

Chin J Plant Ecol ›› 2017, Vol. 41 ›› Issue (6): 632-638.DOI: 10.17521/cjpe.2016.0286
• Research Articles • Previous Articles Next Articles
Ya-Han CHEN, Zong-Qiang XIE*( )
)
Received:2017-02-28
Accepted:2016-09-13
Online:2017-06-10
Published:2017-07-19
Contact:
Zong-Qiang XIE
About author:KANG Jing-yao(1991-), E-mail: Ya-Han CHEN, Zong-Qiang XIE. Effects of storage conditions on total carbon and nitrogen contents of soil and plant samples[J]. Chin J Plant Ecol, 2017, 41(6): 632-638.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2016.0286
| 粒径<0.15 mm Particle size <0.15 mm | 粒径<2 mm Particle size <2 mm 低温 Low temperature | ||
|---|---|---|---|
| 低温 Low temperature | 常温 Room temperature | ||
| 碳质量分数 Carbon mass fraction (%) | |||
| 2012 | 1.56 ± 2.23 | 0.806 ± 0.634 | 1.54 ± 0.65 |
| 2016 | 1.53 ± 2.09 | 0.826 ± 0.633 | 1.69 ± 0.88 |
| 两次测试差异 Difference between the two times | -0.04* | 0.02* | 0.15* |
| 氮质量分数 Nitrogen mass fraction (%) | |||
| 2012 | 0.129 ± 0.154 | 0.072 ± 0.036 | 0.128 ± 0.076 |
| 2016 | 0.129 ± 0.157 | 0.075 ± 0.037 | 0.138 ± 0.087 |
| 两次测试差异 Difference between the two times | ns | 0.003* | 0.01* |
Table 1 Carbon and nitrogen mass fractions of soil samples analyzed in 2012 and 2016 (mean ± SD)
| 粒径<0.15 mm Particle size <0.15 mm | 粒径<2 mm Particle size <2 mm 低温 Low temperature | ||
|---|---|---|---|
| 低温 Low temperature | 常温 Room temperature | ||
| 碳质量分数 Carbon mass fraction (%) | |||
| 2012 | 1.56 ± 2.23 | 0.806 ± 0.634 | 1.54 ± 0.65 |
| 2016 | 1.53 ± 2.09 | 0.826 ± 0.633 | 1.69 ± 0.88 |
| 两次测试差异 Difference between the two times | -0.04* | 0.02* | 0.15* |
| 氮质量分数 Nitrogen mass fraction (%) | |||
| 2012 | 0.129 ± 0.154 | 0.072 ± 0.036 | 0.128 ± 0.076 |
| 2016 | 0.129 ± 0.157 | 0.075 ± 0.037 | 0.138 ± 0.087 |
| 两次测试差异 Difference between the two times | ns | 0.003* | 0.01* |
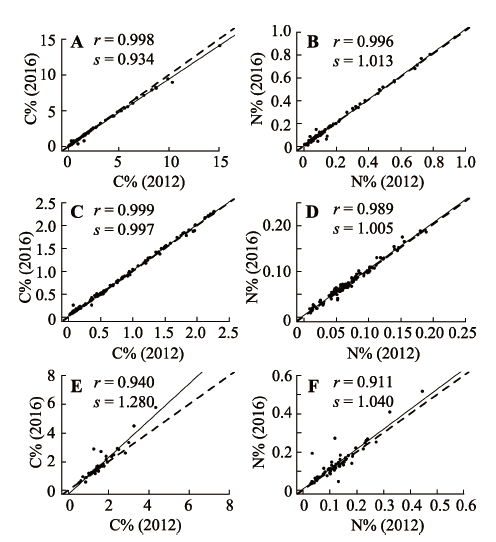
Fig. 1 Linear regression relationship between carbon/nitrogen mass fractions of soil samples analyzed in 2012 and 2016 under different storage conditions. A, B, low temperature (<20 °C), particle size <0.15 mm. C, D, room temperature, particle size <0.15 mm. E, F, low temperature (<20 °C), particle size <2 mm. C%, carbon mass fraction; N%, nitrogen mass fraction; r, correlation coefficient; s, slope of linear regression; solid line, regression line; dashed line, 1:1 line.
| 低温 Low temperature 粒径<2 mm Particle size <2 mm | 常温 Room temperature 粒径<0.15 mm Particle size <0.15 mm | |
|---|---|---|
| 碳质量分数 Carbon mass fraction (%) | ||
| 2012 | 44.30 ± 4.40 | 44.30 ± 4.40 |
| 2016 | 46.09 ± 4.21 | 44.89 ± 4.42 |
| 两次测试差异 Difference between the two times | 1.79* | 0.58* |
| 氮质量分数 Nitrogen mass fraction (%) | ||
| 2012 | 0.789 ± 0.620 | 0.789 ± 0.620 |
| 2016 | 0.796 ± 0.622 | 0.774 ± 0.619 |
| 两次测试差异 Difference between the two times | ns | -0.01* |
Table 2 Carbon and nitrogen mass fractions of plant samples analyzed in 2012 and 2016 (mean ± SD)
| 低温 Low temperature 粒径<2 mm Particle size <2 mm | 常温 Room temperature 粒径<0.15 mm Particle size <0.15 mm | |
|---|---|---|
| 碳质量分数 Carbon mass fraction (%) | ||
| 2012 | 44.30 ± 4.40 | 44.30 ± 4.40 |
| 2016 | 46.09 ± 4.21 | 44.89 ± 4.42 |
| 两次测试差异 Difference between the two times | 1.79* | 0.58* |
| 氮质量分数 Nitrogen mass fraction (%) | ||
| 2012 | 0.789 ± 0.620 | 0.789 ± 0.620 |
| 2016 | 0.796 ± 0.622 | 0.774 ± 0.619 |
| 两次测试差异 Difference between the two times | ns | -0.01* |
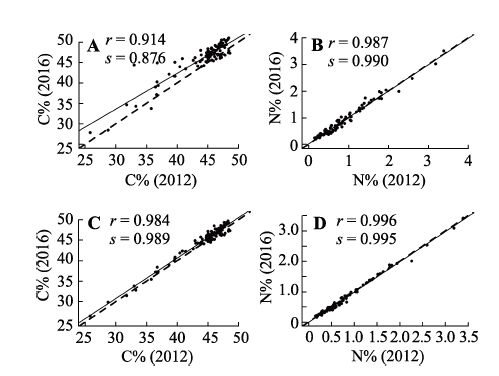
Fig. 2 Linear regression relationship between carbon/nitrogen mass fraction of plant samples analyzed in 2012 and 2016 under different storage conditions. A, B, low temperature (<20 °C), particle size <2 mm. C, D, room temperature, particle size <0.15 mm. C%, carbon mass fraction; N%, nitrogen mass fraction; r, correlation coefficient; s, slope of linear regression; solid line, regression line; dashed line, 1:1 line.
