

Chin J Plant Ecol ›› 2013, Vol. 37 ›› Issue (7): 591-600.DOI: 10.3724/SP.J.1258.2013.00061
Special Issue: 稳定同位素生态学
• Research Articles • Next Articles
FENG Qiu-Hong1,2,CHENG Rui-Mei1,SHI Zuo-Min1,*( ),LIU Shi-Rong1,WANG Wei-Xia1,LIU Xing-Liang2,HE Fei2
),LIU Shi-Rong1,WANG Wei-Xia1,LIU Xing-Liang2,HE Fei2
Received:2012-12-28
Accepted:2013-06-04
Online:2013-12-28
Published:2013-07-05
Contact:
SHI Zuo-Min
FENG Qiu-Hong,CHENG Rui-Mei,SHI Zuo-Min,LIU Shi-Rong,WANG Wei-Xia,LIU Xing-Liang,HE Fei. Response of Rumex dentatus foliar nitrogen and its allocation to altitudinal gradients along Balang Mountain, Sichuan, China[J]. Chin J Plant Ecol, 2013, 37(7): 591-600.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.3724/SP.J.1258.2013.00061
| 海拔 Altitude (m) | 经纬度 Longitude and latitude | 生境情况 Habitat situation |
|---|---|---|
| 2 350 | 103°03′57.3″ E, 30°52′58.3″ N | 阳坡, 高山柳林下缘 Sunny slope, lower edge of alpine willow |
| 2 700 | 102°58′ 33.8″ E, 30°51′47.5″ N | 阳坡, 高山栎林下缘 Sunny slope, lower edge of alpine oak |
| 3 150 | 102°58′ 44.5″ E, 30°52′58.3″ N | 阳坡, 高山柳林下缘 Sunny slope, lower edge of alpine willow |
| 3 530 | 102°57′ 48.1″ E, 30°52′06.8″ N | 阳坡, 高山草甸 Sunny slope, alpine meadow |
Table 1 Geographic location and habitat situation of sites
| 海拔 Altitude (m) | 经纬度 Longitude and latitude | 生境情况 Habitat situation |
|---|---|---|
| 2 350 | 103°03′57.3″ E, 30°52′58.3″ N | 阳坡, 高山柳林下缘 Sunny slope, lower edge of alpine willow |
| 2 700 | 102°58′ 33.8″ E, 30°51′47.5″ N | 阳坡, 高山栎林下缘 Sunny slope, lower edge of alpine oak |
| 3 150 | 102°58′ 44.5″ E, 30°52′58.3″ N | 阳坡, 高山柳林下缘 Sunny slope, lower edge of alpine willow |
| 3 530 | 102°57′ 48.1″ E, 30°52′06.8″ N | 阳坡, 高山草甸 Sunny slope, alpine meadow |
| 海拔 Altitude (m) | A (μmol·m-2·s-1) | Rd (μmol·m-2·s-1) | Amax (μmol·m-2·s-1) | Vcmax (μmol·m-2·s-1) | Jmax (μmol·m-2·s-1) | Jmax/Vcmax |
|---|---|---|---|---|---|---|
| 2 350 | 11.68 ± 1.03Cc | 1.90 ± 0.25Bb | 40.46 ± 8.14Bb | 84.13 ± 12.15Bb | 317.49 ± 73.33Bb | 3.92 ± 0.39Ba |
| 2 700 | 13.90 ± 1.83Cc | 2.21 ± 0.17ABab | 49.75 ± 3.69Aab | 111.31 ± 9.69Aa | 397.14 ± 31.12ABab | 4.21 ± 0.37Ba |
| 3 150 | 17.28 ± 2.12ABab | 2.22 ± 0.17ABab | 50.69 ± 3.55Aa | 90.80 ± 14.24Bab | 414.25 ± 33.22Aa | 4.59 ± 0.51ABa |
| 3 530 | 20.20 ± 4.21Aa | 2.44 ± 0.31Aa | 55.41 ± 4.41Aa | 98.29 ± 13.89ABab | 467.02 ± 60.55Aa | 5.11 ± 1.03Aa |
Table 2 Foliar photosynthetic parameters of Rumex dentatus among different altitudes
| 海拔 Altitude (m) | A (μmol·m-2·s-1) | Rd (μmol·m-2·s-1) | Amax (μmol·m-2·s-1) | Vcmax (μmol·m-2·s-1) | Jmax (μmol·m-2·s-1) | Jmax/Vcmax |
|---|---|---|---|---|---|---|
| 2 350 | 11.68 ± 1.03Cc | 1.90 ± 0.25Bb | 40.46 ± 8.14Bb | 84.13 ± 12.15Bb | 317.49 ± 73.33Bb | 3.92 ± 0.39Ba |
| 2 700 | 13.90 ± 1.83Cc | 2.21 ± 0.17ABab | 49.75 ± 3.69Aab | 111.31 ± 9.69Aa | 397.14 ± 31.12ABab | 4.21 ± 0.37Ba |
| 3 150 | 17.28 ± 2.12ABab | 2.22 ± 0.17ABab | 50.69 ± 3.55Aa | 90.80 ± 14.24Bab | 414.25 ± 33.22Aa | 4.59 ± 0.51ABa |
| 3 530 | 20.20 ± 4.21Aa | 2.44 ± 0.31Aa | 55.41 ± 4.41Aa | 98.29 ± 13.89ABab | 467.02 ± 60.55Aa | 5.11 ± 1.03Aa |
| 海拔 Altitude (m) | 气孔导度 Gs (mol·m-2·s-1) | 叶肉细胞导度 Gm (mol·m-2·s-1) | 羧化位点与外界CO2分压比 Pc/Pa | 稳定碳同位素比值 δ13C (‰) |
|---|---|---|---|---|
| 2 350 | 0.19 ± 0.02Bbc | 0.21 ± 0.06Cc | 0.53 ± 0.02Ba | -30.65 ± 0.27Cc |
| 2 700 | 0.17 ± 0.02Bc | 0.30 ± 0.08Bbc | 0.38 ± 0.04Cb | -28.25 ± 0.45Bb |
| 3 150 | 0.30 ± 0.07Aab | 0.36 ± 0.09ABab | 0.60 ± 0.08ABa | -28.14 ± 0.58Bab |
| 3 530 | 0.31 ± 0.10Aa | 0.44 ± 0.07Aa | 0.57 ± 0.09ABa | -27.52 ± 0.47Aa |
Table 3 Foliar diffusional conductance and stable carbon isotope ratio of Rumex dentatus among different altitudes
| 海拔 Altitude (m) | 气孔导度 Gs (mol·m-2·s-1) | 叶肉细胞导度 Gm (mol·m-2·s-1) | 羧化位点与外界CO2分压比 Pc/Pa | 稳定碳同位素比值 δ13C (‰) |
|---|---|---|---|---|
| 2 350 | 0.19 ± 0.02Bbc | 0.21 ± 0.06Cc | 0.53 ± 0.02Ba | -30.65 ± 0.27Cc |
| 2 700 | 0.17 ± 0.02Bc | 0.30 ± 0.08Bbc | 0.38 ± 0.04Cb | -28.25 ± 0.45Bb |
| 3 150 | 0.30 ± 0.07Aab | 0.36 ± 0.09ABab | 0.60 ± 0.08ABa | -28.14 ± 0.58Bab |
| 3 530 | 0.31 ± 0.10Aa | 0.44 ± 0.07Aa | 0.57 ± 0.09ABa | -27.52 ± 0.47Aa |
| 海拔 Altitude (m) | Nmass (g·100g-1) | Narea (g·m-2) | PR (g·g-1) | PB (g·g-1) | PNUE (μmol·mol-1·s-1) | SLA (cm2·g-1) |
|---|---|---|---|---|---|---|
| 2 350 | 3.59 ± 0.22 Bb | 1.16 ± 0.07Dc | 0.46 ± 0.07ABa | 0.19 ± 0.01Aa | 153.32 ± 31.62Aa | 288.31 ± 34.29Aa |
| 2 700 | 4.10 ± 0.57Aa | 1.68 ± 0.08Cb | 0.49 ± 0.03Aa | 0.20 ± 0.02Aa | 127.28 ± 16.23ABa | 242.61 ± 25.60Bb |
| 3 150 | 3.82 ± 0.16ABab | 1.78 ± 0.08Bb | 0.39 ± 0.09BCa | 0.19 ± 0.02Aa | 122.09 ± 16.94ABa | 217.85 ± 10.52BCbc |
| 3 530 | 3.72 ± 0.09ABab | 1.90 ± 0.04Aa | 0.33 ± 0.10Ca | 0.18 ± 0.01Aa | 114.46 ± 0.09Ba | 197.59 ± 6.78Cc |
Table 4 Leaf nitrogen content, photosynthetic nitrogen use efficiency and special leaf area of Rumex dentatus among different altitudes
| 海拔 Altitude (m) | Nmass (g·100g-1) | Narea (g·m-2) | PR (g·g-1) | PB (g·g-1) | PNUE (μmol·mol-1·s-1) | SLA (cm2·g-1) |
|---|---|---|---|---|---|---|
| 2 350 | 3.59 ± 0.22 Bb | 1.16 ± 0.07Dc | 0.46 ± 0.07ABa | 0.19 ± 0.01Aa | 153.32 ± 31.62Aa | 288.31 ± 34.29Aa |
| 2 700 | 4.10 ± 0.57Aa | 1.68 ± 0.08Cb | 0.49 ± 0.03Aa | 0.20 ± 0.02Aa | 127.28 ± 16.23ABa | 242.61 ± 25.60Bb |
| 3 150 | 3.82 ± 0.16ABab | 1.78 ± 0.08Bb | 0.39 ± 0.09BCa | 0.19 ± 0.02Aa | 122.09 ± 16.94ABa | 217.85 ± 10.52BCbc |
| 3 530 | 3.72 ± 0.09ABab | 1.90 ± 0.04Aa | 0.33 ± 0.10Ca | 0.18 ± 0.01Aa | 114.46 ± 0.09Ba | 197.59 ± 6.78Cc |
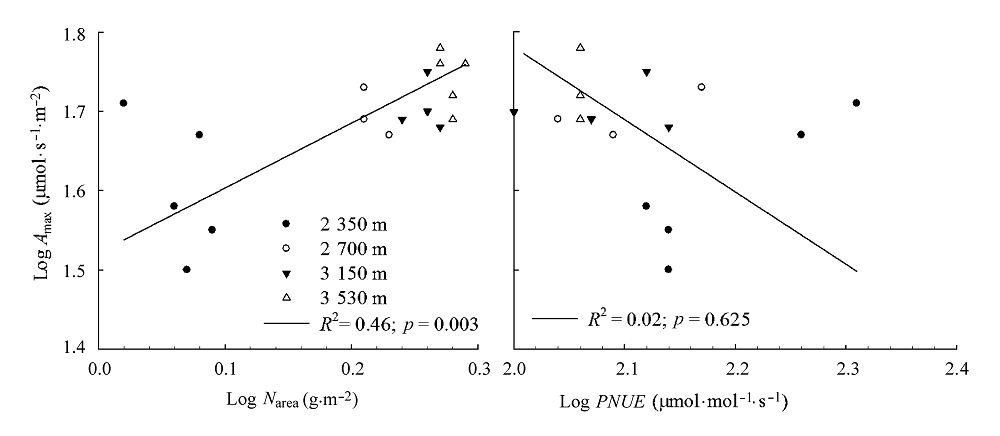
Fig. 1 Relationship between nitrogen content per area (Narea), photosynthetic nitrogen use efficiency (PNUE) and maximum net photosynthetic rate (Amax) of Rumex dentatus.
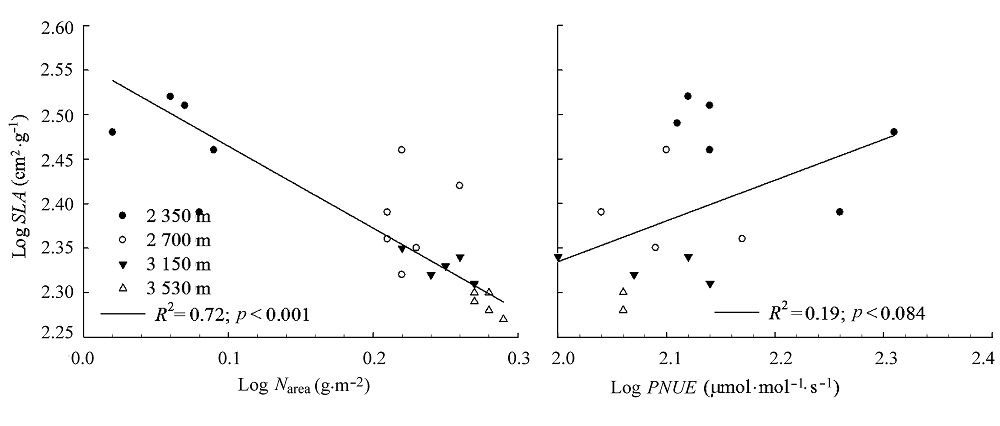
Fig. 2 Relationships between nitrogen content per area (Narea), photosynthetic nitrogen use efficiency (PNUE) and specific leaf area (SLA) of Rumex dentatus.
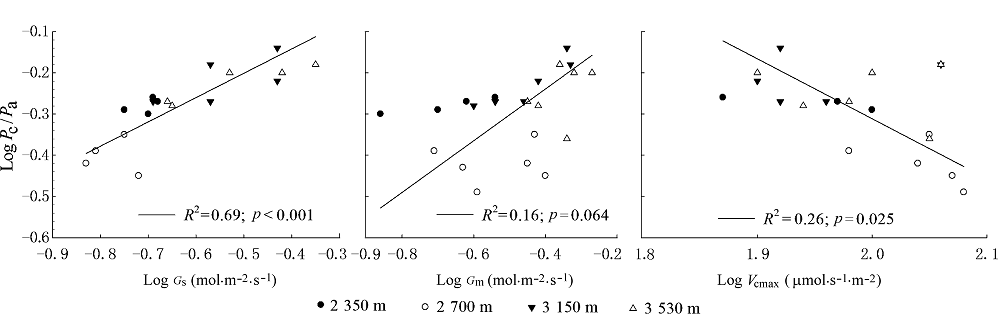
Fig. 3 Relationship between stomatal conductance (Gs), mesophyll conductance (Gm), maximum carboxylation rate (Vcmax) and ratio of chloroplast partial pressure of CO2 to ambient CO2 partial pressure (Pc/Pa) of Rumex dentatus.
| [1] |
Abebe TD, Bauer AM, Léon J (2010). Morphological diversity of Ethiopian barleys (Hordeum vulgare L.) in relation to geographic regions and altitudes. Hereditas, 147, 154-164.
URL PMID |
| [2] | Amthor JS (1984). The role of maintenance respiration in plant growth. Plant, Cell & Environment, 7, 561-569. |
| [3] | Bernacchi CJ, Pimentel C, Long SP (2003). In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell & Environment, 26, 1419-1430. |
| [4] |
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002). Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology, 130, 1992-1998.
URL PMID |
| [5] | Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001). Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment, 24, 253-259. |
| [6] | Cordell S, Goldstein G, Meinzer FC, Handley LL (1999). Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient. Functional Ecology, 13, 811-818. |
| [7] |
Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek M (1998). Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: role of phenotypic plasticity. Oecologia, 113, 188-196.
DOI URL PMID |
| [8] | Evans JR, Seemann JR (1989). The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: Briggs WR ed. Photosynthesis. A.R. Liss, Inc., New York. 183-205. |
| [9] | Falster D, Warton D, Wright I (2006). SMATR: Standardised Major Axis Tests and Routines. http://www.bio.mq.edu.au/ecology/SMATR/index.html. Cited 10 Oct. 2010. |
| [10] | Farquhar GD, von Caemmerer S (1982). Modeling of photosynthetic responses to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H eds. Encyclopedia of Plant Physiology (New Series). Springer-Verlag, Berlin. 549-587. |
| [11] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
URL PMID |
| [12] | Feng QH, Cheng RM, Shi ZM, Liu SR, Liu XL, He F, Cao HM (2011a). Response of foliar δ13C of Quercus spinosa to altitudinal gradients. Acta Ecologica Sinica, 31, 3629-3637. (in Chinese with English abstract) |
| [ 冯秋红, 程瑞梅, 史作民, 刘世荣, 刘兴良, 何飞, 曹慧明 (2011a). 巴郎山刺叶高山栎叶片δ13C对海拔高度的响应. 生态学报, 31, 3629-3637.] | |
| [13] |
Feng QH, Cheng RM, Shi ZM, Liu SR, Liu XL, He F, Cao HM (2011b). Effects of altitudinal gradient on Salix atopantha foliar δ13C. Chinese Journal of Applied Ecology, 22, 2841-2848. (in Chinese with English abstract)
URL PMID |
|
[ 冯秋红, 程瑞梅, 史作民, 刘世荣, 刘兴良, 何飞, 曹慧明 (2011b). 海拔梯度对巴郎山奇花柳叶片δ13C的影响. 应用生态学报, 22, 2841-2848.]
URL PMID |
|
| [14] | Field C, Mooney H (1986). The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ ed. On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, UK. 25-55. |
| [15] |
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008). Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment, 31, 602-621.
URL PMID |
| [16] |
Friend AD, Woodward FI, Switsuer VR (1989). Field measurements of photosynthesis, stomatal conductance, leaf nitrogen and δ13C along altitudinal gradients in Scotland. Functional Ecology, 3, 117-122.
DOI URL |
| [17] | Fujimura S, Shi PL, Iwama K, Zhang XZ, Gopal J, Jitsuyama Y (2010). Effect of altitude on the response of net photosynthetic rate to carbon dioxide increase by spring wheat. Plant Production Science, 13(2), 141-149. |
| [18] |
Gale J (2004). Plants and altitude-revisited. Annals of Botany, 94, 199.
DOI URL PMID |
| [19] | Hachiya T, Terashima I, Noguchi K (2007). Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia × hybrida petals. Plant, Cell & Environment, 30, 1269-1283. |
| [20] |
Hamerlynck EP, Huxman TE, McAuliffe JR, Smith SD (2004). Carbon isotope discrimination and foliar nutrient status of Larrea tridentata (creosote bush) in contrasting Mojave Desert soils. Oecologia, 138, 210-215.
DOI URL PMID |
| [21] |
Harley PC, Thomas RB, Reynolds JF, Strain BR (1992). Modeling photosynthesis of cotton grown in elevated CO2. Plant, Cell & Environment, 15, 271-282.
DOI URL |
| [22] |
Hikosaka K (1997). Modelling optimal temperature acclimation of the photosynthetic apparatus in C3 plants with respect to nitrogen use. Annals of Botany, 80, 721-730.
DOI URL |
| [23] |
Hikosaka K (2004). Interspecific difference in the photosynthesis- nitrogen relationship: patterns, physiological causes, and ecological importance. Journal of Plant Research, 117, 481-494.
DOI URL PMID |
| [24] |
Hikosaka K (2005). Nitrogen partitioning in the photosynthetic apparatus of Plantago asiatica leaves grown under different temperature and light conditions: similarities and differences between temperature and light acclimation. Plant and Cell Physiology, 46, 1283-1290.
DOI URL PMID |
| [25] | Hikosaka K, Murakami A, Hirose T (1999). Balancing carboxylation and regeneration of ribulose-1,5-bisphosphate in leaf photosynthesis: temperature acclimation of an evergreen tree, Quercus myrsinaefolia. Plant, Cell & Environment, 22, 841-849. |
| [26] |
Hultine KR, Marshall JD (2000). Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia, 123, 32-40.
DOI URL PMID |
| [27] |
Johnson FH, Eyring H, Williams RW (1942). The nature of enzyme inhibitions in bacterial luminescence: sulfanilamide, urethane, temperature and pressure. Journal of Cellular and Comparative Physiology, 20, 247-268.
DOI URL |
| [28] |
Jordon DB, Ogren WL (1984). The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Dependence on ribulosebisphosphate concentration, pH and temperature. Planta, 161, 308-313.
DOI URL PMID |
| [29] | Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T (2001). CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant, Cell & Environment, 24, 529-538. |
| [30] |
Körner C (1989). The nutritional status of plants from high altitudes: a worldwide comparison. Oecologia, 81, 379-391.
DOI URL PMID |
| [31] |
Körner C, Diemer M (1987). In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Functional Ecology, 1, 179-194.
DOI URL |
| [32] |
Kumar N, Kumar S, Ahuja PS (2005). Photosynthetic characteristics of Hordeum, Triticum, Rumex, and Trifolium species at contrasting altitudes. Photosynthetica, 43, 195-201.
DOI URL |
| [33] |
Kumar N, Vyas D, Kumar S (2007). Plants at high altitude exhibit higher component of alternative respiration. Journal of Plant Physiology, 164, 31-38.
URL PMID |
| [34] | Lambers H, Chapin FS III, Pons TL (1998). Plant Physiological Ecology. Springer, New York. |
| [35] | Li AR (1998). Flora of China (Volume 25th, 1st fascicle). Science Press, Beijing. 161. (in Chinese) |
| [ 李安仁 (1998). 中国植物志(第25卷第一分册). 科学出版社, 北京. 161.] | |
| [36] |
Loomis RS (1997). On the utility of nitrogen in leaves. Proceedings of the National Academy of Sciences of the United States of America, 94, 13378-13379.
URL PMID |
| [37] |
Loreto F, Harley PC, di Marco G, Sharkey TD (1992). Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology, 98, 1437-1443.
DOI URL PMID |
| [38] | Niinemets Ü, Tenhunen JD (1997). A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant, Cell & Environment, 20, 845-866. |
| [39] | Nolan WG, Smillie RM (1976). Multi-temperature effects on Hill reaction activity of barley chloroplast. Biochimica Biophysica Acta, 440, 461-475. |
| [40] | Onoda Y, Hikosaka K, Hirose T (2004). Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Functional Ecology, 18, 419-425. |
| [41] |
Reich PB, Ellsworth DS, Walters MB (1998). Leaf structure (specific leaf area) modulates photosynthesis-nitrogen relations: evidence from within and across species and functional groups. Functional Ecology, 12, 948-958.
DOI URL |
| [42] |
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991). Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia, 86, 16-24.
DOI URL PMID |
| [43] |
Reich PB, Waiters MB, Kloeppel BD, Ellsworth DS (1995). Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia, 104, 24-30.
URL PMID |
| [44] |
Ryan MG (1991). Effects of climate change on plant respiration. Ecological Applications, 1, 157-167.
DOI URL PMID |
| [45] |
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment, 30, 1035-1040.
URL PMID |
| [46] | Takashima T, Hikosaka K, Hirose T (2004). Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant, Cell & Environment, 27, 1047-1054. |
| [47] |
von Caemmerer S, Farquhar GD (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta, 153, 376-387.
DOI URL PMID |
| [48] |
Wang GA, Han JM, Faiia A, Tan WB, Shi WQ, Liu XZ (2008). Experimental measurements of leaf carbon isotope discrimination and gas exchange in the progenies of Plantago depressa and Setaria viridis collected from a wide altitudinal range. Physiologia Plantarum, 134, 64-73.
DOI URL PMID |
| [49] |
Westbeek MHM, Pons TL, Cambridge ML, Atkin OK (1999). Analysis of differences in photosynthetic nitrogen use efficiency of alpine and lowland Poa species. Oecologia, 120, 19-26.
URL PMID |
| [50] | Wright IJ, Cannon K (2001). Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Functional Ecology, 15, 351-359. |
| [51] |
Wright IJ, Groom PK, Lamont BB, Poot P, Prior LD, Reich PB, Schulze ED, Veneklaas EJ, Westoby M (2004). Leaf trait relationships in Australian plant species. Functional Plant Biology, 31, 551-558.
DOI URL PMID |
| [52] | Wullschleger SD (1993). Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. Journal of Experimental Botany, 44, 907-920. |
| [53] | Yamori W, Noguchi K, Terashima I (2005). Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant, Cell & Environment, 28, 536-547. |
| [1] | ZHANG Peng, WANG Gang, ZHANG Tao, CHEN Nian-Lai. Responses of foliar δ13C in Sabina przewalskii and Picea crassifolia to altitude and its mechanism in the Qilian Mountains, China [J]. Chin J Plant Ecol, 2010, 34(2): 125-133. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn