

Chin J Plant Ecol ›› 2019, Vol. 43 ›› Issue (6): 512-520.DOI: 10.17521/cjpe.2019.0082
• Research Articles • Previous Articles Next Articles
FENG Lu1,3,*( ),BU Zhao-Jun2,3,WU Yu-Huan4,LIU Sha-Sha2,3,LIU Chao2,3
),BU Zhao-Jun2,3,WU Yu-Huan4,LIU Sha-Sha2,3,LIU Chao2,3
Received:2019-04-15
Revised:2019-06-03
Online:2019-06-20
Published:2019-09-30
Contact:
FENG Lu
Supported by:FENG Lu, BU Zhao-Jun, WU Yu-Huan, LIU Sha-Sha, LIU Chao. Characteristic environmental factors in peatlands facilitate the formation of persistent Sphagnum spore banks[J]. Chin J Plant Ecol, 2019, 43(6): 512-520.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2019.0082
| DO (mg·L-1) | pH | Eh (mV) | ||
|---|---|---|---|---|
| 超纯水 Ultrapure water | 不充气 Control | 8.91 ± 0.02Ab | 5.36 ± 0.04Bb | 196.7 ± 3.9Bb |
| 低速率充气 Low | 8.84 ± 0.02c | 5.24 ± 0.06b | 191.0 ± 5.0b | |
| 高速率充气 High | 9.09 ± 0.01a | 5.55 ± 0.07a | 206.2 ± 1.0a | |
| 泥炭地地表水 Peatland surface water | 不充气 Control | 6.91 ± 0.02Cc | 5.80 ± 0.02A | 181.2 ± 3.7Cc |
| 低速率充气 Low | 9.60 ± 0.04b | 7.47 ± 0.06a | 192.8 ± 1.4b | |
| 高速率充气 High | 10.08 ± 0.03a | 7.19 ± 0.01b | 198.6 ± 1.6a | |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 不充气 Control | 7.52 ± 0.17Bc | 4.96 ± 0.06Cc | 247.1 ± 2.5Aa |
| 低速率充气 Low | 9.70 ± 0.02b | 5.91 ± 0.09b | 196.6 ± 5.8b | |
| 高速率充气 High | 10.25 ± 0.02a | 6.14 ± 0.01b | 183.9 ± 0.4c |
Table 1 Dissolved oxygen concentration (DO), pH value and redox potential (Eh) in different water storage solutions with or without air injection (mean ± SE, n = 3)
| DO (mg·L-1) | pH | Eh (mV) | ||
|---|---|---|---|---|
| 超纯水 Ultrapure water | 不充气 Control | 8.91 ± 0.02Ab | 5.36 ± 0.04Bb | 196.7 ± 3.9Bb |
| 低速率充气 Low | 8.84 ± 0.02c | 5.24 ± 0.06b | 191.0 ± 5.0b | |
| 高速率充气 High | 9.09 ± 0.01a | 5.55 ± 0.07a | 206.2 ± 1.0a | |
| 泥炭地地表水 Peatland surface water | 不充气 Control | 6.91 ± 0.02Cc | 5.80 ± 0.02A | 181.2 ± 3.7Cc |
| 低速率充气 Low | 9.60 ± 0.04b | 7.47 ± 0.06a | 192.8 ± 1.4b | |
| 高速率充气 High | 10.08 ± 0.03a | 7.19 ± 0.01b | 198.6 ± 1.6a | |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 不充气 Control | 7.52 ± 0.17Bc | 4.96 ± 0.06Cc | 247.1 ± 2.5Aa |
| 低速率充气 Low | 9.70 ± 0.02b | 5.91 ± 0.09b | 196.6 ± 5.8b | |
| 高速率充气 High | 10.25 ± 0.02a | 6.14 ± 0.01b | 183.9 ± 0.4c |
| TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚 Phenolics | |
|---|---|---|---|---|---|---|---|
| 超纯水 Ultrapure water | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.05 ± 0.01c | 0.00 ± 0.00c | 0.02 ± 0.00c | 0.00 ± 0.00c |
| 泥炭地地表水 Peatland surface water | 0.52 ± 0.05b | 0.03 ± 0.01b | 2.90 ± 0.33b | 5.22 ± 0.70a | 1.64 ± 0.16b | 1.43 ± 0.12a | 6.51 ± 0.05a |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 5.03 ± 0.31a | 0.26 ± 0.04a | 8.89 ± 1.13a | 0.71 ± 0.49b | 4.33 ± 0.18a | 0.39 ± 0.06b | 3.23 ± 0.09b |
Table 2 Main chemical elements and total phenolics concentration (mg·L-1) in three solutions (mean ± SE, n = 3)
| TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚 Phenolics | |
|---|---|---|---|---|---|---|---|
| 超纯水 Ultrapure water | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.05 ± 0.01c | 0.00 ± 0.00c | 0.02 ± 0.00c | 0.00 ± 0.00c |
| 泥炭地地表水 Peatland surface water | 0.52 ± 0.05b | 0.03 ± 0.01b | 2.90 ± 0.33b | 5.22 ± 0.70a | 1.64 ± 0.16b | 1.43 ± 0.12a | 6.51 ± 0.05a |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 5.03 ± 0.31a | 0.26 ± 0.04a | 8.89 ± 1.13a | 0.71 ± 0.49b | 4.33 ± 0.18a | 0.39 ± 0.06b | 3.23 ± 0.09b |
| 来源 Source | d.f. | 尖叶泥炭藓 Sphagnum capillifolium | 喙叶泥炭藓 Sphagnum flexuosum | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| 保存液类型 Water type | 2 | 3.74 | 0.044 | 3.33 | 0.059 |
| 充气速率 Air injection | 2 | 16.31 | 0.000 | 2.90 | 0.081 |
| 保存液类型 × 充气速率 Water type × Air injection | 4 | 5.57 | 0.004 | 3.73 | 0.022 |
Table 3 Two-way ANOVA on the effect of water type, air injection and the interaction between water type and air injection on spore persistence
| 来源 Source | d.f. | 尖叶泥炭藓 Sphagnum capillifolium | 喙叶泥炭藓 Sphagnum flexuosum | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| 保存液类型 Water type | 2 | 3.74 | 0.044 | 3.33 | 0.059 |
| 充气速率 Air injection | 2 | 16.31 | 0.000 | 2.90 | 0.081 |
| 保存液类型 × 充气速率 Water type × Air injection | 4 | 5.57 | 0.004 | 3.73 | 0.022 |
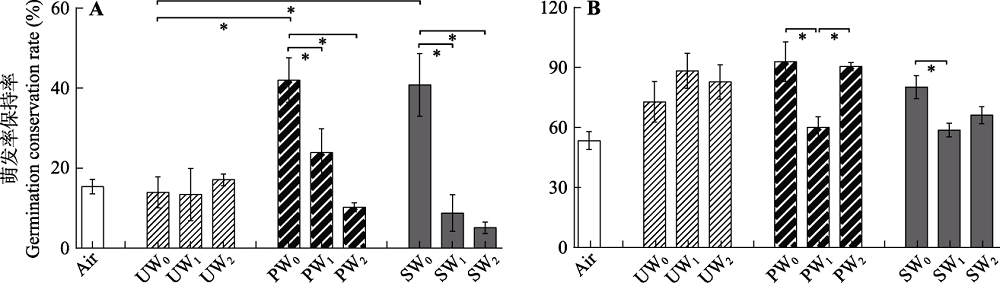
Fig. 1 Germination conservation rate of Sphagnum spores after three types of storage solutions and three levels of air injection. (mean ± SE) A, S. capillifolium. B, S. flexuosum PW, peatland surface water; SW, Sphagnum leachate water; UW, ultrapurewater. 0, no air injection; 1, low air injection; 2, high air injection. * in each group (UW0, PW0 and SW0; UW0, UW1 and UW2; PW0, PW1 and PW2; SW0, SW1 and SW2; Air and UW0) indicated significant differences in one-way ANOVA (p < 0.05).
| 因子 Factor | DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics |
|---|---|---|---|---|---|---|---|---|---|---|
| DO | ||||||||||
| pH | 0.562 | |||||||||
| Eh | -0.322 | -0.401 | ||||||||
| TN | 0.108 | -0.183 | 0.364 | |||||||
| TP | 0.108 | -0.175 | 0.362 | 1.000 | ||||||
| K+ | 0.096 | -0.008 | 0.310 | 0.973 | 0.976 | |||||
| Ca2+ | -0.074 | 0.765 | -0.296 | -0.307 | -0.296 | -0.080 | ||||
| Na+ | 0.091 | 0.038 | 0.293 | 0.958 | 0.961 | 0.998 | -0.022 | |||
| Mg2+ | -0.061 | 0.766 | -0.254 | -0.173 | -0.161 | 0.058 | 0.990 | 0.116 | ||
| 总酚 Phenolics | 0.047 | 0.767 | -0.156 | 0.051 | 0.063 | 0.277 | 0.925 | 0.332 | 0.965 | |
| 尖叶泥炭藓孢子持久性 Sphagnum capillifolium GCR | -0.777 | -0.187 | 0.375 | -0.126 | -0.123 | -0.057 | 0.310 | -0.039 | 0.303 | 0.206 |
| 喙叶泥炭藓孢子持久性 Sphagnum flexuosum GCR | -0.402 | -0.207 | 0.052 | -0.382 | -0.382 | -0.365 | 0.146 | -0.357 | 0.096 | 0.031 |
Table 4 Correlation analysis among water physicochemical indicators and between those indicators with sphagnum spore persistence
| 因子 Factor | DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics |
|---|---|---|---|---|---|---|---|---|---|---|
| DO | ||||||||||
| pH | 0.562 | |||||||||
| Eh | -0.322 | -0.401 | ||||||||
| TN | 0.108 | -0.183 | 0.364 | |||||||
| TP | 0.108 | -0.175 | 0.362 | 1.000 | ||||||
| K+ | 0.096 | -0.008 | 0.310 | 0.973 | 0.976 | |||||
| Ca2+ | -0.074 | 0.765 | -0.296 | -0.307 | -0.296 | -0.080 | ||||
| Na+ | 0.091 | 0.038 | 0.293 | 0.958 | 0.961 | 0.998 | -0.022 | |||
| Mg2+ | -0.061 | 0.766 | -0.254 | -0.173 | -0.161 | 0.058 | 0.990 | 0.116 | ||
| 总酚 Phenolics | 0.047 | 0.767 | -0.156 | 0.051 | 0.063 | 0.277 | 0.925 | 0.332 | 0.965 | |
| 尖叶泥炭藓孢子持久性 Sphagnum capillifolium GCR | -0.777 | -0.187 | 0.375 | -0.126 | -0.123 | -0.057 | 0.310 | -0.039 | 0.303 | 0.206 |
| 喙叶泥炭藓孢子持久性 Sphagnum flexuosum GCR | -0.402 | -0.207 | 0.052 | -0.382 | -0.382 | -0.365 | 0.146 | -0.357 | 0.096 | 0.031 |
| 物种 Species | 因子 Factor | 直接作用 Direct effect | 间接作用 Indirect effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics | |||
| 尖叶泥炭藓 Sphagnum capillifolium | DO | -0.777 | 0.270 | -0.068 | -0.007 | -0.007 | 0.003 | -0.030 | 0.005 | -0.025 | 0.018 | |
| pH | 0.480 | -0.437 | -0.084 | 0.012 | 0.011 | 0.000 | 0.308 | 0.002 | 0.312 | 0.297 | ||
| Eh | 0.210 | 0.250 | -0.192 | -0.024 | -0.022 | 0.008 | -0.119 | 0.015 | -0.103 | -0.061 | ||
| TN | -0.067 | -0.103 | -0.088 | 0.076 | -0.062 | 0.026 | -0.124 | 0.049 | -0.070 | 0.020 | ||
| TP | -0.062 | -0.102 | -0.084 | 0.076 | -0.067 | 0.026 | -0.119 | 0.049 | -0.066 | 0.024 | ||
| K+ | 0.027 | -0.091 | -0.004 | 0.065 | -0.065 | -0.061 | -0.032 | 0.051 | 0.024 | 0.107 | ||
| Ca2+ | 0.403 | 0.070 | 0.367 | -0.062 | 0.021 | 0.018 | -0.002 | -0.001 | 0.403 | 0.358 | ||
| Na+ | 0.051 | -0.087 | 0.018 | 0.062 | -0.064 | -0.060 | 0.027 | -0.009 | 0.047 | 0.129 | ||
| Mg2+ | 0.407 | 0.058 | 0.367 | -0.053 | 0.012 | 0.010 | 0.002 | 0.399 | 0.006 | 0.374 | ||
| 总酚Phenolics | 0.387 | -0.045 | 0.368 | -0.033 | -0.003 | -0.004 | 0.007 | 0.373 | 0.017 | 0.393 | ||
| 喙叶泥炭藓 Sphagnum flexuosum | DO | -0.402 | 0.014 | 0.029 | -0.040 | -0.040 | -0.034 | -0.009 | -0.032 | -0.005 | 0.003 | |
| pH | 0.025 | -0.226 | 0.036 | 0.068 | 0.065 | 0.003 | 0.098 | -0.013 | 0.060 | 0.042 | ||
| Eh | -0.089 | 0.129 | -0.010 | -0.135 | -0.135 | -0.111 | -0.038 | -0.103 | -0.020 | -0.009 | ||
| TN | -0.372 | -0.044 | -0.005 | -0.032 | -0.372 | -0.348 | -0.039 | -0.336 | -0.013 | 0.003 | ||
| TP | -0.372 | -0.043 | -0.004 | -0.032 | -0.372 | -0.349 | -0.038 | -0.337 | -0.013 | 0.003 | ||
| K+ | -0.358 | -0.038 | 0.000 | -0.028 | -0.362 | -0.363 | -0.010 | -0.350 | 0.005 | 0.015 | ||
| Ca2+ | 0.128 | 0.030 | 0.019 | 0.026 | 0.114 | 0.110 | 0.029 | 0.008 | 0.077 | 0.051 | ||
| Na+ | -0.351 | -0.037 | 0.001 | -0.026 | -0.356 | -0.358 | -0.357 | -0.003 | 0.009 | 0.018 | ||
| Mg2+ | 0.078 | 0.024 | 0.019 | 0.023 | 0.064 | 0.060 | -0.021 | 0.127 | -0.041 | 0.053 | ||
| 总酚Phenolics | 0.055 | -0.019 | 0.019 | 0.014 | -0.019 | -0.023 | -0.099 | 0.118 | -0.117 | 0.075 | ||
Table 5 Path analysis of spore persistence and water physicochemical indicators
| 物种 Species | 因子 Factor | 直接作用 Direct effect | 间接作用 Indirect effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics | |||
| 尖叶泥炭藓 Sphagnum capillifolium | DO | -0.777 | 0.270 | -0.068 | -0.007 | -0.007 | 0.003 | -0.030 | 0.005 | -0.025 | 0.018 | |
| pH | 0.480 | -0.437 | -0.084 | 0.012 | 0.011 | 0.000 | 0.308 | 0.002 | 0.312 | 0.297 | ||
| Eh | 0.210 | 0.250 | -0.192 | -0.024 | -0.022 | 0.008 | -0.119 | 0.015 | -0.103 | -0.061 | ||
| TN | -0.067 | -0.103 | -0.088 | 0.076 | -0.062 | 0.026 | -0.124 | 0.049 | -0.070 | 0.020 | ||
| TP | -0.062 | -0.102 | -0.084 | 0.076 | -0.067 | 0.026 | -0.119 | 0.049 | -0.066 | 0.024 | ||
| K+ | 0.027 | -0.091 | -0.004 | 0.065 | -0.065 | -0.061 | -0.032 | 0.051 | 0.024 | 0.107 | ||
| Ca2+ | 0.403 | 0.070 | 0.367 | -0.062 | 0.021 | 0.018 | -0.002 | -0.001 | 0.403 | 0.358 | ||
| Na+ | 0.051 | -0.087 | 0.018 | 0.062 | -0.064 | -0.060 | 0.027 | -0.009 | 0.047 | 0.129 | ||
| Mg2+ | 0.407 | 0.058 | 0.367 | -0.053 | 0.012 | 0.010 | 0.002 | 0.399 | 0.006 | 0.374 | ||
| 总酚Phenolics | 0.387 | -0.045 | 0.368 | -0.033 | -0.003 | -0.004 | 0.007 | 0.373 | 0.017 | 0.393 | ||
| 喙叶泥炭藓 Sphagnum flexuosum | DO | -0.402 | 0.014 | 0.029 | -0.040 | -0.040 | -0.034 | -0.009 | -0.032 | -0.005 | 0.003 | |
| pH | 0.025 | -0.226 | 0.036 | 0.068 | 0.065 | 0.003 | 0.098 | -0.013 | 0.060 | 0.042 | ||
| Eh | -0.089 | 0.129 | -0.010 | -0.135 | -0.135 | -0.111 | -0.038 | -0.103 | -0.020 | -0.009 | ||
| TN | -0.372 | -0.044 | -0.005 | -0.032 | -0.372 | -0.348 | -0.039 | -0.336 | -0.013 | 0.003 | ||
| TP | -0.372 | -0.043 | -0.004 | -0.032 | -0.372 | -0.349 | -0.038 | -0.337 | -0.013 | 0.003 | ||
| K+ | -0.358 | -0.038 | 0.000 | -0.028 | -0.362 | -0.363 | -0.010 | -0.350 | 0.005 | 0.015 | ||
| Ca2+ | 0.128 | 0.030 | 0.019 | 0.026 | 0.114 | 0.110 | 0.029 | 0.008 | 0.077 | 0.051 | ||
| Na+ | -0.351 | -0.037 | 0.001 | -0.026 | -0.356 | -0.358 | -0.357 | -0.003 | 0.009 | 0.018 | ||
| Mg2+ | 0.078 | 0.024 | 0.019 | 0.023 | 0.064 | 0.060 | -0.021 | 0.127 | -0.041 | 0.053 | ||
| 总酚Phenolics | 0.055 | -0.019 | 0.019 | 0.014 | -0.019 | -0.023 | -0.099 | 0.118 | -0.117 | 0.075 | ||
| [1] | Abbott GD, Swain EY, Muhammad AB, Allton K, Belyea LR, Laing CG, Cowie GL (2013). Effect of water-table fluctuations on the degradation of Sphagnum phenols in surficial peats. Geochimica et Cosmochimica Acta, 106, 177-191. |
| [2] | Aerts R, Wallen B, Malmer N (1992). Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. Journal of Ecology, 80, 131-140. |
| [3] | Boatman DJ, Lark PM (1971). Inorganic nutrition of the protonemata of Sphagnum papillosum Lindb., S. magellanicum Brid. and S. cuspidatum Ehrh. New Phytologist, 70, 1053-1059. |
| [4] | Bu ZJ, Li Z, Liu LJ, Sundberg S, Feng YM, Yang YH, Liu S, Song X, Zhang XL (2017a). Bryophyte spore germinability is inhibited by peatland substrates. Acta Oecologica, 78, 34-40. |
| [5] | Bu ZJ, Sundberg S, Feng L, Li HK, Zhao HY, Li HC (2017b). The Methuselah of plant diaspores: Sphagnum spores can survive in nature for centuries. New Phytologist, 214, 1398-1402. |
| [6] | Clymo RS, Duckett JG (1986). Regeneration of Sphagnum. New Phytologist, 102, 589-614. |
| [7] |
Du JJ, Chen ZW (2010). The methods of path analysis by SPSS linear regression. Bulletin of Biology, 45(2), 4-6.
DOI URL |
|
[ 杜家菊, 陈志伟 (2010). 使用SPSS线性回归实现通径分析的方法. 生物学通报, 45(2), 4-6.]
DOI URL |
|
| [8] | Feng L, Bu ZJ, Mallik A, Wang ZC, Liu SS, Wu YH (2017). Continuous waterlogging may not facilitate germinability maintenance of Sphagnum spores. Wetlands, 37, 1015-1022. |
| [9] | Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009). Global patterns in belowground communities. Ecology Letters, 12, 1238-1249. |
| [10] | González-Benito ME, Pérez-García F, Tejeda G, Gómez- Campo C (2011). Effect of the gaseous environment and water content on seed viability of four Brassicaceae species after 36 years storage. Seed Science and Technology, 39, 443-451. |
| [11] | Hättenschwiler S, Vitousek PM (2000). The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends in Ecology & Evolution, 15, 238-243. |
| [12] | Jauhiainen S (1998). Seed and spore banks of two boreal mires. Annales Botanici Fennici, 35, 197-201. |
| [13] | Ke X, Lu W, Conrad R (2015). High oxygen concentration increases the abundance and activity of bacterial rather than archaeal nitrifiers in rice field soil. Microbial Ecology, 70, 961-970. |
| [14] | Li J, He Y, Ma D, He B, Wang Y, Chen B (2018a). Volatile allelochemicals of Chenopodium ambrosioides L. induced mitochondrion-mediated Ca 2+-dependent and caspase-dependent apoptosis signaling pathways in receptor plant cells. Plant and Soil, 425, 297-308. |
| [15] | Li Y, Rashid A, Wang HJ, Hu AY, Lin LF, Yu CP, Chen M, Sun Q (2018b). Contribution of biotic and abiotic factors in the natural attenuation of sulfamethoxazole: A path analysis approach. Science of the Total Environment, 633, 1217-1226. |
| [16] | Liu LJ, Bu ZJ, Liu S, Chen YD, Feng L, Fu B, Yang YH, Wang SZ (2019). Sand and dust deposition may retard the autogenic vegetation succession of peatlands. Scientia Geographica Sinica, 39, 351-358. |
| [ 刘礼洁, 卜兆君, 刘霜, 陈永达, 冯璐, 付彪, 杨云荷, 王升忠 (2019). 沙尘沉降可能阻滞泥炭地植被的自发演替. 地理科学, 39, 351-358.] | |
| [17] | McLetchie DN (1999). Dormancy/Nondormancy cycles in spores of the liverwort Sphaerocarpos texanus. The Bryologist, 102, 15-21. |
| [18] | Michel P, Burritt DJ, Lee WG (2011). Bryophytes display allelopathic interactions with tree species in native forest ecosystems. Oikos, 120, 1272-1280. |
| [19] | Mishler BD, Newton AE (1988). Influences of mature plants and desiccation on germination of spores and gametophyticfragments of Tortula. Journal of Bryology, 15, 327-342. |
| [20] | Montenegro G, Portaluppi MC, Salas FA, Díaz MF (2009). Biological properties of the Chilean native moss Sphagnum magellanicum. Biological Research, 42, 233-237. |
| [21] | Ooi MKJ, Auld TD, Denham AJ (2009). Climate change and bet-hedging: Interactions between increased soil temperatures and seed bank persistence. Global Change Biology, 15, 2375-2386. |
| [22] | Pinsonneault AJ, Moore TR, Roulet NT (2016). Effects of long-term fertilization on peat stoichiometry and associated microbial enzyme activity in an ombrotrophic bog. Biogeochemistry, 129, 149-164. |
| [23] | Proctor MCF, Oliver MJ, Wood AJ, Alpert P, Stark LR, Cleavitt NL, Mishler BD (2007). Desiccation-tolerance in bryophytes: A review. The Bryologist, 110, 595-621. |
| [24] | Rudolph H, Kirchhoff M, Gliesmann S (1988). Sphagnum culture techniques. In: Glime JM ed. Methods in Bryology. Proceedings of the Bryological Methods Workshop, Mainz, Hattori Botanical Laboratory, Nichinan, Japan. |
| [25] | Saatkamp A, Poschlod P, Venable DL (2014). The functional role of soil seed banks in natural communities. In: Gallagher RS ed. Seeds: The Ecology of Regeneration in Plant Communities. CABI, Wallingford. 263-295. |
| [26] | Shen-Miller J, Mudgett MB, Schopf JW, Clarke S, Berger R (1995). Exceptional seed longevity and robust growth: Ancient Sacred Lotus from China. American Journal of Botany, 82, 1367-1380. |
| [27] | Song YY, Song CC, Meng HN, Swarzenski CM, Wang XW, Tan WW (2017). Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecological Engineering, 100, 175-185. |
| [28] | Sundberg S, Rydin H (2000). Experimental evidence for a persistent spore bank in Sphagnum. New Phytologist, 148, 105-116. |
| [29] | Sundberg S, Rydin H (2002). Habitat requirements for establishment of Sphagnum from spores. Journal of Ecology, 90, 268-278. |
| [30] | Taârit MB, Msaada K, Hosni K, Marzouk B (2012). Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. Journal of the Science of Food and Agriculture, 92, 1614-1619. |
| [31] | Tellier A (2019). Persistent seed banking as eco-evolutionary determinant of plant nucleotide diversity: Novel population genetics insights. New Phytologist, 221, 725-730. |
| [32] | Turetsky MR (2003). The role of bryophytes in carbon and nitrogen cycling. The Bryologist, 106, 395-409. |
| [33] | van Zanten BO (1978). Experimental studies on trans-oceanic long-range dispersal of moss spores in the Southern Hemisphere. Journal of the Hattori Botanical Laboratory, 44, 445-482. |
| [34] | Verhoeven JTA, Liefveld WM (1997). The ecological significance of organochemical compounds in Sphagnum. Acta Botanica Neerlandica, 46, 117-130. |
| [35] | Wheeler BD, Proctor MCF (2000). Ecological gradients, subdivisions and terminology of north-west European mires. Journal of Ecology, 88, 187-203. |
| [36] | Whitehead J, Wittemann M, Cronberg N (2018). Allelopathy in bryophytes—A review. Lindbergia, 41, 01097. DOI: 10.25227/linbg.01097. |
| [37] | Yu Z, Dahlgren RA (2000). Evaluation of methods for measuring polyphenols in conifer foliage. Journal of Chemical Ecology, 26, 2119-2140. |
| [38] | Yuan M, Bu ZJ, Liu C, Ma JZ, Wang SZ (2015). Effects of water level and light intensity on capsule production dynamics of Sphagnum capillifolium. Chinese Journal of Plant Ecology, 39, 501-507. |
| [ 袁敏, 卜兆君, 刘超, 马进泽, 王升忠 (2015). 水位与光强变化对尖叶泥炭藓孢蒴生产动态的影响. 植物生态学报, 39, 501-507.] |
| [1] | LI Xiao-Ling, ZHU Dao-Ming, YU Yu-Rong, WU Hao, MOU Li, HONG Liu, LIU Xue- Fei, BU Gui-Jun, XUE Dan, WU Lin. Effects of simulated nitrogen deposition on growth and decomposition of two bryophytes in ombrotrophic peatland, southwestern Hubei, China [J]. Chin J Plant Ecol, 2023, 47(5): 644-659. |
| [2] | LIU Xue-Fei, WU Lin, WANG Han, HONG Liu, XIONG Li-Jun. Growth and decomposition characteristics of Sphagnum in a subalpine wetland, southwestern Hubei, China [J]. Chin J Plant Ecol, 2020, 44(3): 228-235. |
| [3] | YUAN Min,BU Zhao-Jun,LIU Chao,MA Jin-Ze,WANG Sheng-Zhong. Effects of water level and light intensity on capsule production dynamics of Sphagnum capillifolium [J]. Chin J Plan Ecolo, 2015, 39(5): 501-507. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn