土壤是陆地生物最主要的储存库, 孕育了地球上极高的生物多样性(Ahanger et al., 2014; Guerra et al., 2021)。传统上认为生态系统的地上及地下部分相互隔离, 随着研究的不断深入, 人们逐渐意识到陆地生态系统的地上部分与地下部分虽蕴藏着自身独特的生物多样性, 但是彼此间也存在紧密联系, 甚至互为驱动力(Hooper et al., 2000; Wardle et al., 2004; van der Heijden et al., 2008)。早在20世纪90年代, Bever (1994)提出了植物-土壤反馈(plant-soil feedback, PSF)的概念(即植物的出现引起了土壤生物因子或非生物因子的改变, 转而影响植物更新和生长), 将土壤生物(如微生物)的影响整合到地上植物群落动态的研究当中。近年来, 越来越多的研究表明土壤微生物(如菌根真菌、病原真菌等)调控着植物的适合度, 对地上植物多样性维持产生或正或负的影响, 进而调控植物群落物种组成和多度分布(Bever, 1999; Mangan et al., 2010b; Anacker et al., 2014; Teste et al., 2017)。
宿主植物的生长状态受土壤病原菌和菌根真菌的共同影响(Turner et al., 2013; Vandenkoornhuyse et al., 2015; Trivedi et al., 2020)。随着植物的出现, 土壤病原菌的积累将抑制植物的生长与存活, 即引起植物-土壤负反馈(van der Putten et al., 1993; Klironomos, 2002; Liu et al., 2012); 菌根真菌通过提升植物对养分的吸收能力以及拮抗病原菌对植物的危害, 促进植物生长, 即产生植物-土壤正反馈(Bever et al., 2012)。自然条件下, 由于土壤病原菌对植物生长的有害影响易被观察到(Liu et al., 2012; Parker et al., 2015; Jia et al., 2020), 而菌根真菌对植物群落的有益影响通常不明显(Saia et al., 2020), 所以在植物多样性的维持中广泛认为土壤病原菌是驱动植物群落动态变化的重要因素(Comita et al., 2010; Mangan et al., 2010b; Bever et al., 2015; Liu & He, 2019)。目前, 菌根真菌及其多样性对植物共存的影响以及菌根真菌在宿主植物多度格局塑造中扮演的角色尚待揭示。
依据解剖学形态特征, 菌根主要包括4种类型: 丛枝菌根(arbuscular mycorrhiza, AM)、外生菌根(ectomycorrhiza, ECM)、杜鹃花科菌根(ericoid mycorrhiza, ErM)及兰科菌根(orchid mycorrhiza, OrM)。其中, AM在植物与土壤微生物的共生关系中最为普遍, 如陆地植物中有大约80%的植物会与AM真菌形成共生关系(Smith & Read, 2008), 且AM真菌贡献了超过10%的土壤微生物数量(Fitter et al., 2011)。从分布范围来看, AM真菌几乎遍布所有大洲及大多数生物区系(Tedersoo et al., 2014; Davison et al., 2015; Powell & Rillig, 2018)。在热带森林中, 与AM真菌共生的植物则更为普遍(Toussaint et al., 2020)。涉及全球365个位点近15 000个土壤样品的微生物测序分析结果显示, 包括AM真菌在内的多数真菌类群的多样性峰值出现在热带(Tedersoo et al., 2014)。植物多样性假说认为, 热带高的植物多样性增加了土壤有机质的复杂性, 产生了更多的生态位空间, 因而可容纳更多样的土壤生物类群(Hooper et al., 2000; Waldrop et al., 2006)。此外, 在热带地区由于氮限制被磷限制所取代, 植物更倾向于选择与对磷元素吸收更有优势的AM真菌共生(Read, 1991)。尽管如此, 直至21世纪初, 对热带、亚热带森林土壤中AM真菌多样性的研究依旧甚少(Alexander & Selosse, 2009), AM真菌多样性对热带、亚热带森林树种多度格局塑造影响的相关研究则更为匮乏。
关于AM真菌的研究始于19世纪30-40年代, 但当时AM真菌的存在并未得到学界广泛的认同。近年来, 随着高通量测序技术的出现与发展, 植物与微生物间复杂的互作关系被慢慢揭开(Turner et al., 2013), 对AM真菌基因组和转录组信息的挖掘成为研究AM真菌与植物共生关系的重要手段。至此, 关于AM真菌的许多猜想得以更准确地验证, 对AM真菌多样性的相关研究迎来新的发展契机。然而目前有关植物-AM真菌互作效应的研究多集中于农田及草地生态系统(van der Heijden et al., 1998b; Montesinos-Navarro et al., 2012; Liang et al., 2017; 杨文莹等, 2019), 热带、亚热带森林生态系统中虽也开展了不少研究, 但因森林树种组成与森林环境的复杂性以及技术上的限制(如AM真菌具有基因组数量大、可变性高的特点, 大量AM真菌基因无法通过现有的生物信息学技术进行注释), 仍有较大的研究空白。本文结合国内外有关AM真菌研究的进展, 整合了AM真菌在宿主植物群落构建、宿主植物共存及维持稀有种等方面的研究现状, 以期从AM真菌与植物互作、AM真菌多样性对植物群落物种共存的影响角度出发, 为验证“稀有种优势”假说提出新的研究思路(该假说认为植物物种在稀有时具有种群增加的趋势, 确保其在群落中与其他物种长期共存; 戴冬等, 2021)。
1 AM真菌与植物群落构建
1.1 生态位理论与物种共存
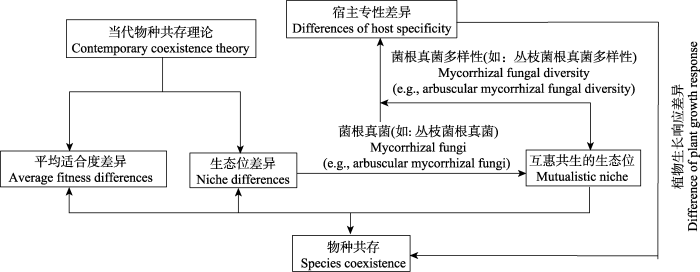
生物多样性决定了陆地生态系统的功能及稳定性(Hooper & Vitousek, 1997; Mi et al., 2021), 关注生物多样性本质上就是关注多物种间的稳定共存(Chesson, 2000)。Grinnell (1917)首次用生态位的概念来描述物种的生存范围及条件, 认为同一地点的两个物种必须存在资源利用上或时间、空间上的生态位分化才能和谐共存, 即经典的生态位理论。除了生态位分化, 当代物种共存理论还强调了竞争能力上的差异, 即平均适合度差异对多样性维持的必要性(Chesson, 2000)。生态位理论的提出为理解控制物种分布的因素及预测种间相互作用(如竞争、捕食、互利共生等)的结果提供了理论基石(Connell, 1971; Chesson, 2000)。植物与菌根真菌共生能拓宽植物及菌根真菌的生态位(Bever et al., 2010), 当存在资源限制时, 菌根的存在提高了宿主植物的适合度(Dybzinski & Tilman, 2007; Rúa et al., 2016)。资源竞争理论(resource competition theory)对有限资源最低需求量(R*)的法则认为, 当多个物种的生长同时受到同一资源的限制时, 对这一限制资源需求量最低的物种能够在竞争中取胜, 而菌根真菌能通过改变土壤中资源的可利用性间接影响植物间的竞争作用(Hodge & Fitter, 2013), 或者通过资源分化扩展生态位空间, 从而在宿主植物生态位的形成中发挥着重要作用(Chase & Leibold, 2004)。Peay等(2016)基于Chase和Leibold (2004)的当代生态位理论(contemporary niche theory, CNT)提出了互惠共生的生态位概念, 并对此做了进一步阐述, 植物与菌根真菌共生能够降低植物对有限资源的最低需求量, 且当土壤中存在多种功能互补的菌根真菌时, 宿主植物通过菌根吸收多种形式的土壤养分, 扩展了植物的需求生态位(requirement niche, 指维持某一特定种群所必需的最低环境条件), 降低植物生态位的重合度, 从而促进物种间的稳定共存。
1.2 AM真菌与物种共存
近几十年来研究愈加深入的Janzen-Connell假说(即J-C效应: 同种成年个体或者同种聚集吸引大量天敌, 导致接近母树的种子和幼苗或者高密度地区的种子和幼苗具有相对高的死亡率的现象) (Janzen, 1970; Connell, 1971; Song et al., 2021)以及植物-土壤负反馈理论(Bever et al., 1997; Bennett & Cahill Jr, 2016; Crawford et al., 2019)是植物物种多样性维持的主流观点。与不同类型菌根共生的植物所受到的植物-土壤反馈强度的差异也会影响局域地区多样性的维持(Bennett et al., 2017; Kadowaki et al., 2018)。由于AM真菌无法在植物根系附近形成菌根鞘, 对病原菌的防御能力有限, 因此相比于ECM树种, AM树种根系周围会积累相对较多的病原菌, 抑制了同种幼苗在其母树周围的生长, 利于该地区植物多样性的维持(Bennett et al., 2017; Johnson et al., 2018; Kadowaki et al., 2018)。除了菌根类型, 植物根系的菌根侵染程度也会影响宿主植物受到的植物-土壤反馈强度, 生长快、竞争能力强的植物其根系AM真菌侵染率较低, 对病原菌的抵御能力有限, 因此会受到更强的植物-土壤负反馈(Moora & Zobel, 2010)。此外, AM植物的菌根及地下菌丝网络能促进弱势竞争对手的生长, 降低了弱势植物被竞争排除的风险, 从而在局域尺度上调节植物共存和多样性, 塑造了植物种群与群落(Tedersoo et al., 2020)。Moora和Zobel (2010)通过一项综合了许多研究的meta分析发现AM真菌能降低植物种间竞争, 尤其当成年植株与幼苗间产生相互作用时, AM真菌通过菌丝网络将从成年树上获取的碳转运给幼苗, 促进其生长。以上研究表明, AM真菌在促进植物物种共存方面的作用同样不容忽视(Grime et al., 1987; Gange et al., 1993)。
由于AM真菌的广布性, 且与植物共生的AM真菌能够减轻宿主植物根系被致病真菌侵染的程度(Newsham et al., 1995), 即减轻植物受到的植物-土壤负反馈, 以往的研究普遍认为AM真菌会对植物施加泛性的正效应(AM真菌不具有或具有低的宿主专性(Law, 1988; Fitter, 1990)且产生正反馈), 从而不利于物种共存(Bever, 2003)。然而, 越来越多的研究表明, 在不同的生境条件下(Hood et al., 2004)、不同的宿主植物中(Kiers et al., 2000), 乃至同一宿主植物的根内或根际土壤中的AM真菌多样性均有显著差异(Herre et al., 2005)。Bever (2002a)将从美国北卡罗来纳州草原分离出的8个常见AM真菌物种分别接种于4个共存植物根部, 结果显示AM真菌与宿主植物的亲和力及AM真菌的产孢率存在明显的宿主依赖性; 也有研究在温带草原、亚热带森林均发现AM真菌存在一定程度的宿主专性(Montesinos- Navarro et al., 2012; Chen et al., 2017)。在植物的不同生长阶段, 由AM真菌调控的植物-土壤反馈也可能发生由正到负的转变, 植物的获益程度往往取决于与其存在相互作用的AM真菌(Johnson et al., 1997; Kiers et al., 2000; Klironomos, 2003)。需要注意的是, AM真菌与宿主植物间由资源交换的不均衡或环境条件改变所致的寄生关系往往不稳定, 相比于刚建立起合作关系的菌根, 已建立长期关系的植物和菌根真菌间更倾向于进化成互惠合作的关系(Johnson et al., 1997; Rúa et al., 2016)。最新的研究却指出与本地植物相比, 非本地植物从AM真菌处获得的生长益处更显著, 即AM真菌加剧了生物入侵(Sheng et al., 2022), 因此在关注植物与AM真菌互作效应时, 除了探讨植物自身因素的影响外, 综合考虑多方因素的潜在影响也尤为重要。Klironomos (2003)认为不同宿主植物对接种的AM真菌较高的偏好性差异可能是影响当地植物多样性维持的关键因素。具体表现在: 植物的生长状态会随着土壤中AM真菌群落物种组成的改变而改变, 宿主植物与AM真菌间不对称的共生关系促进了两个竞争物种间的共存(Bever, 1999, 2002b, 2003)。
因此, AM真菌虽与绝大多数植物共生, 但是其功能的发挥存在环境依赖性及宿主依赖性, 且AM真菌对植物受到的植物-土壤反馈的方向及强度有调节作用, 进而影响着树种间的共存(图1)。AM真菌群落内, 不同的AM真菌在植物根系的定植部位不同(Smith et al., 2000)、养分吸收策略不同(Koide, 2000)以及占据的土壤养分分配的生态位的分化差异降低了AM真菌间的竞争, 促进了AM真菌群落内物种共存(Tedersoo et al., 2020), 塑造了AM真菌的多样群落, 进而影响地上植物群落结构。AM真菌对群落动态的影响毋庸置疑, 但是如何影响及其影响程度正是目前需要深入探讨的问题。
图1
图1
基于当代物种共存理论, 由菌根真菌介导的物种共存理论框架。
Fig. 1
Theoretical framework of species coexistence mediated by mycorrhizal fungi based on contemporary species coexistence theory.
2 AM真菌多样性与植物群落结构
影响植物群落结构(如: 植物多样性、植物的空间分布及多度差异等)的因素主要包括植物种间和种内(Singh & Baruah, 2021)、植物-食草动物(Huisman & Olff, 1998)以及植物-病原菌间的相互作用等(Liu et al., 2012, 2016; 皮磊等, 2018), 而AM真菌及其多样性也会对植物群落结构、多样性和生产力产生影响(Smith & Read, 2008; Mangan et al., 2010a)。但是, 现有的涉及AM真菌多样性的研究多着眼于不同生态系统中AM真菌多样性的差异(Davison et al., 2015; Shi et al., 2019; Vieira et al., 2019), 而非植物对AM真菌多样性的响应差异上, 这很容易掩盖AM真菌多样性对植物群落构建的重要作用。事实上, 同一地区的植物对单一接种及混合接种AM真菌的响应存在很大差异, 且相比于单一接种, 混合接种可使植物获取的效益最大化(van der Heijden et al., 1998a; Rowe et al., 2007), 因此, 亟需将AM真菌多样性纳入对植物群落结构影响的考量中。
早在20世纪末, van der Heijden等(1998b)就提出当宿主植物对接种的AM真菌物种或群落存在生长响应差异时, 这些AM真菌物种及其群落组成也能影响到植物的群落结构。群落水平上植物-植物, 植物-AM真菌相互作用的分析结果显示, 随着根际AM真菌多样性的增加, 存在专性相互作用的植物(某种植物的成年树能促进另一植物的幼苗生长)能相互促进共存, 进而影响植物群落结构(Montesinos- Navarro et al., 2012)。而在退化生态系统的植被及土壤的恢复与重建过程中, AM真菌更是发挥着群落构建者的作用, 维持了植物多样性及群落稳定性(杨宏宇等, 2005; Rowe et al., 2007; 钟思远等, 2017)。近期对美国8 200个森林样地的分析结果显示, 存在单一类型菌根(AM或ECM)的森林展现出较低的植物多样性, 多种菌根类型形成的菌根优势更利于植物群落多样性的维持(Carteron et al., 2022), 该研究弱化了单一菌根类型对植物多样性影响的同时, 却也强调了菌根多样性对植物多样性维持的重要意义。而由于条件限制, 某些研究较少区域的AM真菌多样性可能被严重低估(Kivlin et al., 2011; Öpik et al., 2013), 这势必会影响植物群落结构对AM真菌多样性响应的评估结果。AM真菌无处不在, 那么在热带、亚热带森林生态系统中到底有多少种类的AM真菌在发挥作用? 这些AM真菌功能的发挥是相互补充还是相互叠加(Edathil et al., 1996; Jansa et al., 2008)? AM真菌多样性在植物多样性的维持中究竟发挥何种作用(Hooper et al., 2000; Toussaint et al., 2020)? 如此多样化的AM真菌在物种多度格局的形成与塑造中扮演何种角色? 这些问题都亟需回答。
2.1 AM真菌多样性对森林树种多度的影响
那么AM真菌多样性在森林树种多度格局的塑造中究竟发挥着怎样的作用? 亚热带森林中专性病原真菌导致的同种负密度制约驱动了森林多物种间的共存, 同样具有一定程度宿主专性的AM真菌却能降低甚至抵消病原菌在该过程中的部分不利影响(Liang et al., 2015), 由AM真菌形成的菌根网络更是对植物幼苗密度制约的存活与生长产生促进作用, 从而利于局域地区植物物种多度的增加(Liang et al., 2021), 后者更是突出了AM真菌多样性对森林树种多度维持的积极影响。温带森林中对AM树种及ECM树种在同种树、异种树影响下幼苗及幼树的存活情况的监测结果显示, AM植物幼苗及幼树的多度与CNDD的强度呈正相关关系(Jiang et al., 2020)。这表明植物结合的菌根类型及菌根多样性能够不同程度地影响植物受到的CNDD强度, 进而影响森林树种多度格局形成。
此外, 宿主植物与根际共生真菌间的相容性和共生真菌一定程度的宿主专性可能是影响植物群落结构的先决条件, 且宿主植物的相对多度可能与根际真菌尤其是AM真菌间的宿主专性及彼此间相互作用的强度有密切联系(Schroeder et al., 2018)。然而, 宿主植物多度与根相关真菌(root-associated fungi)多样性的关系, 特别是宿主植物多度与AM真菌多样性间关系的机制其实尚不清楚(Schroeder et al., 2019), 且受限于当前高通量测序新技术平台的缺陷, Illumina MiSeq测序虽然被广泛运用到细菌、真菌多样性的研究中, 但由于其测序长度较短, 除了基于18S rRNA基因片段的AM真菌的检测, 很少有合适片段的AM真菌特异性引物适合于该平台(Jiang et al., 2015; 刘敏等, 2016)。同时由于AM真菌离体培养的难操作性(AM真菌被认为是一种高度依赖宿主植物碳源的共生菌, 无法在脱离寄主根系的情况下实现纯培养), 传统土壤培养条件下难以获得足够纯净的AM真菌菌丝及孢子, 很大程度上限制了对AM真菌的研究(熊天等, 2021)。如果未来能设计出更适配的AM真菌特异性引物, 探寻出实现AM真菌完全离体纯培养的可行性办法(通过脂肪酸的添加在一定程度上实现了纯培养, 但产孢率极低), 关于植物对AM真菌多样性的响应以及AM真菌多样性对植物多度影响的研究都将发生质的飞跃。
2.2 AM真菌及其多样性——稀有种维持的潜在驱动力
全球植物多样性的一大特征是稀有种种类多而常见种少, 目前地球上已知的435 000种陆生植物中约36.5%都是极度稀有的物种, 热带、亚热带森林稳定的气候条件更是孕育了丰富的稀有种, 塑造了极高的生物多样性(Enquist et al., 2019)。自然界中为什么有些物种丰富而有些物种稀少一直困扰着众多生态学研究者(Klironomos, 2002; Chisholm & Muller- Landau, 2011; Chen et al., 2019), 而如此多的稀有种能持久存在的原因更是亟待揭示(Yenni et al., 2012), 这对于更好地保护生物多样性有极为重要的意义(保护稀有种是生物多样性保护的核心内容)。
尽管土壤病原菌引起的J-C效应(植物-土壤负反馈)被认为是促进森林树种多样性维持最主要的驱动因素之一(Klironomos, 2002; Mangan et al., 2010b; Bachelot et al., 2015; Chen et al., 2019), 但是其强度与物种多度的关系目前还存在争议。与此同时, 越来越多的研究发现除了土壤病原菌之外, 与AM真菌共生的植物通过特殊的菌根结构增强植物对养分的吸收, 协助植物产生物理或化学防御, 产生的植物-土壤正反馈削弱了由病原菌引起的植物-土壤负反馈(Cameron et al., 2013), 影响了植物多样性的维持, 并可能对稀有种的维持产生潜在的影响。群落尺度上的研究发现, AM真菌多样性会使稀有种受益(Bachelot et al., 2017)。因此, 土壤病原菌与菌根真菌在稀有种维持机制的研究中同样重要。Connell等(1984)在研究热带雨林树种多样性的维持机制时发现, 当物种数量较低时其种群维持正的增长率, 即稀有物种存在群落补偿趋势(community compensatory trend, CCT)。物种稀有时种群增加的趋势在当代物种共存理论中被称为“可入侵性准则”, Grainger等(2019)将其称为生态学研究上的“通用货币”。AM作为热带森林中最具优势的菌根类型(Newman & Reddell, 1987; Tedersoo et al., 2014), 在其宿主植物稀有时是否发挥着关键作用亟待探索。在AM真菌多样性较低的地区, 当AM真菌群落物种组成发生变化时会导致地上植物群落发生较大的波动, 从而影响了生态系统的稳定性, 而多度较低的非优势物种对AM真菌多样性变化的响应则更为强烈(van der Heijden et al., 1998b), 因此推测AM真菌多样性对稀有树种的影响可能强于常见树种, 且在物种稀有时种群正增长率的维持中可能发挥着至关重要的作用。例如, 以往的研究发现, 稀有植物、入侵植物对AM真菌及病原菌的响应存在明显差异。具体表现在当入侵种入侵某一新地点时, 专性天敌的不利影响被削弱甚至消失(即天敌逃逸), AM真菌维持了其种群正的增长率(Klironomos, 2002), 从而促进了其种群的入侵、建立及长期存在, 该研究强调AM真菌对多度较低的植物种群增长的促进作用, 也否认了AM真菌一定程度上的宿主专性。相反地, Schroeder等(2018)提出, 植物-土壤微生物间专化的相互作用依赖于植物物种的多度(相较于稀有种, 与常见种存在专性相互作用的AM真菌会更为普遍), 即认为AM真菌与宿主植物间的专化相互作用更有利于常见种, 稀有种反而处于劣势地位。然而, 对墨西哥热带森林3对同科但多度差异极大的常见种及稀有种根组织真菌的测序结果显示, 常见种根组织专化真菌的数量并不优于稀有种, 且宿主植物多度与其根组织真菌多样性负相关, 与AM真菌的负相关关系更为显著(Schroeder et al., 2018), 这表明稀有种在与AM真菌的互作上存在优势, 其优势体现在AM真菌多样性上。
尽管如此, 不少研究者长期所持有的观点依旧是稀有种会受到更强的植物-土壤负反馈, 即稀有种劣势(Klironomos, 2002; Mangan et al., 2010b; Kempel et al., 2018)。若事实如此, 稀有种本身由于数量稀少加上受到更强的植物-土壤负反馈作用, 稀有种应当走向灭绝。而真实情况却是地球上稀有种数量众多, 尤其在热带、亚热带森林更是孕育了丰富的稀有种(Enquist et al., 2019)。为了调和稀有种既受到更强的植物-土壤负反馈作用, 而其自身又存在“稀有种优势”二者之间的矛盾, Schroeder等(2020)通过构建空间显式模型追踪了菌根真菌、病原菌的群落动态以及这二者影响下宿主植物的群落动态变化, 他们认为常见种受到的负反馈强度弱的原因是: 同种土壤下积累的有害生物少, 或者更有害的病原菌都积累到了其他常见异种树下, 互惠共生真菌则较多地积累在同种土壤下, 因此在同种土壤下常见种的幼苗存活率要高于稀有种, 异种土壤对稀有种幼苗定植的促进作用反而要优于常见种(Schroeder et al., 2020)。该研究强调了兼性病原菌对植物生长的负影响也不可小觑, 也进一步强调了微生物群落动态在稀有种维持方面的潜力。
目前, 关于AM真菌多样性在稀有种维持方面的实证研究尚且欠缺, 且受限于对野生型AM真菌的分离、鉴定和培养技术, 宿主与AM真菌的亲和力差异及宿主对AM真菌多样性的响应差异如何影响植物群落物种的多度还缺乏更具体的证据, 这也为今后关于“稀有种优势”机制的研究指出了方向。
3 结论
AM是陆地生态系统中最广泛存在的植物-微生物共生关系, AM真菌在维持植物生长、促进植物养分吸收及减缓植物受到的生物、非生物胁迫方面发挥着重要作用。热带、亚热带森林作为AM真菌最主要的分布区, 蕴藏着极高的AM真菌多样性及超高的植物多样性。高多样性森林最典型的特征就是存在大量的稀有树种, 稀有种维持机制一直是诸多研究中的热点问题, 除了考虑病原菌在植物多样性维持、物种多度格局塑造过程中的作用外, AM真菌及其多样性对物种多度格局塑造的影响亦不容忽视。而关于宿主多度与AM真菌多样性间的关系以及相关机制的研究更是未来的关注重点, 这或许会成为AM真菌与植物互作效应及AM真菌多样性对植物物种多度格局塑造、稀有种机制相关研究新的切入点。
致谢
感谢百山祖国家公园管理处给予的大力支持, 感谢白浮镇农林中心刘娟老师对本文文字校对工作的大力帮助。
参考文献
Arbuscular mycorrhiza in crop improvement under environmental stress//Ahmad P, Rasool S
Mycorrhizas in tropical forests: a neglected research imperative
DOI:10.1111/j.1469-8137.2009.02798.x PMID:19291071 [本文引用: 1]
Phylogenetic conservatism in plant-soil feedback and its implications for plant abundance
DOI:10.1111/ele.12378
PMID:25328022
[本文引用: 1]

We examined whether plant-soil feedback and plant-field abundance were phylogenetically conserved. For 57 co-occurring native and exotic plant species from an old field in Canada, we collected a data set on the effects of three soil biota treatments on plant growth: net whole-soil feedback (combined effects of mutualists and antagonists), feedback with arbuscular mycorrhizal fungi (AMF) collected from soils of conspecific plants, and feedback with Glomus etunicatum, a dominant mycorrhizal fungus. We found phylogenetic signal in both net whole-soil feedback and feedback with AMF of conspecifics; conservatism was especially strong among native plants but absent among exotics. The abundance of plants in the field was also conserved, a pattern underlain by shared plant responses to soil biota. We conclude that soil biota influence the abundance of close plant relatives in nature. © 2014 John Wiley & Sons Ltd/CNRS.
Negative density- dependent mortality varies over time in a wet tropical forest, advantaging rare species, common species, or no species
DOI:10.1007/s00442-015-3402-7 URL [本文引用: 1]
Arbuscular mycorrhizal fungal diversity and natural enemies promote coexistence of tropical tree species
DOI:10.1002/ecy.1683
PMID:27984646
[本文引用: 1]

Negative population feedbacks mediated by natural enemies can promote species coexistence at the community scale through disproportionate mortality of numerically dominant (common) tree species. Simultaneously, associations with arbuscular mycorrhizal fungi (AMF) can result in positive effects on tree populations. Coupling data on seedling foliar damage from herbivores and pathogens and DNA sequencing of soil AMF diversity, we assessed the effects of these factors on tree seedling mortality at local (1 m ) and community (16 ha plot) scales in a tropical rainforest in Puerto Rico. At the local scale, AMF diversity in soil counteracted negative effects from foliar damage on seedling mortality. At the community scale, mortality of seedlings of common tree species increased with foliar damage while rare tree species benefited from soil AMF diversity. Together, the effects of foliar damage and soil AMF diversity on seedling mortality might foster tree species coexistence in this forest.© 2016 by the Ecological Society of America.
Fungal effects on plant-plant interactions contribute to grassland plant abundances: evidence from the field
DOI:10.1111/1365-2745.12558 URL [本文引用: 2]
Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics
DOI:10.1126/science.aai8212
PMID:28082590
[本文引用: 2]

Feedback with soil biota is an important determinant of terrestrial plant diversity. However, the factors regulating plant-soil feedback, which varies from positive to negative among plant species, remain uncertain. In a large-scale study involving 55 species and 550 populations of North American trees, the type of mycorrhizal association explained much of the variation in plant-soil feedbacks. In soil collected beneath conspecifics, arbuscular mycorrhizal trees experienced negative feedback, whereas ectomycorrhizal trees displayed positive feedback. Additionally, arbuscular mycorrhizal trees exhibited strong conspecific inhibition at multiple spatial scales, whereas ectomycorrhizal trees exhibited conspecific facilitation locally and less severe conspecific inhibition regionally. These results suggest that mycorrhizal type, through effects on plant-soil feedbacks, could be an important contributor to population regulation and community structure in temperate forests.Copyright © 2017, American Association for the Advancement of Science.
Feedback between plants and their soil communities in an old field community
DOI:10.2307/1941601 URL [本文引用: 1]
Dynamics within mutualism and the maintenance of diversity: inference from a model of interguild frequency dependence
DOI:10.1046/j.1461-0248.1999.21050.x URL [本文引用: 2]
Host-specificity of AM fungal population growth rates can generate feedback on plant growth
DOI:10.1023/A:1020221609080 URL [本文引用: 1]
Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit
DOI:10.1098/rspb.2002.2162 URL [本文引用: 1]
Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests
DOI:10.1046/j.1469-8137.2003.00714.x
PMID:33873396
[本文引用: 2]

A growing body of empirical work suggests that soil organisms can exert a strong role in plant community dynamics and may contribute to the coexistence of plant species. Some of this evidence comes from examining the feedback on plant growth through changes in the composition of the soil community. Host specific changes in soil community composition can generate feedback on plant growth and this feedback can be positive or negative. Previous work has demonstrated that negative soil community feedback can contribute to the coexistence of equivalent competitors. In this paper, I show that negative soil community feedback can also contribute to the coexistence of strong competitors, maintaining plant species that would not coexist in the absence of soil community dynamics. I review the evidence for soil community feedback and find accumulating evidence that soil community feedback can be common, strongly negative, and generated by a variety of complementary soil microbial mechanisms, including host-specific changes in the composition of the rhizosphere bacteria, nematodes, pathogenic fungi, and mycorrhizal fungi. Finally, I suggest topics needing further examination.
Rooting theories of plant community ecology in microbial interactions
DOI:10.1016/j.tree.2010.05.004 URL [本文引用: 1]
Maintenance of plant species diversity by pathogens
DOI:10.1146/ecolsys.2015.46.issue-1 URL [本文引用: 1]
Microbial population and community dynamics on plant roots and their feedbacks on plant communities
DOI:10.1146/annurev-micro-092611-150107
PMID:22726216
[本文引用: 1]

The composition of the soil microbial community can be altered dramatically due to association with individual plant species, and these effects on the microbial community can have important feedbacks on plant ecology. Negative plant-soil feedback plays primary roles in maintaining plant community diversity, whereas positive plant-soil feedback may cause community conversion. Host-specific differentiation of the microbial community results from the trade-offs associated with overcoming plant defense and the specific benefits associated with plant rewards. Accumulation of host-specific pathogens likely generates negative feedback on the plant, while changes in the density of microbial mutualists likely generate positive feedback. However, the competitive dynamics among microbes depends on the multidimensional costs of virulence and mutualism, the fine-scale spatial structure within plant roots, and active plant allocation and localized defense. Because of this, incorporating a full view of microbial dynamics is essential to explaining the dynamics of plant-soil feedbacks and therefore plant community ecology.
Incorporating the soil community into plant population dynamics: the utility of the feedback approach
DOI:10.2307/2960528 URL [本文引用: 1]
Mycorrhiza-induced resistance: more than the sum of its parts
DOI:10.1016/j.tplants.2013.06.004
PMID:23871659
[本文引用: 1]

Plants can develop an enhanced defensive capacity in response to infection by arbuscular mycorrhizal fungi (AMF). This 'mycorrhiza-induced resistance' (MIR) provides systemic protection against a wide range of attackers and shares characteristics with systemic acquired resistance (SAR) after pathogen infection and induced systemic resistance (ISR) following root colonisation by non-pathogenic rhizobacteria. It is commonly assumed that fungal stimulation of the plant immune system is solely responsible for MIR. In this opinion article, we present a novel model of MIR that integrates different aspects of the induced resistance phenomenon. We propose that MIR is a cumulative effect of direct plant responses to mycorrhizal infection and indirect immune responses to ISR-eliciting rhizobacteria in the mycorrhizosphere. Crown Copyright © 2013. Published by Elsevier Ltd. All rights reserved.
Mycorrhizal dominance reduces local tree species diversity across US forests
Phylogenetic relatedness explains highly interconnected and nested symbiotic networks of woody plants and arbuscular mycorrhizal fungi in a Chinese subtropical forest
DOI:10.1111/mec.14061
PMID:28207957
[本文引用: 1]

Elucidating symbiotic relationships between arbuscular mycorrhizal fungi (AMF) and plants contributes to a better understanding of their reciprocally dependent coexistence and community assembly. However, the main drivers of plant and AMF community assembly remain unclear. In this study, we examined AMF communities from 166 root samples of 17 woody plant species from 10 quadrats in a Chinese subtropical forest using 454 pyrosequencing of 18S rRNA gene to describe symbiotic AMF-plant association. Our results show the woody plant-AMF networks to be highly interconnected and nested, but in antimodular and antispecialized manners. The nonrandom pattern in the woody plant-AMF network was explained by plant and AMF phylogenies, with a tendency for a stronger phylogenetic signal by plant than AMF phylogeny. This study suggests that the phylogenetic niche conservatism in woody plants and their AMF symbionts could contribute to interdependent AMF and plant community assembly in this subtropical forest ecosystem.© 2017 John Wiley & Sons Ltd.
Rare and phylogenetically distinct plant species exhibit less diverse root-associated pathogen communities
DOI:10.1111/jec.2019.107.issue-3 URL [本文引用: 2]
Mechanisms of maintenance of species diversity
DOI:10.1146/ecolsys.2000.31.issue-1 URL [本文引用: 3]
A theoretical model linking interspecific variation in density dependence to species abundances
DOI:10.1007/s12080-011-0119-z URL
Asymmetric density dependence shapes species abundances in a tropical tree community
DOI:10.1126/science.1190772
PMID:20576853
[本文引用: 2]

The factors determining species commonness and rarity are poorly understood, particularly in highly diverse communities. Theory predicts that interactions with neighbors of the same (conspecific) and other (heterospecific) species can influence a species' relative abundance, but empirical tests are lacking. By using a hierarchical model of survival for more than 30,000 seedlings of 180 tropical tree species on Barro Colorado Island, Panama, we tested whether species' sensitivity to neighboring individuals relates to their relative abundance in the community. We found wide variation among species in the effect of conspecific, but not heterospecific, neighbors on survival, and we found a significant relationship between the strength of conspecific neighbor effects and species abundance. Specifically, rare species suffered more from the presence of conspecific neighbors than common species did, suggesting that conspecific density dependence shapes species abundances in diverse communities.
On the role of the natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees//Boer PJD, Gradwell GR
Compensatory recruitment, growth, and mortality as factors maintaining rain forest tree diversity
DOI:10.2307/1942659
URL
[本文引用: 1]

One general hypothesis to explain how forest tree diversity is maintained is that rarer species are favored over commoner species in their reproduction, growth, and/or mortality. Mechanisms acting in this way would continually compensate for the tendency of some species to increase at the expense of others, and would reduce the chance of local extinction of rare species. Two hypotheses concerning such compensatory mechanisms were tested in subtropical and tropical evergreen rain forests in Queensland, Australia.
When and where plant-soil feedback may promote plant coexistence: a meta-analysis
DOI:10.1111/ele.13278
PMID:31149765
[本文引用: 1]

Plant-soil feedback (PSF) theory provides a powerful framework for understanding plant dynamics by integrating growth assays into predictions of whether soil communities stabilise plant-plant interactions. However, we lack a comprehensive view of the likelihood of feedback-driven coexistence, partly because of a failure to analyse pairwise PSF, the metric directly linked to plant species coexistence. Here, we determine the relative importance of plant evolutionary history, traits, and environmental factors for coexistence through PSF using a meta-analysis of 1038 pairwise PSF measures. Consistent with eco-evolutionary predictions, feedback is more likely to mediate coexistence for pairs of plant species (1) associating with similar guilds of mycorrhizal fungi, (2) of increasing phylogenetic distance, and (3) interacting with native microbes. We also found evidence for a primary role of pathogens in feedback-mediated coexistence. By combining results over several independent studies, our results confirm that PSF may play a key role in plant species coexistence, species invasion, and the phylogenetic diversification of plant communities.© 2019 John Wiley & Sons Ltd/CNRS.
Advances in mechanisms of rare species maintenance and plant-soil feedback in plant communities
DOI:10.17520/biods.2021141
[本文引用: 1]

Background & Aim: Since the Janzen-Connell (J-C) hypothesis was proposed half a century ago, a mounting number of studies have been conducted to test the hypothesis in tropical and subtropical forests. These studies have since greatly improved our understanding of how high biodiversity is maintained. In particular, the pathogenic fungi-induced J-C effect, a type of negative plant-soil feedback (PSF), has been well-recognized as a mechanism to maintain biodiversity and structure community composition, though the overall contribution of PSF to the persistence of a large number of rare species in nature remains controversial. As predicted by the modern species coexistence theory, the “invasion criterion” should be met for rare species to co-exist with other species such that one species will increase in abundance when rare. However, previous studies show results contrary to the prediction of such theory and have thus sparked debates on the mechanism underlying rare species maintenance. Progresses: In this work, we review PSF and the potential factors associated with PSF, including mycorrhizal fungi, soil nutrient content, and fine root functional traits. We discuss their contributions in maintaining rare species and determining species abundance via PSF. In addition to PSF, some other perspectives about rare species maintenance are also covered in this review. Prospects: We propose that the advantages in maintaining the long persistence of rare species and the limitations in restricting population expansion of rare species may be of equal importance for rare species. The combination of modern species coexistence theory and new techniques and methodologies provide promising future directions to fully understand rare species and to better conserve rare species in the future.
植物群落稀有种维持机制与土壤反馈的研究进展
DOI:10.17520/biods.2021141
[本文引用: 1]

自Janzen-Connell (J-C)假说提出后半个世纪以来, 生态学家在热带及亚热带森林对该假说开展的大量实证研究表明, 由专性天敌导致的J-C效应所引起的负密度制约是维持森林多样性和决定群落组成的重要驱动力, 该假说成功地解释了热带及亚热带森林的丰富多样性。土壤病原真菌所引起的植物-土壤负反馈是J-C效应最主要的表现形式。然而, 对于植物-土壤负反馈是否能够维持森林群落中的大量稀有种仍然存在许多争议。基于当代物种共存理论的“稀有种优势”假说认为, 只有在满足“可入侵准则” (即物种在稀有时具有种群增加的趋势)的前提下, 稀有种才能在群落中与其他物种长期共存。然而, 当前基于土壤反馈的实验结果与该理论预测相悖, 因此在稀有种的维持机制方面仍存在较大的分歧。本文通过介绍植物-土壤反馈理论, 整合了可能对稀有种维持有较大影响的因素, 包括共生菌根真菌、土壤养分以及植物细根性状等在影响土壤负反馈方面的相关研究, 并对这些因素如何影响群落中物种多度和稀有种在群落中的维持进行了探讨。最后, 我们也从其他角度探讨了一些对稀有种维持的研究。我们认为在未来对稀有种的研究中, 探讨使其长期存续的“优势”和制约其种群扩大的“限制”同等重要, 将当代物种共存理论与新技术、新方法相结合对于探究稀有种的维持机制具有重要的意义, 可为稀有种保护提供理论依据。
Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism
DOI:10.1126/science.aab1161
PMID:26315436
[本文引用: 2]

The global biogeography of microorganisms remains largely unknown, in contrast to the well-studied diversity patterns of macroorganisms. We used arbuscular mycorrhizal (AM) fungus DNA from 1014 plant-root samples collected worldwide to determine the global distribution of these plant symbionts. We found that AM fungal communities reflected local environmental conditions and the spatial distance between sites. However, despite AM fungi apparently possessing limited dispersal ability, we found 93% of taxa on multiple continents and 34% on all six continents surveyed. This contrasts with the high spatial turnover of other fungal taxa and with the endemism displayed by plants at the global scale. We suggest that the biogeography of AM fungi is driven by unexpectedly efficient dispersal, probably via both abiotic and biotic vectors, including humans. Copyright © 2015, American Association for the Advancement of Science.
Resource use patterns predict long-term outcomes of plant competition for nutrients and light
An 11-year competition experiment among combinations of six prairie perennial plant species showed that resource competition theory generally predicted the long-term outcome of competition. We grew each species in replicated monocultures to determine its requirements for soil nitrate (R*) and light (I*). In six pairwise combinations, the species with the lower R* and I* excluded its competitor, as predicted by theory. In the remaining two pairwise combinations, one species had a lower R*, and the second had a lower I*; these species pairs coexisted, although it is unclear whether resource competition alone was responsible for their coexistence. Smaller differences in R* or I* between competing species led to slower rates of competitive exclusion, and the influence of R* differences on the rate of competitive exclusion was more pronounced on low-nitrogen soils, while the influence of I* differences was more pronounced on high-nitrogen (low-light) soils. These results were not explained by differences in initial species abundances or neutrality. However, only a few of our paired species coexisted under our experimentally imposed conditions (homogeneous soils, high seeding densities, minimal disturbance, regular water, and low herbivory levels), suggesting that other coexistence mechanisms help generate the diversity observed in natural communities.
Interaction of multiple VAM fungal species on root colonization, plant growth and nutrient status of tomato seedlings (Lycopersicon esculentum Mill.)
DOI:10.1016/0167-8809(96)01040-7 URL [本文引用: 1]
The commonness of rarity: global and future distribution of rarity across land plants
DOI:10.1126/sciadv.aaz0414 [本文引用: 2]
The role of ecological significance of vesicular-arbuscular mycorrhizas in temperate ecosystems
DOI:10.1016/0167-8809(90)90268-I URL [本文引用: 1]
Nutritional exchanges in the arbuscular mycorrhizal symbiosis: implications for sustainable agriculture
DOI:10.1016/j.fbr.2011.01.002 URL [本文引用: 1]
Vesicular-arbuscular mycorrhizal fungi: a determinant of plant community structure in early succession
DOI:10.2307/2390139 URL [本文引用: 1]
The invasion criterion: a common currency for ecological research
DOI:10.1016/j.tree.2019.05.007 URL [本文引用: 1]
Floristic diversity in a model system using experimental microcosms
DOI:10.1038/328420a0 [本文引用: 1]
The niche-relationships of the California thrasher
DOI:10.2307/4072271 URL [本文引用: 1]
Tracking, targeting, and conserving soil biodiversity
DOI:10.1126/science.abd7926 PMID:33446546 [本文引用: 1]
An overview of arbuscular mycorrhizal fungal composition, distribution and host effects from a tropical moist forest//Burslem D, Pinard M, Hartley S
Microbial mediation of plant competition and community structure
DOI:10.1111/1365-2435.12002 URL [本文引用: 1]
The influence of spatial patterns of damping-off disease and arbuscular mycorrhizal colonization on tree seedling establishment in Ghanaian tropical forest soil
DOI:10.1111/jec.2004.92.issue-5 URL [本文引用: 1]
Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: patterns, mechanisms, and feedbacks
DOI:10.1641/0006-3568(2000)050[1049:IBAABB]2.0.CO;2 URL [本文引用: 3]
The effects of plant composition and diversity on ecosystem processes
DOI:10.1126/science.277.5330.1302
URL
[本文引用: 1]

The relative effects of plant richness (the number of plant functional groups) and composition (the identity of the plant functional groups) on primary productivity and soil nitrogen pools were tested experimentally. Differences in plant composition explained more of the variation in production and nitrogen dynamics than did the number of functional groups present. Thus, it is possible to identify and differentiate among potential mechanisms underlying patterns of ecosystem response to variation in plant diversity, with implications for resource management.
Competition and facilitation in multispecies plant-herbivore systems of productive environments
DOI:10.1046/j.1461-0248.1998.00015.x URL [本文引用: 1]
Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi
DOI:10.1111/j.1469-8137.2007.02294.x
PMID:18042204
[本文引用: 1]

Arbuscular mycorrhizal fungal (AMF) communities were established in pots using fungal isolates from a single field in Switzerland. It was tested whether multispecies mixtures provided more phosphorus and supported greater plant growth than single AMF species. Two host plants, medic (Medicago truncatula) and leek (Allium porrum), were inoculated with three AMF species (Glomus mosseae, G. claroideum and G. intraradices), either separately or in mixtures. The composition of the AMF communities in the roots was assessed using real-time PCR to determine the copy number of large ribosomal subunit genes. Fungal communities in the roots were usually dominated by one AMF species (G. mosseae). The composition of the communities depended on both plant identity and the time of harvest. Leek colonized by a mixture of G. claroideum and G. intraradices acquired more P than with either of the two AMF separately. Direct evidence is provided for functional complementarity among species within the AMF community colonizing a single root system. Competition among the species poses a major challenge in interpreting experiments with mixed inoculations, but this is greatly facilitated by use of real-time PCR.
Herbivores and the number of tree species in tropical forests
DOI:10.1086/282687 URL [本文引用: 1]
Tree species traits affect which natural enemies drive the Janzen-Connell effect in a temperate forest
DOI:10.1038/s41467-019-14140-y [本文引用: 1]
Tree mycorrhizal type mediates the strength of negative density dependence in temperate forests
DOI:10.1111/jec.v108.6 URL [本文引用: 1]
Comparison of different PCR primers on detecting arbuscular mycorrhizal communities inside plant roots
Communities of arbuscular mycorrhizal fungi (AMF) colonizing roots have been increasingly investigated by molecular approaches with AMF-specific PCR primers. However, it is difficult to compare the species diversity and species compositions of AMF communities across various studies due to the PCR primers used differently, and also little is known if significant difference of community compositions is characterized by different primers. We aim to compare the difference of efficiency of four primers for AMF.We chose four commonly used AMF-specific primer combinations (NS31-AM1, AMLl-AML2, NS31-AML2 and SSUmCf-LSUmBr), and used 18S rDNA clone libraries to describe the AMF diversity and community.Our results showed that the specificity and coverage varied among the tested primers, different primer combinations would yield distinct patterns of species diversity and composition of AMF community. SSUmCf-LSUmBr had the best specificity and coverage in amplifying AMF sequences, followed by NS31-AML2 and NS31-AM1, and AML1-AML2 showed the lowest specificity towards AMF sequences.SSUmCf-LSUmBr is not the optimal primer pair for AMF community study in current stage due to limited reference sequences and large DNA size. As an alternative, NS31-AML2 is more suitable in AMF community study, because its target rDNA region could well match the increasingly used virtual taxonomy database (http://maarjam. botany.ut.ee) and also its suitable DNA size could be efficiently used in high-throughput sequencing.
Mycorrhizal associations and the spatial structure of an old-growth forest community
DOI:10.1007/s00442-017-3987-0
PMID:29086005
[本文引用: 1]

Plant-soil feedbacks are known to play a central role in species co-existence, but conceptual frameworks for predicting their magnitude and direction are lacking. We ask whether co-occurring trees that associate with different types of mycorrhizal fungi, which are hypothesized to differ in terms of nutrient use and plant-soil feedbacks, differ in sapling establishment densities and probability of co-occurrence. Given that ectomycorrhizal (ECM) trees typically have fungal structures that protect roots from pathogens whereas arbuscular mycorrhizal (AM) trees do not, we hypothesized that ECM saplings would be clustered around ECM trees, while AM saplings would be suppressed near AM trees. Most previous studies have focused on seedlings, but here we examine whether the spatial signal is evident in later life stages. We measured the spatial associations of ~ 28,000 trees using point pattern analysis in a 25-ha old-growth forest where ECM trees comprised 72% of total basal area and 42% of the total stems, while AM trees comprised the remainder. Supporting our hypothesis, AM saplings were more inhibited by AM trees, while ECM saplings were more clustered around ECM trees. The spatial patterns of AM and ECM trees on saplings of the alternate mycorrhizal type were inhibited. To the extent that similar types of feedbacks occur for other AM and ECM trees, our results suggest that fundamental differences in the nature of local-scale biotic interactions between trees and their fungal symbionts may influence forest community assembly and ecosystem dynamics.
Functioning of mycorrhizas associations along the mutualism-parasitism continuum
DOI:10.1046/j.1469-8137.1997.00729.x URL [本文引用: 2]
Mycorrhizal fungi mediate the direction and strength of plant-soil feedbacks differently between arbuscular mycorrhizal and ectomycorrhizal communities
DOI:10.1038/s42003-018-0201-9 [本文引用: 2]
Plant soil feedback strength in relation to large-scale plant rarity and phylogenetic relatedness
DOI:10.1002/ecy.2145
PMID:29493787
[本文引用: 2]

Understanding why some species are rare while others are common remains a central and fascinating question in ecology. Recently, interactions with soil organisms have been shown to affect local abundances of plant species within communities, however, it is not known whether they might also drive patterns of rarity at large scales. Further, little is known about the specificity of soil-feedback effects, and whether closely related plants share more soil pathogens than more distantly related plants. In a multi-species soil-feedback experiment (using 19 species) we tested whether regionally and locally rare species differed in their response to soil biota. Regional rarity was measured using range size or IUCN status and local rarity by typical abundance within an area. All species were grown on soils trained by a variety of regionally and locally rare and common species, which also varied in their degree of relatedness to the target. We found that, in general, regionally rare species suffered more than twice as much from soil biota than regionally common species. Soil cultured by regionally rare species also had a more negative effect on subsequent plant growth, suggesting they may have also accumulated more pathogens. Local rarity did not predict feedback strength. Further, soil trained by closely related plants had a more negative effect on growth than soil trained by distant relatives, which indicates a phylogenetic signal in the host range of soil biota. We conclude that soil biota may well contribute to plant rarity at large spatial scales, which offers a novel explanation for plant rarity and commonness. Moreover, our results show that phylogenetic relatedness between plants was a good predictor of the likelihood that two plant species interacted negatively via soil biota, which might mean that soil pathogens could prevent the coexistence of closely related plants and could drive patterns of phylogenetic overdispersion. Our results suggest that soil pathogens could restrict the ability of rare species to shift their ranges and might need to be considered by conservation biologists seeking to protect populations of rare plants.© 2018 by the Ecological Society of America.
Differential effects of tropical arbuscular mycorrhizal fungal inocula on root colonization and tree seedling growth: implications for tropical forest diversity
DOI:10.1046/j.1461-0248.2000.00126.x URL [本文引用: 2]
Global diversity and distribution of arbuscular mycorrhizal fungi
DOI:10.1016/j.soilbio.2011.07.012 URL [本文引用: 1]
Feedback with soil biota contributes to plant rarity and invasiveness in communities
Variation in plant response to native and exotic arbuscular mycorrhizal fungi
DOI:10.1890/02-0413 URL [本文引用: 2]
Functional complementarity in the arbuscular mycorrhizal symbiosis
DOI:10.1046/j.1469-8137.2000.00710.x
URL
[本文引用: 1]

The causes and consequences of biodiversity are central themes in ecology. Perhaps one reason for much of the current interest in biodiversity is the belief that the loss of species (by extinction) or their gain (by invasion) will significantly influence ecosystem function. Arbuscular mycorrhizal (AM) fungi are components of most terrestrial ecosystems and, while many research programs have shown that variability among species or isolates of AM fungi does occur (Giovannetti & Gianinazzi‐Pearson, 1994), the basis for this variability and its consequences to the function of communities and ecosystems remains largely unexplored. Smith et al. (pp. 357–366 in this issue) now show clearly that ecologically significant functional diversity exists among AM fungal species in the regions of the soil from which they absorb phosphate, and their results suggest that such diversity may have significant ecological consequences.
Some ecological properties of intimate mutualisms involving plants
Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens
Soilborne pathogens can contribute to diversity maintenance in tree communities through the Janzen-Connell effect, whereby the pathogenic reduction of seedling performance attenuates with distance from conspecifics. By contrast, arbuscular mycorrhizal fungi (AMF) have been reported to promote seedling performance; however, it is unknown whether this is also distance dependent. Here, we investigate the distance dependence of seedling performance in the presence of both pathogens and AMF. In a subtropical forest in south China, we conducted a four-year field census of four species with relatively large phylogenetic distances and found no distance-dependent mortality for newly germinated seedlings. By experimentally separating the effects of AMF and pathogens on seedling performance of six subtropical tree species in a shade house, we found that soil pathogens significantly inhibited seedling survival and growth while AMF largely promoted seedling growth, and these effects were host specific and declined with increasing conspecific distance. Together, our field and experimental results suggest that AMF can neutralize the negative effect of pathogens and that the Janzen-Connell effect may play a less prominent role in explaining diversity of nondominant tree species than previously thought.
Soil fungal networks moderate density-dependent survival and growth of seedlings
DOI:10.1111/nph.17237
PMID:33506513
[本文引用: 1]

Pathogenic and mutualistic fungi have contrasting effects on seedling establishment, but it remains unclear whether density-dependent survival and growth are regulated by access to different types of mycorrhizal fungal networks supported by neighbouring adult trees. Here, we conducted an extensive field survey to test how mycorrhizal and pathogenic fungal colonization of arbuscular mycorrhizal (AM) and ectomycorrhizal (ECM) seedlings in a subtropical forest respond to density of neighbouring adult trees. In addition, we undertook a hyphal exclusion experiment to explicitly test the role of soil fungal networks in driving density-dependent effects on seedling growth and survival. Conspecific adult density was a strong predictor for the relative abundance of putative pathogens, which was greater in roots of AM than of ECM seedlings, while mycorrhizal fungal abundance and colonization were not consistently affected by conspecific adult density. Both ECM and AM fungal networks counteracted conspecific density-dependent mortality, but ECM fungi were more effective at weakening the negative effects of high seedling density than AM fungi. Our findings reveal a critical role of common fungal networks in mitigating negative density-dependent effects of pathogenic fungi on seedling establishment, which provides mechanistic insights into how soil fungal diversity shapes plant community structure in subtropical forests.© 2021 The Authors. New Phytologist © 2021 New Phytologist Foundation.
Molecular diversity of arbuscular mycorrhizae in roots of Juniperus virginiana invasive to grasslands
DOI:10.2136/sssaj2016.05.0133
URL
[本文引用: 1]

\nCore Ideas\nGlomus spp. dominate the arbuscular mycorrhizal fungal (AMF) community in eastern red cedar (ERC) roots.
Advances of species diversity of arbuscular mycorrhizal fungi
丛枝菌根真菌物种多样性研究进展
Phylogenetic congruence between subtropical trees and their associated fungi
DOI:10.1002/ece3.2503
PMID:28031793

Recent studies have detected phylogenetic signals in pathogen-host networks for both soil-borne and leaf-infecting fungi, suggesting that pathogenic fungi may track or coevolve with their preferred hosts. However, a phylogenetically concordant relationship between multiple hosts and multiple fungi in has rarely been investigated. Using next-generation high-throughput DNA sequencing techniques, we analyzed fungal taxa associated with diseased leaves, rotten seeds, and infected seedlings of subtropical trees. We compared the topologies of the phylogenetic trees of the soil and foliar fungi based on the internal transcribed spacer (ITS) region with the phylogeny of host tree species based on,, and genes. We identified 37 foliar and 103 soil pathogenic fungi belonging to the Ascomycota and Basidiomycota phyla and detected significantly nonrandom host-fungus combinations, which clustered on both the fungus phylogeny and the host phylogeny. The explicit evidence of congruent phylogenies between tree hosts and their potential fungal pathogens suggests either diffuse coevolution among the plant-fungal interaction networks or that the distribution of fungal species tracked spatially associated hosts with phylogenetically conserved traits and habitat preferences. Phylogenetic conservatism in plant-fungal interactions within a local community promotes host and parasite specificity, which is integral to the important role of fungi in promoting species coexistence and maintaining biodiversity of forest communities.
The effect of soil-borne pathogens depends on the abundance of host tree species
DOI:10.1038/ncomms10017 [本文引用: 1]
Incorporating the disease triangle framework for testing the effect of soil-borne pathogens on tree species diversity
DOI:10.1111/1365-2435.13345
[本文引用: 1]

1. The enemy-induced Janzen-Connell ( JC) effect, a classic model invoking conspecific negative density dependence (CNDD) and distance dependence, is a primary biodiversity maintenance hypothesis. Yet, conflicting evidence for the JC effect leads to disagreement about its role in maintaining forest diversity. 2. We focus this review on soil-borne pathogens, which are the primary agent inducing the JC effect in many forest ecosystems. Although the test of the pathogeninduced JC effect in ecology critically rests on the seedling mortality caused by soil pathogens, what has not been explicitly explored in the early literature but has increasingly received attention is the long-recognized fact that the environment can alter virulence of pathogens and host susceptibility (thus pathogen-host interactions), as predicted by the classic disease triangle framework enlightened by pathology research in agricultural systems. 3. Here, following the disease triangle framework we review evidence on how the pathogen-induced JC effect may be contingent on context (e.g. environmental conditions, pathogen inoculum load and genetic divergence in host and pathogen populations). The reviewed evidence reveals and clarifies the conditions where pathogens may or may not cause disease to hosts, thus contributing to reconciling the inconsistent results about the pathogen-induced JC effect in the literature. The context dependence of the disease triangle predicts that the pathogen-induced JC effect would change under global change. 4. Gaining insights from evidence that the pathogen-induced JC effect is context-dependent, we suggest that future tests on the JC hypothesis be conducted under the framework of disease triangle, and we stress the necessity by controlling the effect of context factors on plant-pathogen interactions when testing for the JC effect. We conclude the review by proposing three lines of future research for testing the importance of the JC effect in maintaining global forest tree species diversity, with a particular emphasis on testing the effect of global warming on the strength of pathogen-host interactions for better predicting changes of forest biodiversity under climate change.
Analysis of a negative plant-soil feedback in a subtropical monsoon forest
DOI:10.1111/jec.2012.100.issue-4 URL [本文引用: 3]
Coexistence and relative abundance in plant communities are determined by feedbacks when the scale of feedback and dispersal is local
Negative plant-soil feedback occurs when the presence of an individual of a particular species at a particular site decreases the relative success of individuals of the same species compared to those other species at that site. This effect favors heterospecifics thereby facilitating coexistence and maintaining diversity. Empirical work has demonstrated that the average strengths of these feedbacks correlate with the relative abundance of species within a community, suggesting that feedbacks are an important driver of plant community composition. Understanding what factors contribute to the generation of this relationship is necessary for diagnosing the dynamic forces that maintain diversity in plant communities. We used a spatially explicit, individual-based computer simulation to test the effects of dispersal distance, the size of feedback neighbourhoods, the strength of pairwise feedbacks and community wide variation of feedbacks, community richness, as well as life-history differences on the dependence of relative abundance on strength of feedback. We found a positive dependence of relative abundance of a species on its average feedback for local scale dispersal and feedback. However, we found that the strength of this dependence decreased as either the spatial scale of dispersal and/or the spatial scale of feedback increased. We also found that for spatially local (i.e. relatively small) scale interaction and dispersal, as the mean strength of feedbacks in the community becomes less negative, the greater the increase in abundance produced by a comparable increase in species-specific average feedback. We found that life-history differences such as mortality rate did not generate a pattern with abundance, nor did they affect the relationship between abundance and average feedback.. Our results support the claim that empirical observations of a positive correlation between relative abundance and strength of average feedback serves as evidence that local scale negative feedbacks play a prominent role in structuring plant communities. We also identify that this relationship depends upon local scale plant dispersal and feedback which generates clumping and magnifies the negative feedbacks.
Specificity between Neotropical tree seedlings and their fungal mutualists leads to plant-soil feedback
DOI:10.1890/09-0396.1 URL [本文引用: 1]
Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest
DOI:10.1038/nature09273 [本文引用: 4]
The global significance of biodiversity science in China: an overview
DOI:10.1093/nsr/nwab032 [本文引用: 1]
Plant facilitation occurs between species differing in their associated arbuscular mycorrhizal fungi
DOI:10.1111/j.1469-8137.2012.04290.x
PMID:22943426
[本文引用: 2]

Complementary beneficial effects of different arbuscular mycorrhizal fungi (AMF) can result in a more efficient exploitation of the soil nutrients available, thus influencing plant communities. Here, we hypothesize that plant-AMF specificity is mediated by phylogenetic constraints defining possible interactions, and that plant-AMF interaction patterns can influence plant-plant facilitation specificity. We reanalyzed previous data describing plant-plant and plant-AMF interaction at the community level to specifically test for a phylogenetic signal on plant and AMF interactions and for a relationship between plant-plant facilitation specificity and plant species differences in their AMF associates. Closely related AMF operational taxonomical units (OTUs) tend to interact with the same plant species, but there is not a significant signal in the interaction through the plant phylogeny. This indicates that the similarity in the AMF associates of two plant species is independent of their phylogenetic relatedness. Interestingly, plant-AMF interactions match plant facilitation specificity, with pairs of plant species recruiting more frequently under each other tending to have different AMF associates. An increment of AMF diversity in the rhizosphere, as a result of plant-AMF and plant-plant selectivity, is suggested as a potential driver of plant-plant facilitation. This study highlights the role of plant-AMF interactions in shaping plant community assemblages.© 2012 The Authors. New Phytologist © 2012 New Phytologist Trust.
Arbuscular mycorrhizae and plant-plant interactions impact of invisible world on visible patterns//Pugnaire F
The distribution of mycorrhizas among families of vascular plants
DOI:10.1111/j.1469-8137.1987.tb00175.x
PMID:33874079
[本文引用: 1]

From a literature search, information has been compiled on the mycorrhizal status under field conditions of 20 or more species in each of 25 families. The percentage of species which are mycorrhizal ranged from 100% in seven families to 8% in Cruciferae, many families having additional species that are sometimes mycorrhizal. No family in the list was consistently non-mycorrhizal. Apart from the Ericaceae, the families were either predominantly ectomycorrhizal or predominantly VA mycorrhizal. However, almost all families had at least one example of each of these mycorrhizal types.
Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field
DOI:10.2307/2261180 URL [本文引用: 1]
Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi
DOI:10.1007/s00572-013-0482-2
PMID:23422950
[本文引用: 1]

We aimed to enhance understanding of the molecular diversity of arbuscular mycorrhizal fungi (AMF) by building a new global dataset targeting previously unstudied geographical areas. In total, we sampled 96 plant species from 25 sites that encompassed all continents except Antarctica. AMF in plant roots were detected by sequencing the nuclear SSU rRNA gene fragment using either cloning followed by Sanger sequencing or 454-sequencing. A total of 204 AMF phylogroups (virtual taxa, VT) were recorded, increasing the described number of Glomeromycota VT from 308 to 341 globally. Novel VT were detected from 21 sites; three novel but nevertheless widespread VT (Glomus spp. MO-G52, MO-G53, MO-G57) were recorded from six continents. The largest increases in regional VT number were recorded in previously little-studied Oceania and in the boreal and polar climatic zones - this study providing the first molecular data from the latter. Ordination revealed differences in AM fungal communities between different continents and climatic zones, suggesting that both biogeographic history and environmental conditions underlie the global variation of those communities. Our results show that a considerable proportion of Glomeromycota diversity has been recorded in many regions, though further large increases in richness can be expected in remaining unstudied areas.
Phylogenetic structure and host abundance drive disease pressure in communities
DOI:10.1038/nature14372 [本文引用: 1]
The mutualistic niche: mycorrhizal symbiosis and community dynamics
DOI:10.1146/ecolsys.2016.47.issue-1 URL [本文引用: 1]
Research progress on the interaction between plant pathogen effectors and their host plants
植物病原物效应子与寄主植物互作的研究进展
Biodiversity of arbuscular mycorrhizal fungi and ecosystem function
DOI:10.1111/nph.15119
PMID:29603232
[本文引用: 1]

Contents Summary 1059 I. Introduction: pathways of influence and pervasiveness of effects 1060 II. AM fungal richness effects on ecosystem functions 1062 III. Other dimensions of biodiversity 1062 IV. Back to basics - primary axes of niche differentiation by AM fungi 1066 V. Functional diversity of AM fungi - a role for biological stoichiometry? 1067 VI. Past, novel and future ecosystems 1068 VII. Opportunities and the way forward 1071 Acknowledgements 1072 References 1072 SUMMARY: Arbuscular mycorrhizal (AM) fungi play important functional roles in ecosystems, including the uptake and transfer of nutrients, modification of the physical soil environment and alteration of plant interactions with other biota. Several studies have demonstrated the potential for variation in AM fungal diversity to also affect ecosystem functioning, mainly via effects on primary productivity. Diversity in these studies is usually characterized in terms of the number of species, unique evolutionary lineages or complementary mycorrhizal traits, as well as the ability of plants to discriminate among AM fungi in space and time. However, the emergent outcomes of these relationships are usually indirect, and thus context dependent, and difficult to predict with certainty. Here, we advocate a fungal-centric view of AM fungal biodiversity-ecosystem function relationships that focuses on the direct and specific links between AM fungal fitness and consequences for their roles in ecosystems, especially highlighting functional diversity in hyphal resource economics. We conclude by arguing that an understanding of AM fungal functional diversity is fundamental to determine whether AM fungi have a role in the exploitation of marginal/novel environments (whether past, present or future) and highlight avenues for future research.© 2018 The Authors. New Phytologist © 2018 New Phytologist Trust.
Mycorrhizas in ecosystems
DOI:10.1007/BF01972080 URL [本文引用: 1]
Comparisons of mycorrhizal responsiveness with field soil and commercial inoculum for six native montane species and Bromus tectorum
DOI:10.1111/rec.2007.15.issue-1 URL [本文引用: 2]
Home-field advantage? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis
DOI:10.1186/s12862-016-0698-9 [本文引用: 2]
Arbuscular mycorrhizal fungi and nutrient cycling in cropping systems//Datta R, Meena RS, Pathan SI, Ceccherini MT
Community composition and diversity of Neotropical root-associated fungi in common and rare trees
DOI:10.1111/btp.2018.50.issue-4 URL [本文引用: 3]
Mutualist and pathogen traits interact to affect plant community structure in a spatially explicit model
DOI:10.1038/s41467-020-16047-5 [本文引用: 2]
Host plant phylogeny and abundance predict root- associated fungal community composition and diversity of mutualists and pathogens
DOI:10.1111/1365-2745.13166
[本文引用: 1]

Interactions between plants and their root-associated fungi (RAF) may influence the relative abundance of tree species and determine forest community diversity. Such plant-soil feedbacks in turn depend on the degree to which spatial distance and phylogenetic relatedness of host trees structure pathogen and mutualist communities, but research detailing these aspects of RAF communities is lacking. Here, we characterize plant-RAF associations across a diverse plant community, focusing on the degree to which RAF communities are structured by spatial distance, host phylogenetic relatedness, and host abundance. We compare results for different functional groups, including both putative mutualists and pathogens, an aspect poorly examined hitherto. We collected roots at regular intervals along ten 50 m by 2 m transects, then used DNA barcoding to identify host plants, and characterize the associated fungal community. Variance partitioning was used to measure the relative contributions of host phylogenetic relatedness and spatial distance to explaining RAF community composition. A weighted linear regression was used to measure the correlation between host abundance and RAF diversity. Phylogenetic distance among hosts was a better predictor of RAF community composition than spatial distance, but this relationship was stronger for putative pathogens than for mutualists, suggesting that pathogens show stronger host preference than mutualists. Across all functional groups, RAF showed similar levels of spatial structure. Additionally, RAF communities of locally abundant plants were less diverse than RAF communities of rare plants. Synthesis. We found that RAF communities are structured by the phylogenetic relatedness of hosts and, to a lesser extent, by spatial distance, with pathogens showing stronger host preference than mutualists. Abundant hosts had less diverse RAF communities than rare hosts, which is notable because abundant plants tend to experience weaker negative plant-soil feedback. Going forward, mechanisms underlying the host abundance-RAF diversity relationship warrant further investigation. Additionally, the survey approach presented here could be paired with experiments linking RAF community composition to plant recruitment.
Acquisition and evolution of enhanced mutualism—An underappreciated mechanism for invasive success
DOI:10.1038/s41396-022-01293-w [本文引用: 1]
Alterations of arbuscular mycorrhizal fungal diversity in soil with elevation in tropical forests of China
DOI:10.3390/d11100181 [本文引用: 1]
Higher order interactions and species coexistence
DOI:10.1007/s12080-020-00481-8
[本文引用: 1]

Higher order interactions (HOIs) have been suggested to stabilize diverse ecological communities. However, their role in maintaining species coexistence from the perspective of modern coexistence theory is not known. Here, using generalized Lotka-Volterra model, we derive a general rule for species coexistence modulated by HOIs. We show that where pairwise species interactions fail to promote species coexistence in regions of extreme fitness differences, negative HOIs that intensify pairwise competition, however, can promote coexistence provided that HOIs strengthen intraspecific competition more than interspecific competition. In contrast, positive HOIs that alleviate pairwise competition can stabilize coexistence across a wide range of fitness differences, irrespective of differences in strength of inter- and intraspecific competition. In addition, we extend our three-species analytical result to multispecies communities and show, using simulations, that multispecies coexistence is possible provided that strength of negative intraspecific HOIs is higher than interspecific HOIs. Our work sheds light on the underlying mechanisms through which HOIs can maintain species diversity.
Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula
DOI:10.1046/j.1469-8137.2000.00695.x
URL
[本文引用: 1]

Responses of Medicago truncatula to colonization by two arbuscular mycorrhizal fungi, Scutellospora calospora \nisolate WUM 12(2) and Glomus caledonium isolate RIS 42, were compared in the light of previous findings that \nthe former fungus can be ineffective as a beneficial microsymbiont with some host plants. The plants were grown \nindividually in two‐compartment systems in which a lateral side arm containing soil labelled with 33P was \nseparated from the main soil compartment by a nylon mesh that prevented penetration by roots but not fungal \nhyphae. Fungal inoculum was applied as a root–soil mixture in a band opposite the side arm. Nonmycorrhizal \ncontrols were set up similarly, without inoculum. There were harvests at 28, 35, 42 and 49 d. Both sets of \nmycorrhizal plants grew better than nonmycorrhizal plants and initially had higher concentrations of P in shoots \nand roots. Plants grown with S. calospora grew better than plants grown with G. caledonium, and this was \nassociated with somewhat greater fungal colonization in terms of intraradical hyphae and numbers of arbuscules. \nScutellospora calospora formed denser hyphae at root surfaces than G. caledonium. By 28 d there were extensive \nhyphae of both fungi in the side arms, and after 35 d S. calospora produced denser hyphae there than G. \ncaledonium. Nevertheless, there was very little transfer of 33P via S. calospora to the plant at 28 d, and thereafter \nits transfer increased at a rate only c. 33% of that via G. caledonium. The results showed that plants colonized by \nS. calospora preferentially obtained P from sites in the main soil chamber relatively close to the roots, compared \nwith plants colonized by G. caledonium. Hence formation of a highly beneficial arbuscular mycorrhizal symbiosis \ndoes not necessarily depend on development of hyphae at a distance from the roots or on large‐scale translocation \nof P from distant sites. The results are discussed in relation to previous studies with compartmented systems that \nhave involved the same fungi. Possible causes of the variable effects of S. calospora in symbiosis with different host plants are briefly assessed. Differences in spatial abilities of individual arbuscular mycorrhizal fungi to acquire P might have strong ecological implications for plant growth in soils low in P.
The symbionts forming arbuscular mycorrhizas//Smith SE, Read D
When do Janzen-Connell effects matter? A phylogenetic meta- analysis of conspecific negative distance and density dependence experiments
DOI:10.1111/ele.v24.3 URL [本文引用: 1]
Global diversity and geography of soil fungi
DOI:10.1126/science.1256688 [本文引用: 3]
How mycorrhizal associations drive plant population and community biology
DOI:10.1126/science.aba1223 [本文引用: 2]
Plant-soil feedback and the maintenance of diversity in Mediterranean-climate shrublands
DOI:10.1126/science.aai8291
PMID:28082588
[本文引用: 1]

Soil biota influence plant performance through plant-soil feedback, but it is unclear whether the strength of such feedback depends on plant traits and whether plant-soil feedback drives local plant diversity. We grew 16 co-occurring plant species with contrasting nutrient-acquisition strategies from hyperdiverse Australian shrublands and exposed them to soil biota from under their own or other plant species. Plant responses to soil biota varied according to their nutrient-acquisition strategy, including positive feedback for ectomycorrhizal plants and negative feedback for nitrogen-fixing and nonmycorrhizal plants. Simulations revealed that such strategy-dependent feedback is sufficient to maintain the high taxonomic and functional diversity characterizing these Mediterranean-climate shrublands. Our study identifies nutrient-acquisition strategy as a key trait explaining how different plant responses to soil biota promote local plant diversity.Copyright © 2017, American Association for the Advancement of Science.
Asymmetric patterns of global diversity among plants and mycorrhizal fungi
DOI:10.1111/jvs.12837
[本文引用: 2]

Questions Although the roles of mycorrhizal fungi in different vegetation types are widely acknowledged, it is still largely unknown how the diversity and frequency of different symbiotic partners vary among plant assemblages globally. We asked (1) how the global distribution of vascular plants correlates with the diversity (i.e. number of species) and frequency (i.e. relative abundance) of different plant mycorrhizal types (i.e. arbuscular mycorrhizal [AM], ectomycorrhizal [ECM], ericoid mycorrhizal [ERM], orchid mycorrhizal [ORM] and non-mycorrhizal [NM]); and (2) how the diversities of the most dominant plant mycorrhizal types (AM and ECM) correlate with those of their respective mycorrhizal fungal partners. Location Worldwide. Methods We retrieved all vascular plant occurrences available in the Global Biodiversity Information Facility database from sites worldwide where AM and ECM fungal diversity has been examined. Plant mycorrhizal types were assigned to plant species using expert-based imputation. Diversity and frequency indices were calculated using extrapolation and bootstrapping procedures in order to account for the heterogeneity and uncertainty of the datasets. Results Each plant mycorrhizal type correlated differently with the global diversity pattern of vascular plants, with higher total plant diversity in AM-dominated vegetation, compared with vegetation containing a larger share of ECM, ERM or NM plant species. The diversities of AM and ECM fungi were positively correlated with the frequency, but not diversity, of their respective plant mycorrhizal types; and weakly correlated with the frequency and diversity of other plant mycorrhizal types. Conclusions At the global scale, vascular plant distribution correlates, among other factors, with the frequency, and to a lesser extent diversity, of different mycorrhizal types of plants and fungi. Recognizing these relationships may help to predict changes in the frequency of ECM and AM plant mycorrhizal types under the different ongoing global changes.
Plant-microbiome interactions: from community assembly to plant health
DOI:10.1038/s41579-020-0412-1 [本文引用: 1]
The plant microbiome
DOI:10.1186/gb-2013-14-6-209 [本文引用: 2]
The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems
DOI:10.1111/j.1461-0248.2007.01139.x
PMID:18047587
[本文引用: 1]

Microbes are the unseen majority in soil and comprise a large portion of life's genetic diversity. Despite their abundance, the impact of soil microbes on ecosystem processes is still poorly understood. Here we explore the various roles that soil microbes play in terrestrial ecosystems with special emphasis on their contribution to plant productivity and diversity. Soil microbes are important regulators of plant productivity, especially in nutrient poor ecosystems where plant symbionts are responsible for the acquisition of limiting nutrients. Mycorrhizal fungi and nitrogen-fixing bacteria are responsible for c. 5-20% (grassland and savannah) to 80% (temperate and boreal forests) of all nitrogen, and up to 75% of phosphorus, that is acquired by plants annually. Free-living microbes also strongly regulate plant productivity, through the mineralization of, and competition for, nutrients that sustain plant productivity. Soil microbes, including microbial pathogens, are also important regulators of plant community dynamics and plant diversity, determining plant abundance and, in some cases, facilitating invasion by exotic plants. Conservative estimates suggest that c. 20 000 plant species are completely dependent on microbial symbionts for growth and survival pointing to the importance of soil microbes as regulators of plant species richness on Earth. Overall, this review shows that soil microbes must be considered as important drivers of plant diversity and productivity in terrestrial ecosystems.
Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure
DOI:10.1890/0012-9658(1998)079[2082:DAMFSA]2.0.CO;2 URL [本文引用: 1]
Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity
Plant- specific soil-borne diseases contribute to succession in foredune vegetation
DOI:10.1038/362053a0
The importance of the microbiome of the plant holobiont
DOI:10.1111/nph.13312
PMID:25655016
[本文引用: 1]

Plants can no longer be considered as standalone entities and a more holistic perception is needed. Indeed, plants harbor a wide diversity of microorganisms both inside and outside their tissues, in the endosphere and ectosphere, respectively. These microorganisms, which mostly belong to Bacteria and Fungi, are involved in major functions such as plant nutrition and plant resistance to biotic and abiotic stresses. Hence, the microbiota impact plant growth and survival, two key components of fitness. Plant fitness is therefore a consequence of the plant per se and its microbiota, which collectively form a holobiont. Complementary to the reductionist perception of evolutionary pressures acting on plant or symbiotic compartments, the plant holobiont concept requires a novel perception of evolution. The interlinkages between the plant holobiont components are explored here in the light of current ecological and evolutionary theories. Microbiome complexity and the rules of microbiotic community assemblage are not yet fully understood. It is suggested that the plant can modulate its microbiota to dynamically adjust to its environment. To better understand the level of plant dependence on the microbiotic components, the core microbiota need to be determined at different hierarchical scales of ecology while pan-microbiome analyses would improve characterization of the functions displayed.© 2015 The Authors New Phytologist © 2015 New Phytologist Trust.
Ecological aspects of arbuscular mycorrhizal fungal communities in different habitat types of a Brazilian mountainous area
DOI:10.1111/ere.2019.34.issue-1 URL [本文引用: 1]
Resource availability controls fungal diversity across a plant diversity gradient
DOI:10.1111/j.1461-0248.2006.00965.x
PMID:16972876
[本文引用: 1]

Despite decades of research, the ecological determinants of microbial diversity remain poorly understood. Here, we test two alternative hypotheses concerning the factors regulating fungal diversity in soil. The first states that higher levels of plant detritus production increase the supply of limiting resources (i.e. organic substrates) thereby increasing fungal diversity. Alternatively, greater plant diversity increases the range of organic substrates entering soil, thereby increasing the number of niches to be filled by a greater array of heterotrophic fungi. These two hypotheses were simultaneously examined in experimental plant communities consisting of one to 16 species that have been maintained for a decade. We used ribosomal intergenic spacer analysis (RISA), in combination with cloning and sequencing, to quantify fungal community composition and diversity within the experimental plant communities. We used soil microbial biomass as a temporally integrated measure of resource supply. Plant diversity was unrelated to fungal diversity, but fungal diversity was a unimodal function of resource supply. Canonical correspondence analysis (CCA) indicated that plant diversity showed a relationship to fungal community composition, although the occurrence of RISA bands and operational taxonomic units (OTUs) did not differ among the treatments. The relationship between fungal diversity and resource availability parallels similar relationships reported for grasslands, tropical forests, coral reefs, and other biotic communities, strongly suggesting that the same underlying mechanisms determine the diversity of organisms at multiple scales.
Ecological linkages between aboveground and belowground biota
DOI:10.1126/science.1094875
PMID:15192218
[本文引用: 1]

All terrestrial ecosystems consist of aboveground and belowground components that interact to influence community- and ecosystem-level processes and properties. Here we show how these components are closely interlinked at the community level, reinforced by a greater degree of specificity between plants and soil organisms than has been previously supposed. As such, aboveground and belowground communities can be powerful mutual drivers, with both positive and negative feedbacks. A combined aboveground-belowground approach to community and ecosystem ecology is enhancing our understanding of the regulation and functional significance of biodiversity and of the environmental impacts of human-induced global change phenomena.
Genomic and transcriptomic studies of several species of arbuscular mycorrhizal fungi
丛枝菌根真菌数个种的基因组和转录组研究概况
Function of arbuscular mycorrhiza in the restoration and reconstruction of degraded ecosystems
丛枝菌根在退化生态系统恢复和重建中的作用
Research advances in species diversity of arbuscular mycorrhizal fungi in terrestrial agro-ecosystem
陆地农业生态系统丛枝菌根真菌物种多样性研究进展
DOI:10.13287/j.1001-9332.201911.036
[本文引用: 1]

丛枝菌根真菌(AMF)是一种古老的、在自然界中普遍存在的土壤微生物,能与大部分陆生植物形成互惠互利的菌根共生体.在这种共生关系中,AMF从植物获取自身生长所需碳源的同时,帮助宿主吸收氮、磷等营养物质.AMF在农业生态系统中具有重要作用,能够促进植物生长、改善作物品质、提高植物抗逆性、稳定土壤结构、维护生态平衡和维持农业可持续发展.本文总结了近几年来陆地农业生态系统AMF的研究进展,着重从我国陆地农业生态系统AMF物种多样性、AMF生物多样性时空分布特征及影响AMF多样性的因素等几个方面,综述了陆地农业生态系统AMF的物种多样性,并对以后的研究进行了展望.
Strong self-limitation promotes the persistence of rare species
Theory has recognized a combination of niche and neutral processes each contributing, with varying importance, to species coexistence. However, long-term persistence of rare species has been difficult to produce in trait-based models of coexistence that incorporate stochastic dynamics, raising questions about how rare species persist despite such variability. Following recent evidence that rare species may experience significantly different population dynamics than dominant species, we use a plant community model to simulate the effect of disproportionately strong negative frequency dependence on the long-term persistence of the rare species in a simulated community. This strong self-limitation produces long persistence times for the rare competitors, which otherwise succumb quickly to stochastic extinction. The results suggest that the mechanism causing species to be rare in this case is the same mechanism allowing those species to persist.
Soil aggregate composition and its relationship with arbuscular mycorrhizal fungi in different restoration stages on severely eroded lands
沿海侵蚀台地不同恢复阶段土壤团聚体组成及其与丛枝菌根真菌的关系
DOI:10.16258/j.cnki.1674-5906.2017.02.006 [本文引用: 1]