| [1] | Alef K, Nannipieri P (1995). Methods in Applied Soil Microbiology and Biochemistry. Academic Press, London. |
| [2] | Ali AA, Xu CG, Rogers A, McDowell NG, Medlyn BE, Fisher RA, Wullschleger SD, Reich PB, Vrugt JA, Bauerle WL, Santiago LS, Wilson CJ (2015). Global-scale environmental control of plant photosynthetic capacity. Ecological Applications, 25, 2349-2365. |
| [3] | Amaral CDB, Nobrega JA, Nogueira ARA (2014). Investigation of arsenic species stability by HPLC-ICP-MS in plants stored under different conditions for 12 months. Microchemical Journal, 117, 122-126. |
| [4] | Blake L, Goulding KWT, Mott CJB, Poulton PR (2000). Temporal changes in chemical properties of air-dried stored soils and their interpretation for long-term experiments. European Journal of Soil Science, 51, 345-353. |
| [5] | Cernohlavkova J, Jarkovsky J, Negporova M, Hofman J (2009). Variability of soil microbial properties: Effects of sampling, handling and storage. Ecotoxicology and Environmental Safety, 72, 2102-2108. |
| [6] | Cui H, Wang CH, Gu ZH, Zhu HH, Fu SL, Yao Q (2014). Evaluation of soil storage methods for soil microbial community using genetic and metabolic fingerprintings. European Journal of Soil Biology, 63, 55-63. |
| [7] | Dadenko EV, Kazeev KS, Kolesnikov SI, Val’kov VF (2009). Changes in the enzymatic activity of soil samples upon their storage. Eurasian Soil Science, 42, 1380-1385. |
| [8] | de Nobili M, Contin M, Brookes PC (2006). Microbial biomass dynamics in recently air-dried and rewetted soils compared to others stored air-dry for up to 103 years. Soil Biology & Biochemistry, 38, 2871-2881. |
| [9] | Dolfing J, Vos A, Bloem J, Ehlert PAI, Naumova NB, Kuikman PJ (2004). Microbial diversity in archived soils. Science, 306, 813-813. |
| [10] | Eichenberg D, Ristok C, Krober W, Bruelheide H (2014). Plant polyphenols—Implications of different sampling, storage and sample processing in biodiversity-ecosystem functioning experiments. Chemistry and Ecology, 30, 676-692. |
| [11] | Fang F, Hu YK, Gong YM, Tang HP (2015). Soil organic carbon of different decomposition rate and its relation to microbial activity in saline-alkali desert ecosystem. Polish Journal of Ecology, 63, 102-109. |
| [12] | Ginn BR, Habteselassie MY, Meile C, Thompson A (2014). Effects of sample storage on microbial Fe-reduction in tropical rainforest soils. Soil Biology & Biochemistry, 68, 44-51. |
| [13] | Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, Schmid HP, Richardson AD (2013). Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature, 499, 324-327. |
| [14] | Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, Mellado-Vazquez PG, Malik AA, Roy J, Scheu S, Steinbeiss S, Thomson BC, Trumbore SE, Gleixner G (2015). Plant diversity increases soil microbial activity and soil carbon storage. Nature Communications, 6, 6707. |
| [15] | Moon-van der Staay SY, Tzeneva VA, van der Staay GWM, de Vos WM, Smidt H, Hackstein JHP (2006). Eukaryotic diversity in historical soil samples. Fems Microbiology Ecology, 57, 420-428. |
| [16] | Niu SL, Sherry RA, Zhou XH, Wan SQ, Luo YQ (2010). Nitrogen regulation of the climate-carbon feedback: Evidence from a long-term global change experiment. Ecology, 91, 3261-3273. |
| [17] | Pence VC (2014). Tissue cryopreservation for plant conservation: Potential and challenges. International Journal of Plant Sciences, 175, 40-45. |
| [18] | Prodromou KP, Pavlatou-Ve AS (1998). Changes in soil pH due to the storage of soils. Soil Use and Management, 14, 182-183. |
| [19] | Rubin BER, Gibbons SM, Kennedy S, Hampton-Marcell J, Owens S, Gilbert JA (2013). Investigating the impact of storage conditions on microbial community composition in soil samples. PLOS ONE, 8, e70460. doi:10.1371/journal. pone.0070460. |
| [20] | Shan H, Zhang QZ, Zhang XJ, Han RY, Feng ZH (2013). Effects of preservation, analysis method on determination of nitrate in soils.Journal of Instrumental Analysis, 12, 1466-1471. (in Chinese with English abstract)[陕红, 张庆忠, 张晓娟, 韩瑞芸, 封朝晖 (2013). 保存、分析方法等因素对土壤中硝态氮测定的影响. 分析测试学报, 12, 1466-1471.] |
| [21] | Staats M, Cuenca A, Richardson JE, Vrielink-van Ginkel R, Petersen G, Seberg O, Bakker FT (2011). DNA damage in plant herbarium tissue. PLOS ONE, 6, e28448. doi:10.1371/journal.pone.0028448. |
| [22] | Techinical Manual Writing Group of Ecosystem Carbon Sequestration Project (2015).Observation and Investigation for Carbon Sequestration in Terrestrial Ecosystems.Science Press, Beijing. 11-16. (in Chinese)[生态系统固碳项目技术规范编写组 (2015). 生态系统固碳观测与调查技术规范.科学出版社, 北京. 11-16.] |
| [23] | Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C (2001). Diversity and productivity in a long-term grassland experiment. Science, 294, 843-845. |
| [24] | Tzeneva VA, Salles JF, Naumova N, de Vos WA, Kuikman PJ, Dolfing J, Smidt H (2009). Effect of soil sample preservation, compared to the effect of other environmental variables, on bacterial and eukaryotic diversity. Research in Microbiology, 160, 89-98. |
| [25] | Vlassak K (1970). Total soil nitrogen and nitrogen mineralization. Plant and Soil, 32, 27-32. |
| [26] | Wallenius K, Rita H, Simpanen S, Mikkonen A, Niemi RM (2010). Sample storage for soil enzyme activity and bacterial community profiles. Journal of Microbiological Methods, 81, 48-55. |
| [27] | Warton D ( |
| [28] | Weiss CL, Schuenemann VJ, Devos J, Shirsekar G, Reiter E, Gould BA, Stinchcombe JR, Krause J, Burbano HA (2016). Temporal patterns of damage and decay kinetics of DNA retrieved from plant herbarium specimens. Royal Society Open Science, 3, 160239. doi: 10.1098/rsos.160239. |
| [29] | Worm B, Sandow M, Oschlies A, Lotze HK, Myers RA (2005). Global patterns of predator diversity in the open oceans. Science, 309, 1365-1369. |
| [30] | Yan XL, Chen TB, Liao XY, Xie H, Zhai LM (2005). Transformation of arsenic species in soil samples stored under different conditions: Recommended technique of soil sample storage for arsenic species analysis.Acta Scientiae Circumstantiae, 25, 976-981. (in Chinese with English abstract)[阎秀兰, 陈同斌, 廖晓勇, 谢华, 翟丽梅 (2005). 土壤样品保存过程中无机砷的形态变化及其样品保存方法. 环境科学学报, 25, 976-981.] |
| [31] | Yu QA, Elser JJ, He NP, Wu HH, Chen QS, Zhang GM, Han XG (2011). Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland.Oecologia, 166, 1-10. |
| [32] | Zhao BZ, Chen J, Zhang JB, Qin SW (2011). Effect of storage time of air-drying soil and incubation period following rewetting on soil enzyme activities in North China Plain.Soils, 43, 418-425. (in Chinese with English abstract)[赵炳梓, 陈吉, 张佳宝, 钦绳武 (2011) 风干土保存时间和湿土培育时间对黄淮海平原潮土酶活性的影响. 土壤, 43, 418-425.] |
| [33] | Zhou Y, Cui H, Zhu HH, Fu SL, Yao Q (2015). Effects of long- term soil storage on the metabolic activity and functional groups of soil microbial community.Microbiology China, 42, 1017-1024. (in Chinese with English abstract)[周杨, 崔航, 朱红惠, 傅声雷, 姚青 (2015). 土壤样品长期保存对微生物群落代谢活性和功能类群的影响. 微生物学通报, 42, 1017-1024.] |
| [34] | Zhou ZY, Zhang ZQ, Zha TG, Luo ZK, Zheng JM, Sun OJ (2013). Predicting soil respiration using carbon stock in roots, litter and soil organic matter in forests of Loess Plateau in China. Soil Biology & Biochemistry, 57, 135-143. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn