percentage, and up to 60 % higher than whole-leaf N percentage, while lamina density calculated without mid-rib was up to 80 % less than that with the mid-rib. For all leaf compartments, N percentage was negatively associated with density and dry to fresh mass ratio, while C percentage was positively linked to these characteristics, reflecting the overall inverse scaling between structural and physiological characteristics. However, the correlations between N and C percentages and structural characteristics differed among mid-ribs, petioles and laminas, implying that the mass-weighted average leaf N and C percentage, density, and dry to fresh mass ratio can have different functional values depending on the importance of within-leaf support investments.These data demonstrate that variation in leaf size is associated with major changes in within-leaf support investments and in large modifications in integrated leaf chemical and structural characteristics. These size-dependent alterations can importantly affect general leaf structure vs. function scaling relationships. These data further demonstrate important life-form effects on and climatic differentiation in foliage support costs.
Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants
The implications of extensive variation in leaf size for biomass distribution between physiological and support tissues and for overall leaf physiological activity are poorly understood. Here, we tested the hypotheses that increases in leaf size result in enhanced whole-plant support investments, especially in compound-leaved species, and that accumulation of support tissues reduces average leaf nitrogen (N) content per unit dry mass (N(M)), a proxy for photosynthetic capacity. Leaf biomass partitioning among the lamina, mid-rib and petiole, and whole-plant investments in leaf support (within-leaf and stem) were studied in 33 simple-leaved and 11 compound-leaved species. Support investments in mid-ribs and petioles increased with leaf size similarly in simple leaves and leaflets of compound leaves, but the overall support mass fraction within leaves was larger in compound-leaved species as a result of prominent rachises. Within-leaf and within-plant support mass investments were negatively correlated. Therefore, the total plant support fraction was independent of leaf size and lamina dissection. Because of the lower N(M) of support biomass, the difference in N(M) between the entire leaf and the photosynthetic lamina increased with leaf size. We conclude that whole-plant support costs are weakly size-dependent, but accumulation of support structures within the leaf decreases whole-leaf average N(M), potentially reducing the integrated photosynthetic activity of larger leaves.
Light capture efficiency decreases with increasing tree age and size in the southern hemisphere gymnosperm Agathis australis
DOI:10.1007/s00468-004-0379-y URL [本文引用: 2]
“Diminishing returns” in the scaling of functional leaf traits across and within species groups
More than 5,000 measurements from 1,943 plant species were used to explore the scaling relationships among the foliar surface area and the dry, water, and nitrogen/phosphorus mass of mature individual leaves. Although they differed statistically, the exponents for the relationships among these variables were numerically similar among six species groups (ferns, graminoids, forbs, shrubs, trees, and vines) and within 19 individual species. In general, at least one among the many scaling exponents was <1.0, such that increases in one or more features influencing foliar function (e.g., surface area or living leaf mass) failed to keep pace with increases in mature leaf size. Thus, a general set of scaling relationships exists that negatively affects increases in leaf size. We argue that this set reflects a fundamental property of all plants and helps to explain why annual growth fails to keep pace with increases in total body mass across species.
Optimum leaf size predicted by a novel leaf energy balance model incorporating dependencies of photosynthesis on light and temperature
DOI:10.1007/s11284-011-0905-5 URL [本文引用: 9]
A global study of relationships between leaf traits, climate and soil measures of nutrient fertility
DOI:10.1111/geb.2009.18.issue-2 URL [本文引用: 1]
Optimal leaf size in relation to environment
DOI:10.2307/2258359 URL [本文引用: 4]
Stomatal density, leaf area and plant size variation of Rhizophora mangle (Malpighiales:Rhizophoraceae) along a salinity gradient in the Mexican Caribbean
Can leaf shape be represented by the ratio of leaf width to length? Evidence from nine species of Magnolia and Michelia (Magnoliaceae
).DOI:10.3390/f12010041 URL [本文引用: 1]
Biomechanical and leaf-climate relationships: a comparison of ferns and seed plants
DOI:10.3732/ajb.1300220 URL [本文引用: 2]
Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications
DOI:10.1111/nph.2011.190.issue-3 URL [本文引用: 24]
Leaf size and leaf display of thirty-eight tropical tree species
DOI:10.1007/s00442-008-1131-x URL [本文引用: 1]
Foliar venation of angiosperms. II. Histogenesis of the venation of Liriodendron
DOI:10.1002/j.1537-2197.1955.tb11089.x URL [本文引用: 1]
Scaling and structure of dicotyledonous leaf venation networks
DOI:10.1111/ele.2011.15.issue-2 URL [本文引用: 1]
Leaf morphological and stomatal variations in paper birch populations along environmental gradients in Canada
DOI:10.4236/ajps.2014.511166 URL [本文引用: 3]
Key canopy traits drive forest productivity
DOI:10.1098/rspb.2011.2270 URL [本文引用: 1]
The evolution of plant functional variation: traits, spectra, and strategies
Variation and genetic parameters of leaf morphological traits of eight families from Populus simonii × P. nigra
DOI:10.3390/f11121319 URL [本文引用: 1]
Survey of plant leaf area measurement methods
植物叶面积测量方法综述
Modeling individual leaf area of rose (Rosa hybrida L.) based on leaf length and width measurement
DOI:10.1007/s11099-010-0003-x URL [本文引用: 1]
Ecology of leaf teeth: a multi-site analysis from an Australian subtropical rainforest
DOI:10.3732/ajb.0800282 URL [本文引用: 4]
Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum
DOI:10.1371/journal.pone.0007653 URL [本文引用: 2]
Roles of climate and functional traits in controlling toothed vs. untoothed leaf margins
DOI:10.3732/ajb.1100428
PMID:22494908
[本文引用: 6]

Leaf-margin state (toothed vs. untoothed) forms the basis of several popular methods for reconstructing temperature. Some potential confounding factors have not been investigated with large data sets, limiting our understanding of the adaptive significance of leaf teeth and their reliability to reconstruct paleoclimate. Here we test the strength of correlations between leaf-margin state and deciduousness, leaf thickness, wood type (ring-porous vs. diffuse-porous), height within community, and several leaf economic variables.We assembled a trait database for 3549 species from six continents based on published and original data. The strength of associations between traits was quantified using correlational and principal axes approaches.Toothed species, independent of temperature, are more likely to be deciduous and to have thin leaves, a high leaf nitrogen concentration, a low leaf mass per area, and ring-porous wood. Canopy trees display the highest sensitivity between leaf-margin state and temperature; subcanopy plants, especially herbs, are less sensitive.Our data support hypotheses linking the adaptive significance of teeth to leaf thickness and deciduousness (in addition to temperature). Toothed species associate with the "fast-return" end of the leaf economic spectrum, providing another functional link to thin leaves and the deciduous habit. Accounting for these confounding factors should improve climate estimates from tooth-based methods.
Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy
DOI:10.1086/497995 URL [本文引用: 9]
Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record
DOI:10.3732/ajb.92.7.1141
PMID:21646136
[本文引用: 9]

The sizes and shapes (physiognomy) of fossil leaves are widely applied as proxies for paleoclimatic and paleoecological variables. However, significant improvements to leaf-margin analysis, used for nearly a century to reconstruct mean annual temperature (MAT), have been elusive; also, relationships between physiognomy and many leaf ecological variables have not been quantified. Using the recently developed technique of digital leaf physiognomy, correlations of leaf physiognomy to MAT, leaf mass per area, and nitrogen content are quantified for a set of test sites from North and Central America. Many physiognomic variables correlate significantly with MAT, indicating a coordinated, convergent evolutionary response of fewer teeth, smaller tooth area, and lower degree of blade dissection in warmer environments. In addition, tooth area correlates negatively with leaf mass per area and positively with nitrogen content. Multiple linear regressions based on a subset of variables produce more accurate MAT estimates than leaf-margin analysis (standard errors of ±2 vs. ±3°C); improvements are greatest at sites with shallow water tables that are analogous to many fossil sites. The multivariate regressions remain robust even when based on one leaf per species, and the model most applicable to fossils shows no more signal degradation from leaf fragmentation than leaf-margin analysis.
A common developmental program can produce diverse leaf shapes
DOI:10.1111/nph.14449
PMID:28248421
[本文引用: 1]

Eudicot leaves have astoundingly diverse shapes. The central problem addressed in this paper is the developmental origin of this diversity. To investigate this problem, we propose a computational model of leaf development that generalizes the largely conserved molecular program for the reference plants Arabidopsis thaliana, Cardamine hirsuta and Solanum lycopersicum. The model characterizes leaf development as a product of three interwoven processes: the patterning of serrations, lobes and/or leaflets on the leaf margin; the patterning of the vascular system; and the growth of the leaf blade spanning the main veins. The veins play a significant morphogenetic role as a local determinant of growth directions. We show that small variations of this model can produce diverse leaf shapes, from simple to lobed to compound. It is thus plausible that diverse shapes of eudicot leaves result from small variations of a common developmental program.© 2017 The Authors. New Phytologist © 2017 New Phytologist Trust.
Developmentally based scaling of leaf venation architecture explains global ecological patterns
DOI:10.1038/ ncomms1835 URL [本文引用: 5]
On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora
Trait patterns of epiphytes compared to other plant life-forms along a tropical elevation gradient
DOI:10.1111/fec.2018.32.issue-8 URL
Evolution of leaf form correlates with tropical-temperate transitions in Viburnum (Adoxaceae)
DOI:10.1098/rspb.2012.1110 URL [本文引用: 1]
Water supply and demand remain coordinated during breakdown of the global scaling relationship between leaf size and major vein density
DOI:10.1111/nph.14382
PMID:28005294
[本文引用: 4]

Vein networks that disobey the global scaling of major vein density with leaf size shed light on functional constraints of vein network formation in dicotyledons. Understanding their evolution, distribution and impact on vein-stomata-climate associations is an important contribution to our global view of vein network organization. Based on vein traits of 55 species of pantropical Ochnaceae, stomata and climatic niche data, and a dated molecular phylogeny, we unveil major structural shifts in vein networks through deep time, relationships between leaf size, vein and stomata traits, and their interplay with climate. Dense 2° veins, reduction of minor veins and the associated breakdown of vein-leaf size scaling evolved multiple times independently in Ochnaceae. In spite of the drastic changes in vein architecture in this venation type, vein and stomatal densities remain correlated. Our study demonstrates that shortening the major vein-stomata distance is economically not less advantageous than by increasing minor vein density, as illustrated by the same degree of coordination between vein and stomatal densities and the similar construction costs across networks with dense 2° veins and those with 'normally' spaced 2° veins.© 2016 The Authors. New Phytologist © 2016 New Phytologist Trust.
Leaf boundary layers
DOI:10.1111/j.1469-8137.1993.tb03898.x
PMID:33874584
[本文引用: 3]

Studies of heat and mass exchange between leaves and their local environment are central to our understanding of plant-atmosphere interactions. The transfer across aerodynamic leaf boundary layers is generally described by non-dimensional expressions which reflect largely empirical adaptations of engineering models derived for flat plates. This paper reviews studies on leaves, and leaf models with varying degrees of abstraction, in free and forced convection. It discusses implecations of finding for leaf morphology as it affects - and is affected by - the local microclimate. Predictions of transfer from many leaves in plant communities are complicated by physical and physiological feedback mechanisms between leaves and their environment. Some common approaches, and the current challenge of integrating leaf-atmosphere interactions into models of global relevance, are also briefly addressed. Contents Summary 477 I. Introduction 478 II. Early studies 479 III. The formal description of leaf transfer 480 IV. Effects of turbulence on idealized shapes 484 V. Effects of aspect ratio and inclination 486 VI. Leaves and leaf models in forced convection 491 VII. Leaves and leaf models in mixed and free convection 493 VIII. Interpretation of leaf shape 494 IX. Leaves in plant canopies 499 X. Synopsis and conclusions 502 References 502.
Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture
DOI:10.1104/pp.111.173856 URL [本文引用: 7]
Convergence in leaf size versus twig leaf area scaling: Do plants optimize leaf area partitioning
DOI:10.1093/aob/mcw231 URL [本文引用: 4]
A geographic perspective on the ecology of compound leaves
DOI:10.1111/evo.1981.35.issue-4 URL [本文引用: 2]
Leaf margin analysis: a new equation from humid to mesic forests in China
DOI:10.2110/palo.2009.p09-129r URL [本文引用: 1]
The TIE1 transcriptional repressor links TCP transcription factors with TOPLESS/TOPLESS-RELATED corepressors and modulates leaf development in Arabidopsis
DOI:10.1105/tpc.113.109223 URL [本文引用: 1]
Comparative ecophysiology of leaf and canopy photosynthesis
Leaf morphological and anatomical traits from tropical to temperate coniferous forests: mechanisms and influencing factors
DOI:10.1038/srep19703 URL [本文引用: 1]
Environmental signals from leaves-A physiognomic analysis of European vegetation
DOI:10.1111/nph.2005.166.issue-2 URL [本文引用: 13]
Leaf traits as indicators of resource-use strategy in floras with succulent species
DOI:10.1046/j.1469-8137.2002.00357.x URL [本文引用: 1]
The smaller the leaf is, the faster the leaf water loses in a temperate forest
DOI:10.3389/fpls.2019.00058 URL [本文引用: 2]
The China Plant Trait Database: toward a comprehensive regional compilation of functional traits for land plants
DOI:10.1002/ecy.2091
PMID:29155446
[本文引用: 1]

<空>
Not so simple after all: searching for ecological advantages of compound leaves
DOI:10.1111/more.2011.120.issue-6 URL [本文引用: 2]
A physiognomic classification of Australian rain forests
DOI:10.2307/2257290 URL [本文引用: 1]
Leaf size and leaf consistence of a riverine forest formation along a climatic gradient
DOI:10.1007/BF00344908
PMID:28309876
[本文引用: 4]

The riverine forest formation on the levees along the Orange River in South Africa shows a shift in floristic composition as the river traverses various climatic zones on its course through the temperate area in the eastern parts of the subcontinent, the central semi-desert region, and the desert area near the Atlantic in the west. Leaf size classes and leaf consistency types of the woody species in the riverine forest were determined for each community. Analysis of these data revealed a diversification of leaf sizes as the climate changed from temperate to hot and arid and particularly microphylls became relatively less important and were replaced by smaller leaves in the hot areas. In the same direction malacophylls, which are of the "low-cost, quick-profit" strategy type and are well represented in the temperate, frosty areas, disappear and xeromorphic leaves ("high-cost, slow-profit" strategy type) increase in importance. It is suggested that the cooler conditions with higher wind speeds and higher degrees of average air humidity near the Atlantic coast are reflected by an increase in mesophylls which are sclerophyllous as an adaptation to the frequently and rapidly changing temperature conditions here.
The leaf size/number trade-off in herbaceous angiosperms
DOI:10.1093/jpe/rtp018 URL [本文引用: 1]
Estimation of temperature and precipitation from morphological characters of dicotyledonous leaves
The utility of regression and correspondence models for deducing climate from leaf physiognomy was evaluated by the comparative application of different predictive models to the same three leaf assemblages. Mean annual temperature (MAT), mean annual precipitation (MAP), and growing season precipitation (GSP) were estimated from the morphological characteristics of samples of living leaves from two extant forests and an assemblage of fossil leaves. The extant forests are located near Gainesville, Florida, and in the Florida Keys; the fossils were collected from the Eocene Clarno Nut Beds, Oregon. Simple linear regression (SLR), multiple linear regression (MLR), and canonical correspondence analysis (CCA) were used to estimate temperature and precipitation. The SLR models used only the percentage of species having entire leaf margins as a predictor for MAT and leaf size as a predictor for MAP. The MLR models used from two to six leaf characters as predictors, and the CCA used 31 characters. In comparisons between actual and predicted values for the extant forests, errors in prediction of MAT were 0.6°-5.7°C, and errors in prediction of precipitation were 6-89 cm (=6-66%). At the Gainesville site, seven models underestimated MAT and only one overestimated it, whereas at the Keys site, all eight models overestimated MAT. Precipitation was overestimated by all four models at Gainesville, and by three of them at the Keys. The MAT estimates from the Clarno leaf assemblage ranged from 14.3° to 18.8°C, and the precipitation estimates from 227 to 363 cm for MAP and from 195 to 295 cm for GSP.
When are leaves good thermometers? A new case for leaf margin analysis
DOI:10.1017/S0094837300019746 URL [本文引用: 5]
Using fossil leaves as paleoprecipitation indicators: an Eocene example
DOI:10.1130/0091-7613(1998)026<0203:UFLAPI>2.3.CO;2 URL [本文引用: 4]
Computer vision cracks the leaf code
Light mediates the relationship between community diversity and trait plasticity in functionally and phylogenetically diverse tree mixtures
DOI:10.1111/jec.v108.4 URL [本文引用: 4]
Paleoclimatic estimates from Tertiary leaf assemblages
DOI:10.1146/earth.1995.23.issue-1 URL [本文引用: 2]
Using fossil leaves as paleoprecipitation indicators: an Eocene example: comment and reply
DOI:10.1130/0091-7613(1999)027<0091:UFLAPI>2.3.CO;2 URL [本文引用: 1]
Global climatic drivers of leaf size
DOI:10.1126/science.aal4760
PMID:28860384
[本文引用: 9]

Leaf size varies by over a 100,000-fold among species worldwide. Although 19th-century plant geographers noted that the wet tropics harbor plants with exceptionally large leaves, the latitudinal gradient of leaf size has not been well quantified nor the key climatic drivers convincingly identified. Here, we characterize worldwide patterns in leaf size. Large-leaved species predominate in wet, hot, sunny environments; small-leaved species typify hot, sunny environments only in arid conditions; small leaves are also found in high latitudes and elevations. By modeling the balance of leaf energy inputs and outputs, we show that daytime and nighttime leaf-to-air temperature differences are key to geographic gradients in leaf size. This knowledge can enrich "next-generation" vegetation models in which leaf temperature and water use during photosynthesis play key roles.Copyright © 2017, American Association for the Advancement of Science.
The worldwide leaf economics spectrum
DOI:10.1038/nature02403 URL [本文引用: 1]
Differences in leaf functional traits between simple and compound leaves of Canavalia maritime
DOI:10.15244/pjoes/85946 URL [本文引用: 3]
Quantitative climate reconstructions of the late Miocene Xiaolongtan megaflora from Yunnan, southwest China
DOI:10.1016/j.palaeo.2009.02.024 URL [本文引用: 4]
Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves
DOI:10.1016/j.pnsc.2009.10.001 URL [本文引用: 3]
Size-dependent leaf area ratio in plant twigs: implication for leaf size optimization
DOI:10.1093/aob/mcp262 URL [本文引用: 3]
Compound leaves are associated with high hydraulic conductance and photosynthetic capacity: evidence from trees in Northeast China
DOI:10.1093/treephys/tpy147 URL [本文引用: 2]
Trait-based climate change predictions of vegetation sensitivity and distribution in China
DOI:10.3389/fpls.2019.00908 URL [本文引用: 1]
A novel approach for modelling vegetation distributions and analysing vegetation sensitivity through trait-climate relationships in China
DOI:10.1038/ srep24110 URL [本文引用: 1]
Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region
DOI:10.1111/j.1365-2435.2009.01678.x URL [本文引用: 1]
Trait identity and functional diversity co-drive response of ecosystem productivity to nitrogen enrichment
DOI:10.1111/jec.v107.5 URL [本文引用: 1]
The Arabidopsis RING-Type E3 ligase TEAR1 controls leaf development by targeting the TIE1 transcriptional repressor for degradation
DOI:10.1105/tpc.16.00771 URL [本文引用: 2]
Analysis of photosynthesis-water relationship between simple- and compound-leafed leguminous trees
豆科复叶和单叶树种的光合-水分关系分析
Warming alters plant phylogenetic and functional community structure
DOI:10.1111/jec.v108.6 URL [本文引用: 1]