

Chin J Plant Ecol ›› 2020, Vol. 44 ›› Issue (3): 228-235.DOI: 10.17521/cjpe.2019.0316
• Research Articles • Previous Articles Next Articles
LIU Xue-Fei1,2,WU Lin1,2,*( ),WANG Han1,2,HONG Liu1,2,XIONG Li-Jun3
),WANG Han1,2,HONG Liu1,2,XIONG Li-Jun3
Received:2019-11-19
Accepted:2020-01-19
Online:2020-03-20
Published:2020-02-24
Contact:
Lin WU
Supported by:LIU Xue-Fei, WU Lin, WANG Han, HONG Liu, XIONG Li-Jun. Growth and decomposition characteristics of Sphagnum in a subalpine wetland, southwestern Hubei, China[J]. Chin J Plant Ecol, 2020, 44(3): 228-235.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2019.0316
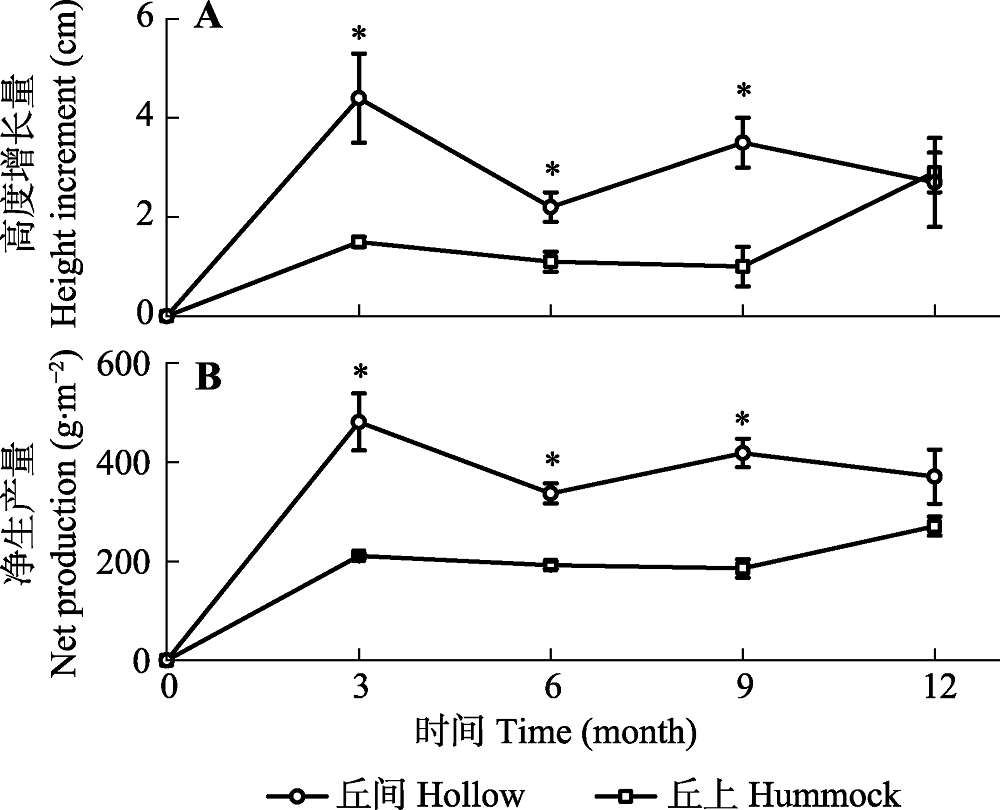
Fig. 2 Increment of height growth (A) and net production (B) of Sphagnum in hollow and hummock of Sphagnum-dominated subalpine wetlands in southwestern Hubei (mean ± SE, n = 3). * refers a significant difference between the two microhabitats at the same time (p < 0.05).
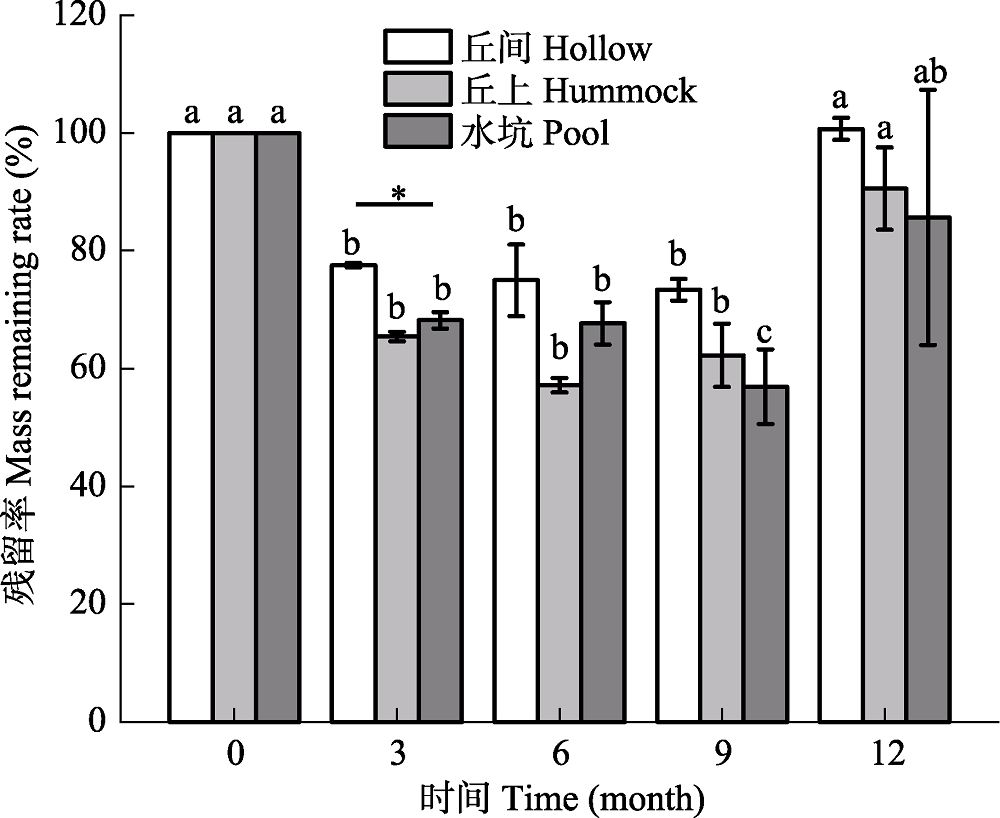
Fig. 3 Changes in litters residual rate of Sphagnum under three microhabitats of Sphagnum-dominated subalpine wetlands in southwestern Hubei (mean ± SE, n = 3). * refers a significant difference among the three microhabitats at the same time (p < 0.05); different lowercase letters indicate significant differences at different time periods in the same microhabitat (p < 0.05).
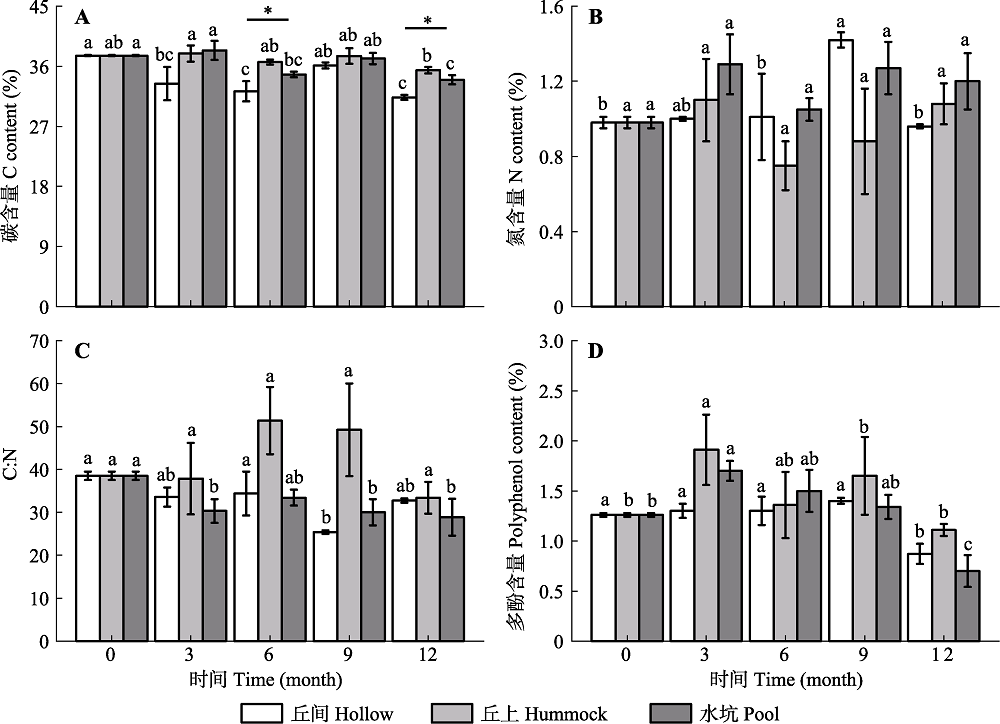
Fig. 4 Contents of C (A), N (B), C:N (C) and polyphenols (D) in the litters of Sphagnum of the three microhabitats of Sphagnum- dominated subalpine wetlands in southwestern Hubei (mean ± SE, n = 3). * refers a significant difference among the three microhabitats at the same time (p < 0.05); different lowercase letters indicate significant differences at different time periods in the same microhabitat(p < 0.05)
| 纬度 Latitude (°) | 经度 Longitude (°) | 年平均气温 Annual mean temperature (℃) | 年降水量 Precipitation (mm) | 物种 Species | 生长速率 Growth rate (mm·d-1) | 净生产力 Net primary productivity (g·m-2·d-1) | 参考文献 Reference |
|---|---|---|---|---|---|---|---|
| 68.35 | 18.82 | 0.5 | 320 | S. fus | 0.04 | 1.51 | |
| 60.52 | 17.92 | 3.1 | 544 | S. fus | 0.10 | 0.64 | |
| 59.90 | 15.83 | 1.4 | 515 | S. fus, S. mag | 0.13 | 1.12 | |
| 57.15 | -111.98 | 2.1 | 387 | S. fus | 0.09 | 1.68 | |
| 56.63 | -110.20 | 1.1 | 420 | S. fus | 0.09 | 1.72 | |
| 55.68 | -111.83 | 2.1 | 421 | S. fus | 0.10 | 1.96 | |
| 54.68 | -113.47 | 1.7 | 500 | S. fus | 0.03 | 1.15 | |
| 49.67 | -93.72 | 2.6 | 714 | S. mag, S. fus, S. ang | 0.12 | 0.86 | |
| 46.32 | 11.67 | 5.0 | 1 100 | S. cap, S. mag, S. fal | 0.26 | 1.70 | |
| 39.12 | -79.58 | 7.9 | 1 330 | S. mag, S. rec | 0.42 | 3.44 | |
| 30.17 | 109.73 | 7.2 | 1 590 | S. pal | 0.33 | 3.84 | 本研究 This study |
Table 1 Growth rate and net primary productivity of Sphagnum spp. in areas of different latitude
| 纬度 Latitude (°) | 经度 Longitude (°) | 年平均气温 Annual mean temperature (℃) | 年降水量 Precipitation (mm) | 物种 Species | 生长速率 Growth rate (mm·d-1) | 净生产力 Net primary productivity (g·m-2·d-1) | 参考文献 Reference |
|---|---|---|---|---|---|---|---|
| 68.35 | 18.82 | 0.5 | 320 | S. fus | 0.04 | 1.51 | |
| 60.52 | 17.92 | 3.1 | 544 | S. fus | 0.10 | 0.64 | |
| 59.90 | 15.83 | 1.4 | 515 | S. fus, S. mag | 0.13 | 1.12 | |
| 57.15 | -111.98 | 2.1 | 387 | S. fus | 0.09 | 1.68 | |
| 56.63 | -110.20 | 1.1 | 420 | S. fus | 0.09 | 1.72 | |
| 55.68 | -111.83 | 2.1 | 421 | S. fus | 0.10 | 1.96 | |
| 54.68 | -113.47 | 1.7 | 500 | S. fus | 0.03 | 1.15 | |
| 49.67 | -93.72 | 2.6 | 714 | S. mag, S. fus, S. ang | 0.12 | 0.86 | |
| 46.32 | 11.67 | 5.0 | 1 100 | S. cap, S. mag, S. fal | 0.26 | 1.70 | |
| 39.12 | -79.58 | 7.9 | 1 330 | S. mag, S. rec | 0.42 | 3.44 | |
| 30.17 | 109.73 | 7.2 | 1 590 | S. pal | 0.33 | 3.84 | 本研究 This study |
| [1] |
Asada T, Warner BG, Banner A (2003). Growth of mosses in relation to climate factors in a hypermaritime coastal peatland in British Columbia, Canada. The Bryologist, 106, 516-527.
DOI URL |
| [2] |
Bell MC, Ritson JP, Verhoef A, Brazier RE, Templeton MR, Graham NJD, Freeman C, Clark JM (2018). Sensitivity of peatland litter decomposition to changes in temperature and rainfall. Geoderma, 331, 29-37.
DOI URL |
| [3] |
Bengtsson F, Granath G, Rydin H (2016). Photosynthesis, growth, and decay traits in Sphagnum—A multispecies comparison. Ecology and Evolution, 6, 3325-3341.
DOI URL |
| [4] |
Bengtsson F, Rydin H, Hájek T (2018). Biochemical determinants of litter quality in 15 species of Sphagnum. Plant and Soil, 425, 161-176.
DOI URL |
| [5] |
Bragazza L, Siffi C, Iacumin P, Gerdol R (2007). Mass loss and nutrient release during litter decay in peatland: the role of microbial adaptability to litter chemistry. Soil Biology & Biochemistry, 39, 257-267.
DOI URL |
| [6] |
Breeuwer A, Heijmans M, Robroek BJM, Limpens J, Berendse F (2008). The effect of increased temperature and nitrogen deposition on decomposition in bogs. Oikos, 117, 1258-1268.
DOI URL |
| [7] |
Clymo RS (1970). The growth of Sphagnum: methods of measurement. Journal of Ecology, 58, 13-49.
DOI URL |
| [8] |
Clymo RS, Hayward PM (1982). The ecology of Sphagnum// Smith AJE. Bryophyte Ecology. Chapman and Hall, London.
DOI URL PMID |
| [9] |
Dorrepaal E, Aerts R, Cornelissen JHC, Callaghan TV, van Logtestijn RSP (2003). Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Global Change Biology, 10, 93-104.
DOI URL |
| [10] | Editorial Board of Wetland Vegetation in China (1999). Wetland Vegetation in China. Science Press, Beijing. |
| [ 中国湿地植被编辑委员会(1999). 中国湿地植被. 科学出版社, 北京.] | |
| [11] |
Furness SB, Grime JP (1982). Growth rate and temperature responses in bryophytes: I. An investigation of Brachythecium rutabulum. Journal of Ecology, 70, 513-523.
DOI URL |
| [12] |
Genet H, Oberbauer SF, Colby SJ, Staudhammer CL, Starr G (2013). Growth responses of Sphagnum hollows to a growing season lengthening manipulation in Alaskan Arctic tundra. Polar Biology, 36, 41-50.
DOI URL |
| [13] |
Gerdol R (1995). The growth dynamics of Sphagnum based on field measurements in a temperate bog and on laboratory cultures. Journal of Ecology, 83, 431-437.
DOI URL |
| [14] |
Gerdol R, Petraglia A, Bragazza L, Iacumin P, Brancaleoni L (2007). Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Global Change Biology, 13, 1810-1821.
DOI URL |
| [15] |
Gorham E (1991). Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications, 1, 182-195.
DOI URL |
| [16] |
Gunnarsson U (2005). Global patterns of Sphagnum productivity. Journal of Bryology, 27, 269-279.
DOI URL |
| [17] |
Ho YS, Mckay G (2000). The kinetics of sorption of divalent metal ions onto Sphagnum moss peat. Water Research, 34, 735-742.
DOI URL |
| [18] |
Jassey VEJ, Chiapusio G, Gilbert D, Buttler A, Toussaint ML, Binet P (2011). Experimental climate effect on seasonal variability of polyphenol/phenoloxidase interplay along a narrow fen-bog ecological gradient in Sphagnum fallax. Global Change Biology, 17, 2945-2957.
DOI URL |
| [19] | Johnson LC, Damman AWH (1993). Decay and its regulation in Sphagnum peatlands. Advances in Bryology, 5, 249-296. |
| [20] |
Kosykh NP, Koronatova NG, Granath G (2017). Effect of temperature and precipitation on linear increment of Sphagnum fuscum and S. magellanicum in Western Siberia. Russian Journal of Ecology, 2017,48, 203-211.
DOI URL |
| [21] |
Lang SI, Cornelissen JHC, Klahn T, van Logtestijn RSP, Broekman R, Schweikert W, Aerts R (2009). An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. Journal of Ecology, 97, 886-900.
DOI URL |
| [22] |
Leroy F, Gogo S, Guimbaud C, Bernard-Jannin L, Hu Z, Laggoun-Défarge F (2017). Vegetation composition controls temperature sensitivity of CO2 and CH4 emissions and DOC concentration in peatlands. Soil Biology & Biochemistry, 107, 164-167.
DOI URL |
| [23] |
Li H, Parent LE, Karam A, Tremblay C (2004). Potential of Sphagnum peat for improving soil organic matter, water holding capacity, bulk density and potato yield in a sandy soil. Plant and Soil, 265, 355-365.
DOI URL |
| [24] | Li W, Bu ZJ, Zhang BJ, Long C, Tang RJ, Cui QW (2013). Decomposition of Sphagnum litter in 4 peatlands of the Changbai Mountains along an altitudinal gradient. Journal of Mountain Science, 31, 442-447. |
| [ 李伟, 卜兆君, 张兵将, 龙川, 唐瑞江, 崔钱王 (2013). 长白山不同海拔泥炭地泥炭藓残体的分解. 山地学报, 31, 442-447.] | |
| [25] |
Limpens J, Berendse F (2003). How litter quality affects mass loss and N loss from decomposing Sphagnum. Oikos, 103, 537-547.
DOI URL |
| [26] |
Liu YY, Ma JZ, Bu ZJ, Wang SZ, Zhang XB, Zhang TY, Liu SS, Fu B, Kang Y (2018). Effect of geographical sources and biochemical traits on plant litter decomposition in a peatland. Chinese Journal of Plant Ecology, 42, 713-722.
DOI URL |
|
[ 刘媛媛, 马进泽, 卜兆君, 王升忠, 张雪冰, 张婷玉, 刘莎莎, 付彪, 康媛 (2018). 地理来源与生物化学属性对泥炭地植物残体分解的影响. 植物生态学报, 42, 713-722.]
DOI URL |
|
| [27] |
Loisel J, Gallego-Sala AV, Yu Z (2012). Global-scale pattern of peatland Sphagnum growth driven by photosynthetically active radiation and growing season length. Biogeosciences, 9, 2737-2746.
DOI URL |
| [28] | Ma JZ (2018). The Simulation Study on Effects of Climate Warming on Plant Litter Decomposition in Peatlands Basing on Three Experimental Modes. Master degree dissertation, Northeast Normal University, Changchun. |
| [ 马进泽, (2018) . 基于三种实验方式的气候变暖对泥炭地植物凋落物分解影响的模拟研究. 硕士学位论文, 东北师范大学, 长春.] | |
| [29] |
Mironov VL, Kondratev AY (2017). Peat moss Sphagnum riparium follows a circatrigintan growth rhythm in situ: a case report. Chronobiology International, 34, 981-984.
DOI URL |
| [30] |
Moore TR (1989). Growth and net production of Sphagnum at five fen sites, subarctic eastern Canada. Canadian Journal of Botany, 67, 1203-1207.
DOI URL |
| [31] |
Newman TR, Wright N, Wright B, Sjögersten S (2018). Interacting effects of elevated atmospheric CO2 and hydrology on the growth and carbon sequestration of Sphagnum moss. Wetlands Ecology and Management, 26, 763-774.
DOI URL |
| [32] |
Orwin KH, Ostle NJ (2012). Moss species effects on peatland carbon cycling after fire. Functional Ecology, 26, 829-836.
DOI URL |
| [33] |
Philben M, Holmquist J, MacDonald G, Duan DD, Kaiser, K, Benner R (2015). Temperature, oxygen, and vegetation controls on decomposition in a James Bay peatland. Global Biogeochemical Cycles, 29, 729-743.
DOI URL |
| [34] |
Rochefort L, Vitt DH, Bayley SE (1990). Growth, production, and decomposition dynamics of Sphagnum under natural and experimentally acidified conditions. Ecology, 71, 1986-2000.
DOI URL |
| [35] | Singleton VL, Orthofer R, Lamuela-Raventós RM (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152-178. |
| [36] |
Tahvanainen T, Haraguchi A (2013). Effect of pH on phenol oxidase activity on decaying Sphagnum mosses. European Journal of Soil Biology, 54, 41-47.
DOI URL |
| [37] |
Thormann MN, Bayley SE (1997). Aboveground net primary production along a bog-fen-marsh gradient in southern boreal Alberta, Canada. Écoscience, 4, 374-384.
DOI URL |
| [38] | Waddington JM, Rochefort L, Campeau S (2003). Sphagnum production and decomposition in a restored cutover peatland. Wetlands Ecology & Management, 11, 85-95. |
| [39] | Wang LS, Jia Y, Zhang XC, Qin HN (2018). Species Catalogue of China. Volume 1 Plants A Synoptic Checklist (I). Science Press, Beijing. |
| [ 王立松, 贾谕, 张宪春, 覃海宁 (2018). 中国生物名录第一卷植物总名录(上册). 科学出版社, 北京.] | |
| [40] |
Wieder RK, Lang GE (1983). Net primary production of the dominant bryophytes in a Sphagnum-dominated wetland in West Virginia. The Bryologist, 86, 280-286.
DOI URL |
| [41] |
Wieder RK, Vitt DH, Burke-Scoll M, Scott KD, House M, Vile MA (2010). Nitrogen and sulphur deposition and the growth of Sphagnum fuscum in bogs of the Athabasca Oil Sands Region, Alberta. Journal of Limnology, 69, 161-170.
DOI URL |
| [42] |
Yazaki T, Yabe K (2012). Effects of snow-load and shading by vascular plants on the vertical growth of hummocks formed by Sphagnum papillosum in a mire of northern Japan. Plant Ecology, 213, 1055-1067.
DOI URL |
| [43] |
Yu ZC (2012). Northern peatland carbon stocks and dynamics: a review. Biogeosciences, 9, 4071-4085.
DOI URL |
| [44] | Yu ZC, Loisel J, Brosseau DP, Beilman DW, Hunt SJ (2010). Global peatland dynamics since the last glacial maximum. Geophysical Research Letters, 37, L13402. DOI: 10.1029/2010gl043584. |
| [45] |
Zhang XH, Sun XX, Mao R (2017). Effects of litter evenness, nitrogen enrichment and temperature on short-term litter decomposition in freshwater marshes of Northeast China. Wetlands, 37, 145-152.
DOI URL |
| [1] | DENG Wen-Jie, WU Hua-Zheng, LI Tian-Xiang, ZHOU Li-Na, HU Ren-Yong, JIN Xin-Jie, ZHANG Yong-Pu, ZHANG Yong-Hua, LIU Jin-Liang. Main vegetation types and characteristics in Dongtou National Marine Park, Zhejiang, China [J]. Chin J Plant Ecol, 2024, 48(2): 254-268. |
| [2] | ZHANG Hui-Ling, ZHANG Yao-Yi, PENG Qing-Qing, YANG Jing, NI Xiang-Yin, WU Fu-Zhong. Variations of trace-elements resorption efficiency in leaves of different tree species as affected by life forms in a mid-subtropical common garden [J]. Chin J Plant Ecol, 2023, 47(7): 978-987. |
| [3] | LI Xiao-Ling, ZHU Dao-Ming, YU Yu-Rong, WU Hao, MOU Li, HONG Liu, LIU Xue- Fei, BU Gui-Jun, XUE Dan, WU Lin. Effects of simulated nitrogen deposition on growth and decomposition of two bryophytes in ombrotrophic peatland, southwestern Hubei, China [J]. Chin J Plant Ecol, 2023, 47(5): 644-659. |
| [4] | ZHONG Qi, LI Zeng-Yan, MA Wei, KUANG Yu-Xiao, QIU Ling-Jun, LI Yun-Jie, TU Li-Hua. Effects of nitrogen addition and litter manipulations on leaf litter decomposition in western edge of Sichuan Basin, China [J]. Chin J Plant Ecol, 2023, 47(5): 629-643. |
| [5] | WAN Chun-Yan, YU Jun-Rui, ZHU Shi-Dan. Differences in leaf traits and trait correlation networks between karst and non-karst forest tree species [J]. Chin J Plant Ecol, 2023, 47(10): 1386-1397. |
| [6] | GAN Zi-Ying, WANG Hao, DING Chi, LEI Mei, YANG Xiao-Gang, CAI Jing-Yan, QIU Qing-Yan, HU Ya-Lin. Effects of dissolved organic matter derived from different plant and tissues in a subtropical forest on soil priming effect and the underlying mechanisms [J]. Chin J Plant Ecol, 2022, 46(7): 797-810. |
| [7] | WU Qiu-Xia, WU Fu-Zhong, HU Yi, KANG Zi-Jia, ZHANG Yao-Yi, YANG Jing, YUE Kai, NI Xiang-Yin, YANG Yu-Sheng. Difference in non-structural carbohydrates between fresh and senescent leaves of 11 tree species in a subtropical common-garden [J]. Chin J Plant Ecol, 2021, 45(7): 771-779. |
| [8] | MOU Li, WU Lin, LIU Xue-Fei, LI Xiao-Ling, WANG Han, WU Hao, YU Yu-Rong, DU Sheng-Lan. Characteristics and environmental factors controlling methane emission from a Sphagnum bog with different plant cover types in a subalpine area, southwest of Hubei, China [J]. Chin J Plant Ecol, 2021, 45(2): 131-143. |
| [9] | CAO Jia-Yu, LIU Jian-Feng, YUAN Quan, XU De-Yu, FAN Hai-Dong, CHEN Hai-Yan, TAN Bin, LIU Li-Bin, YE Duo, NI Jian. Traits of shrubs in forests and bushes reveal different life strategies [J]. Chin J Plant Ecol, 2020, 44(7): 715-729. |
| [10] | CHEN Si-Lu, CAI Jin-Song, LIN Cheng-Fang, SONG Hao-Wei, YANG Yu-Sheng. Response of leaf litter decomposition of different tree species to nitrogen addition in a subtropical forest [J]. Chin J Plant Ecol, 2020, 44(3): 214-227. |
| [11] | MEI Kong-Can, CHENG Lei, ZHANG Qiu-Fang, LIN Kai-Miao, ZHOU Jia-Cong, ZENG Quan-Xin, WU Yue, XU Jian-Guo, ZHOU Jin-Rong, CHEN Yue-Min. Effects of dissolved organic matter from different plant sources on soil enzyme activities in subtropical forests [J]. Chin J Plant Ecol, 2020, 44(12): 1273-1284. |
| [12] | LI Xu, WU Ting, CHENG Yan, TAN Na-Dan, JIANG Fen, LIU Shi-Zhong, CHU Guo-Wei, MENG Ze, LIU Ju-Xiu. Ecophysiological adaptability of four tree species in the southern subtropical evergreen broad-leaved forest to warming [J]. Chin J Plant Ecol, 2020, 44(12): 1203-1214. |
| [13] | MO Dan, WANG Zhen-Meng, ZUO You-Lu, XIANG Shuang. Trade-off between shooting and leaf developing of woody species saplings in subtropical evergreen broad-leaved forests [J]. Chin J Plant Ecol, 2020, 44(10): 995-1006. |
| [14] | CHE Jian, ZHENG Jie, JIANG Ya, JIN Yi, YI Yin. Separation of phylogeny and ecological behaviors between evergreen and deciduous woody angiosperms in the subtropical forest dynamics plots of China [J]. Chin J Plant Ecol, 2020, 44(10): 1007-1014. |
| [15] | FENG Lu, BU Zhao-Jun, WU Yu-Huan, LIU Sha-Sha, LIU Chao. Characteristic environmental factors in peatlands facilitate the formation of persistent Sphagnum spore banks [J]. Chin J Plant Ecol, 2019, 43(6): 512-520. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn