

植物生态学报 ›› 2023, Vol. 47 ›› Issue (7): 1020-1031.DOI: 10.17521/cjpe.2022.0335
所属专题: 微生物生态学
张仲富1,2, 王四海1,2,*( ), 杨卫1, 陈剑1,2
), 杨卫1, 陈剑1,2
收稿日期:2022-08-18
接受日期:2023-03-13
出版日期:2023-07-20
发布日期:2023-07-21
通讯作者:
*王四海(作者简介:ORCID: 王四海: 0000-0002-4143-4919
基金资助:
ZHANG Zhong-Fu1,2, WANG Si-Hai1,2,*( ), YANG Wei1, CHEN Jian1,2
), YANG Wei1, CHEN Jian1,2
Received:2022-08-18
Accepted:2023-03-13
Online:2023-07-20
Published:2023-07-21
Contact:
*WANG Si-Hai(Supported by:摘要:
为了解蒜头果(Malania oleifera)植株根际微生物组成和功能特征与蒜头果健康状态的关系, 该研究采集了阔叶林、人工种植林和喀斯特森林下的5种不同生境的健康和非健康蒜头果植株根际土壤样品, 运用Illumina高通量测序技术进行细菌测序, 并用FAPROTAX进行功能预测分析。研究结果表明: 1)扩增子序列变异体(ASV)分析结果显示, 在门水平上不同生境的细菌组成存在一定差异, 其中相对丰度最高的前5个门的细菌类群为酸杆菌门、变形菌门、放线菌门、绿弯菌门和黏菌门。健康植株和非健康植株的根际细菌组成有显著的差异, 且细菌群落的优势菌群发生显著的变化。2)非度量多维尺度分析显示不同健康状态蒜头果的根际细菌组成有显著差异, 冗余分析结果显示健康植株的样本沿第一轴分布, 两轴共解释了25.83%的细菌群落变异, 土壤速效磷含量、总钾含量和pH是影响健康植株根际细菌群落物种组成的主要因子。非健康植株的冗余分析结果显示两轴累积解释了51.84%的细菌群落物种组成变异, 土壤总钾含量和速效磷含量是影响其物种组成的重要因子。3)相关性热图显示健康植株根际土壤pH、速效磷含量和全钾含量与绿弯菌门、浮霉菌门、Methylomirabilota和Desulfobacterota等主要类群的相对丰度显著相关。非健康植株的土壤pH、速效氮含量、速效磷含量、总磷含量和全钾含量与绿弯菌门、酸杆菌门、Desulfobacterota、Latescibacterota和芽单胞菌门等主要类群的相对丰度显著相关。4) FAPROTAX功能预测结果显示光营养、光能自养、芳香化合物降解、蓝藻细菌和含氧光能自养等细菌功能群相对丰度在健康植株的根际土壤中明显下降, 而发酵、尿素分解和人类病原菌等细菌功能群相对丰度则明显升高, 说明不同健康状态植株的根际细菌发生了显著的变化。
张仲富, 王四海, 杨卫, 陈剑. 蒜头果根际细菌群落结构与功能特征对其健康状态的响应. 植物生态学报, 2023, 47(7): 1020-1031. DOI: 10.17521/cjpe.2022.0335
ZHANG Zhong-Fu, WANG Si-Hai, YANG Wei, CHEN Jian. Response of rhizosphere microbial community structure and functional characteristics to health status of Malania oleifera. Chinese Journal of Plant Ecology, 2023, 47(7): 1020-1031. DOI: 10.17521/cjpe.2022.0335
| 采集地点 Sample location | 海拔 Altitude (m) | 生境 Biotope | 生境特征 Habitat characteristic | 采样数量 Sample size |
|---|---|---|---|---|
| 广南县拖董村 Tuodong village, Guangnan County | 1 280 | 自然分布 Natural distribution | 黄壤, 常绿阔叶林 Yellow soil, evergreen broadleaf forest | 健康和非健康植株各6株 6 healthy and 6 non-healthy individuals |
| 广南县岩腊村 Aila village, Guangnan County | 1 270 | 自然分布 Natural distribution | 石灰岩山地, 蒜头果为主的疏林地 Limestone mountain, Malania oleifera sparse woods | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
| 广南县中就村人工基地 Artificial cultivation in Zhongjiu village, Guangnan County | 1 410 | 人工种植 Artificial cultivation | 黄壤, 人工种植疏杉木林地 Yellow soil, artificial cultivation Cunninghamia lanceolata sparse woods | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
| 富宁县木都村 Mudu village, Funing County | 850 | 自然分布 Natural distribution | 赤红壤, 季风常绿阔叶林 Lateritic red soil, monsoon evergreen broadleaf forest | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
| 富宁县那见村 Najian village, Funing County | 550 | 人工种植 Artificial cultivation | 赤红壤, 次生季风常绿阔叶林 Lateritic red soil, secondary monsoon evergreen broadleaf forest | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
表1 蒜头果采样点信息
Table 1 Sampling site information of Malania oleifera
| 采集地点 Sample location | 海拔 Altitude (m) | 生境 Biotope | 生境特征 Habitat characteristic | 采样数量 Sample size |
|---|---|---|---|---|
| 广南县拖董村 Tuodong village, Guangnan County | 1 280 | 自然分布 Natural distribution | 黄壤, 常绿阔叶林 Yellow soil, evergreen broadleaf forest | 健康和非健康植株各6株 6 healthy and 6 non-healthy individuals |
| 广南县岩腊村 Aila village, Guangnan County | 1 270 | 自然分布 Natural distribution | 石灰岩山地, 蒜头果为主的疏林地 Limestone mountain, Malania oleifera sparse woods | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
| 广南县中就村人工基地 Artificial cultivation in Zhongjiu village, Guangnan County | 1 410 | 人工种植 Artificial cultivation | 黄壤, 人工种植疏杉木林地 Yellow soil, artificial cultivation Cunninghamia lanceolata sparse woods | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
| 富宁县木都村 Mudu village, Funing County | 850 | 自然分布 Natural distribution | 赤红壤, 季风常绿阔叶林 Lateritic red soil, monsoon evergreen broadleaf forest | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |
| 富宁县那见村 Najian village, Funing County | 550 | 人工种植 Artificial cultivation | 赤红壤, 次生季风常绿阔叶林 Lateritic red soil, secondary monsoon evergreen broadleaf forest | 健康和非健康植株各3株 3 healthy and 3 non-healthy individuals |

图1 健康和非健康的蒜头果植株。A, 健康植株: 树冠开展, 枝叶茂盛, 枯枝少。B, 非健康植株: 树冠小, 枝叶稀疏, 较多枯枝。
Fig. 1 Healthy and non-healthy plant morphology of Malania oleifera. A, Healthy individual: spreading crown, lush branches and leaves, less dead branches. B, Non-healthy individual: small crown, sparse branches and leaves, more dead branches.
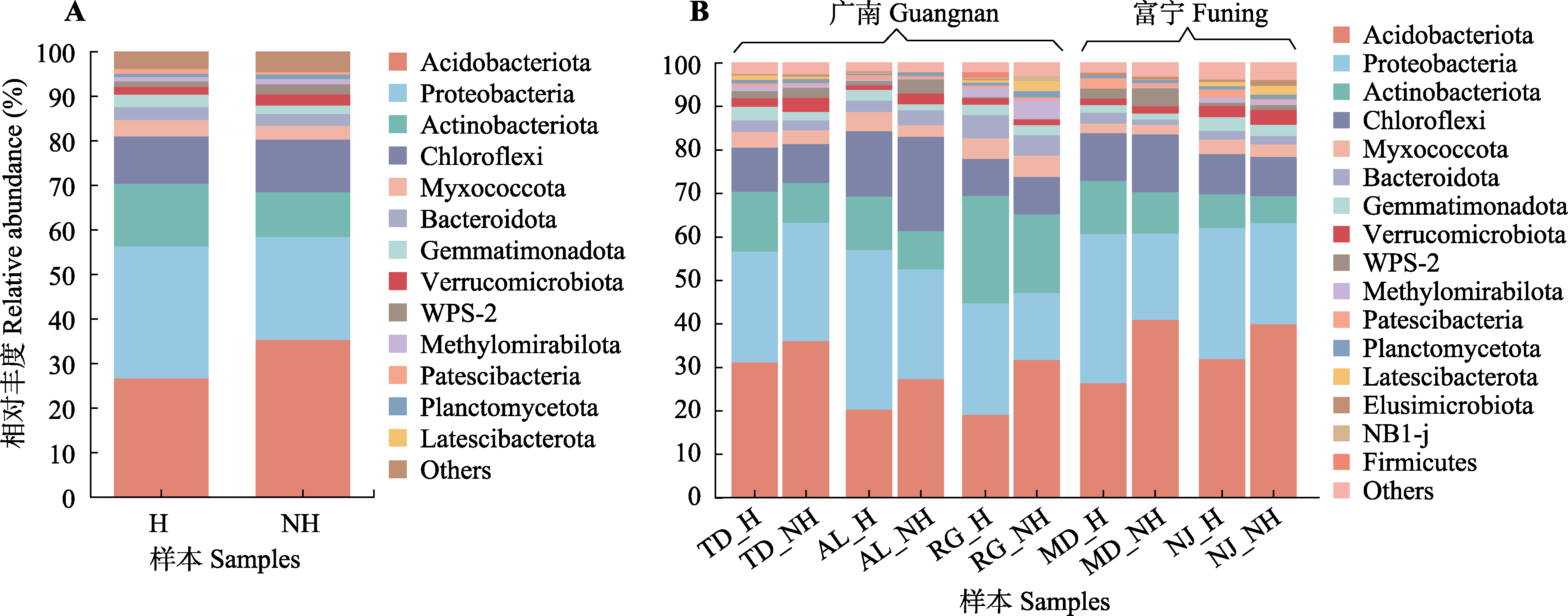
图2 蒜头果根际细菌群落组成。A, 健康植株与非健康植株的物种组成。B, 5种生境的细菌物种组成。H, 健康植株; NH, 非健康植株。AL, 岩腊村; MD, 木都村; NJ, 那见村; RG, 中就村人工基地; TD, 拖董村。Acidobacteriota, 酸杆菌门; Actinobacteriota, 放线菌门; Bacteroidota, 拟杆菌门; Chloroflexi, 绿弯菌门; Firmicutes, 厚壁菌门; Gemmatimonadota, 芽单胞菌门; Myxococcota, 黏菌门; Planctomycetota, 浮霉菌门; Proteobacteria, 变形菌门; Verrucomicrobiota, 疣微菌门; Others, 其他。
Fig. 2 Root rhizosphere bacteria relative abundance in Malania oleifera. A, Rhizosphere bacteria composition of healthy and non-healthy plants. B, Rhizosphere bacteria composition of five habitats. H, healthy individual; NH, non-healthy individual. AL, Aila village; MD, Mudu village; NJ, Najian village; RG, human planted forest of Zhongjiu village; TD, Tuodong village.
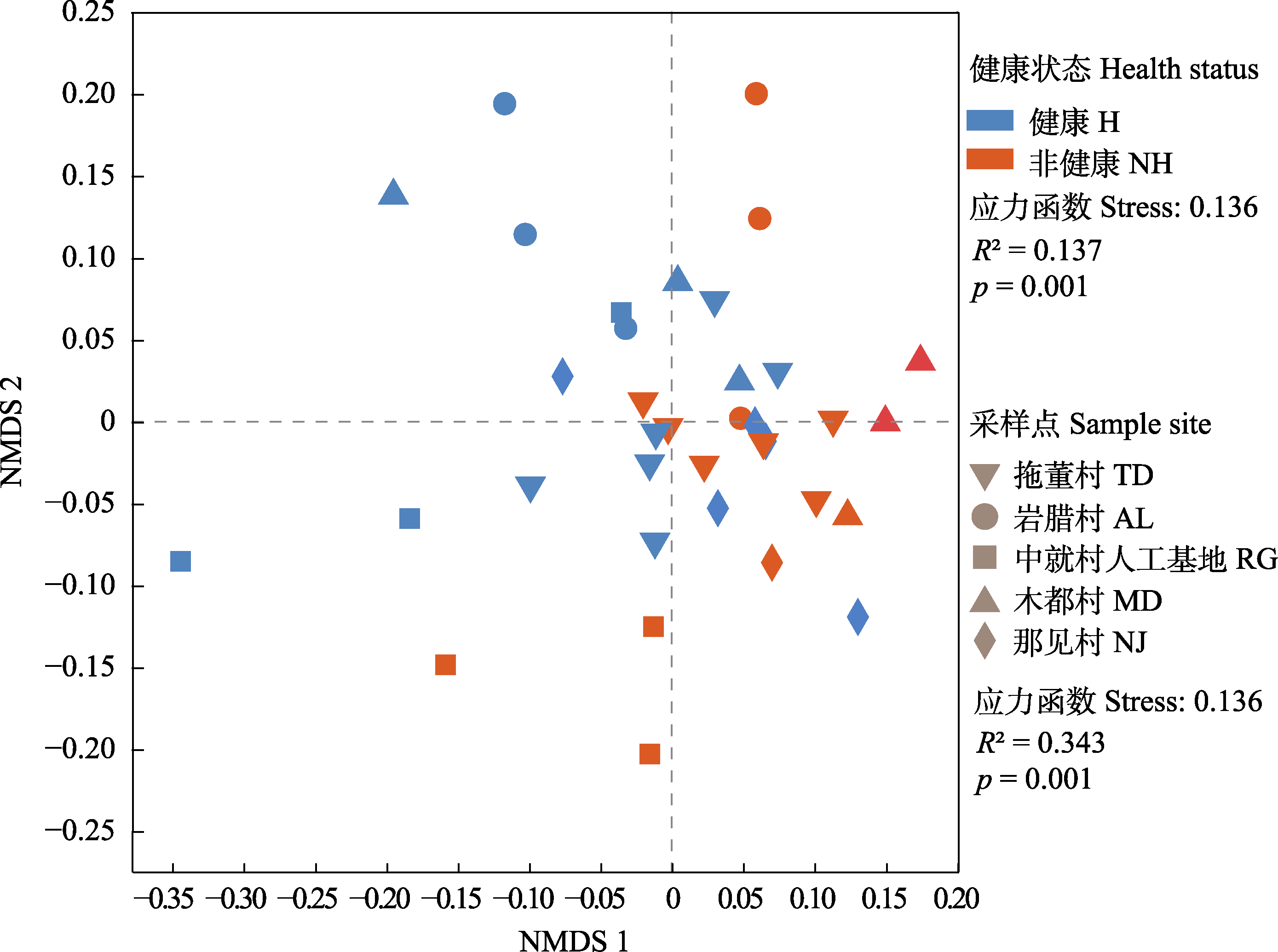
图3 蒜头果根际细菌群落组成的非度量多维尺度(NMDS)分析。
Fig. 3 Root rhizosphere bacteria community of non-metric multidimensional scaling (NMDS) analysis in Malania oleifera. H, healthy individual; NH, non-healthy individual. AL, Aila village; MD, Mudu village; NJ, Najian village; RG, human planted forest of Zhongjiu village; TD, Tuodong village.
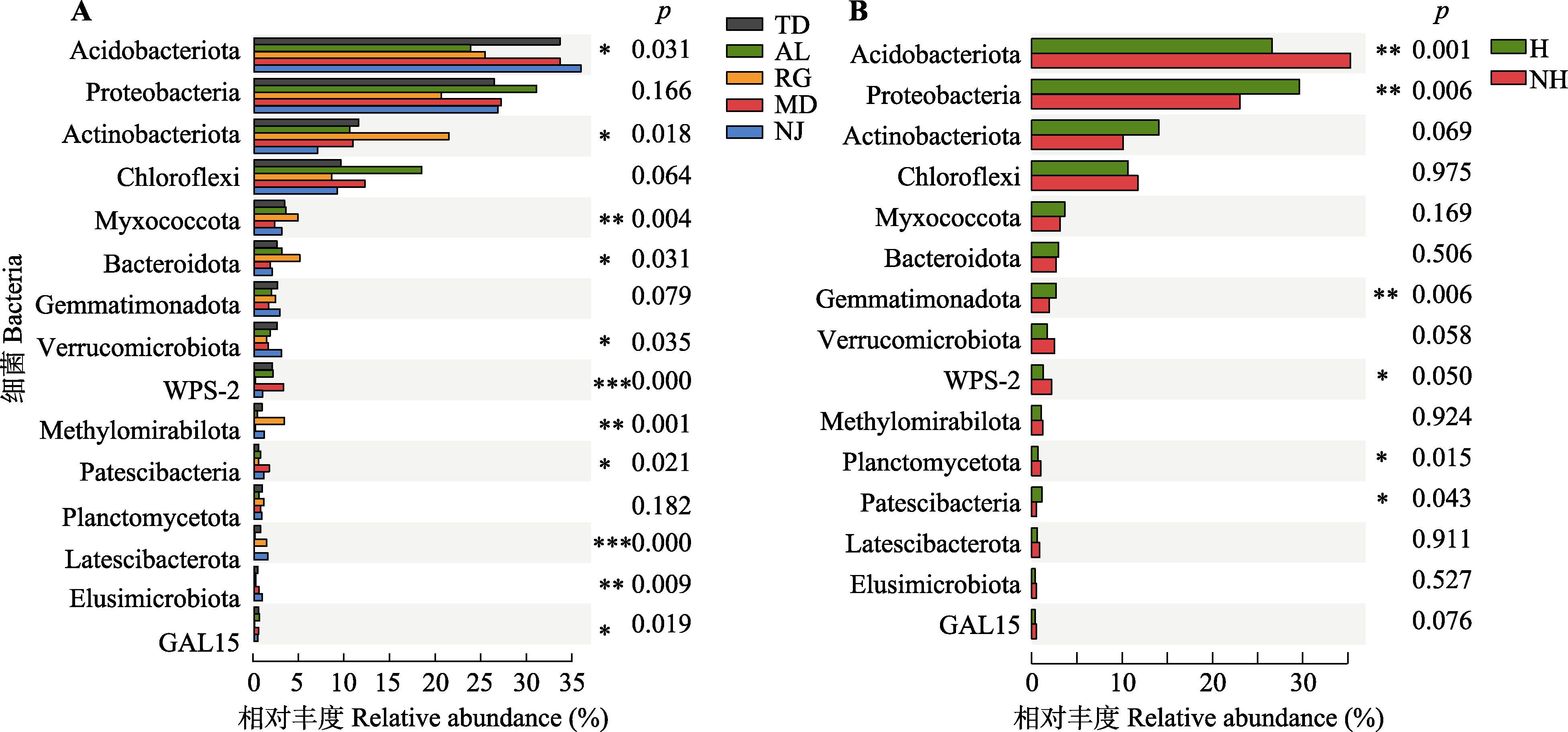
图4 蒜头果根际细菌群落的差异性检验。A, 不同地区差异性。B, 健康状态间差异性。*, 0.01 < p ≤ 0.05, **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001。H, 健康植株; NH, 非健康植株。AL, 岩腊村; MD, 木都村; NJ, 那见村; RG, 中就村人工基地; TD, 拖董村。细菌同图2。
Fig. 4 Difference of root rhizosphere bacteria community in Malania oleifera. A, Bacteria relative abundance in five habitats. B, Healthy and non-healthy individuals in five habitats. *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001. H, healthy individual; NH, non-healthy individual. AL, Aila village; MD, Mudu village; NJ, Najian village; RG, human planted forest of Zhongjiu village; TD, Tuodong village.
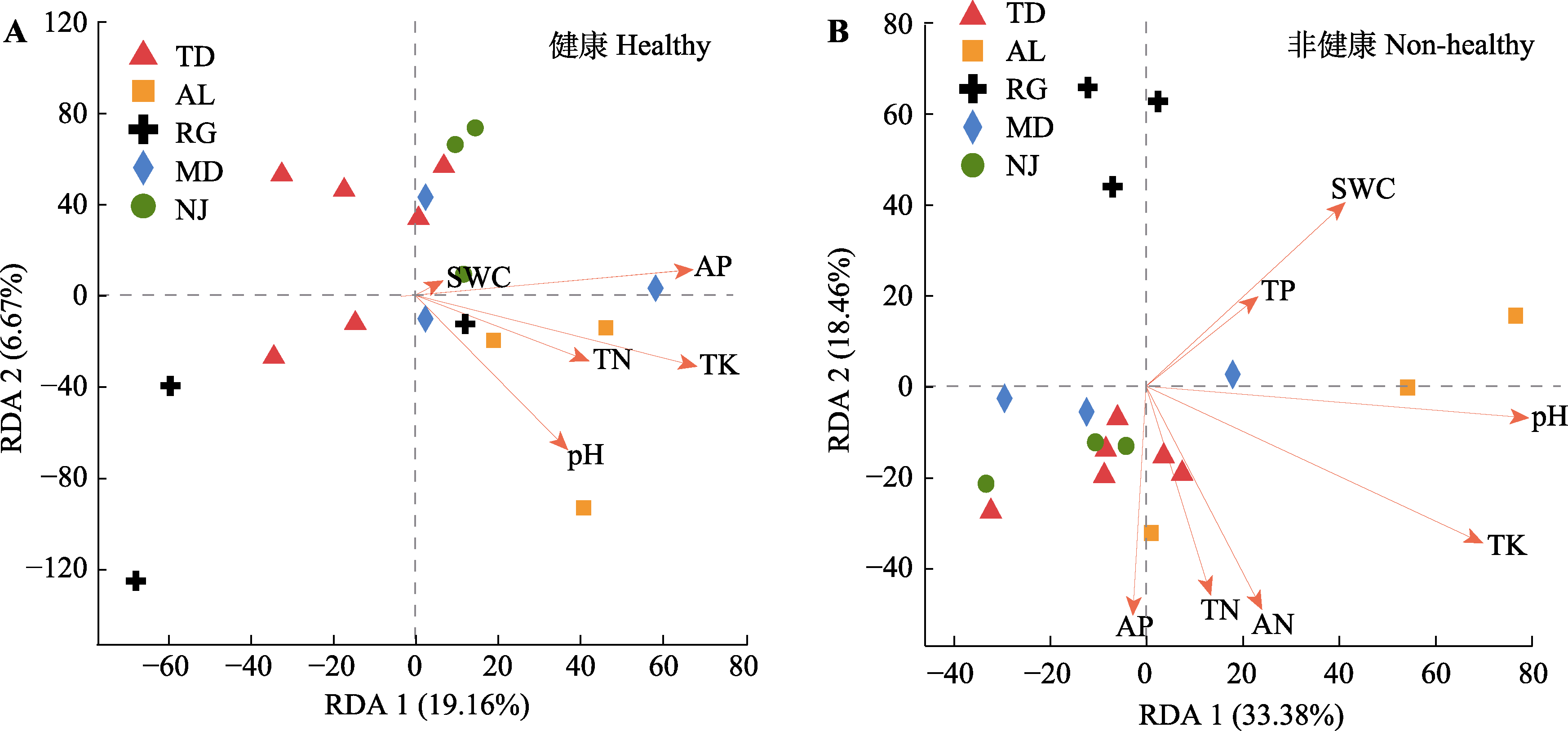
图5 蒜头果根际细菌的冗余分析(RDA)。A, 健康植株。B, 非健康植株。AN, 土壤速效氮含量; AP, 土壤速效磷含量; pH, 土壤pH; SWC, 土壤含水率; TK, 土壤总钾含量; TN, 土壤总氮含量; TP, 土壤总磷含量。AL, 岩腊村; MD, 木都村; NJ, 那见村; RG, 中就村人工基地; TD, 拖董村。
Fig. 5 Rhizosphere bacteria community of redundancy analysis (RDA) in Malania oleifera. A, Healthy individuals in five habitat. B, Non-healthy individuals in five habitats. AN, soil available nitrogen content; AP, soil available phosphorus content; pH, soil pH; SWC, soil water content; TK, soil total potassium content; TN, soil total nitrogen content; TP, soil total phosphorus content. AL, Aila village; MD, Mudu village; NJ, Najian village; RG, human planted forest of Zhongjiu village; TD, Tuodong village.
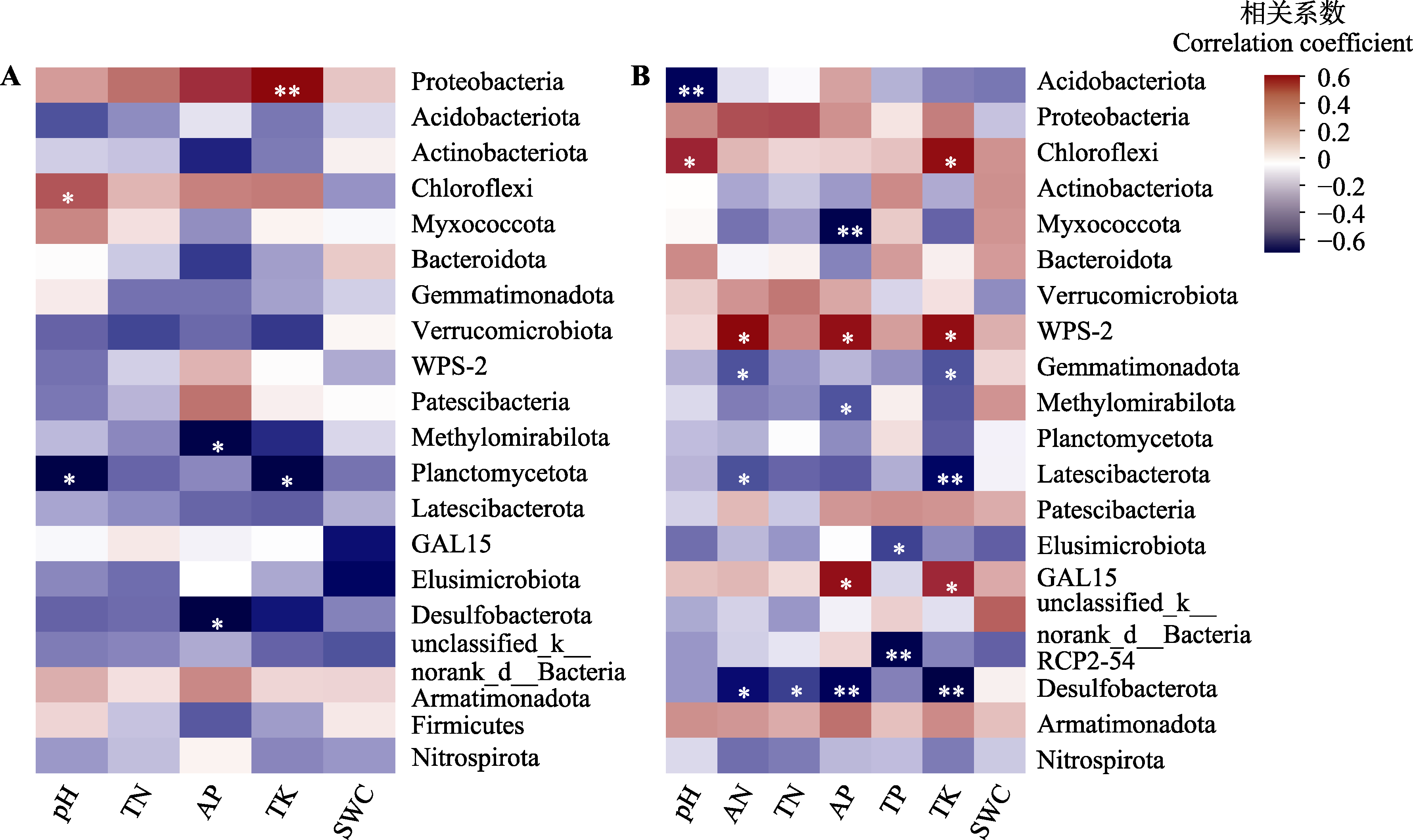
图6 环境因子与蒜头果根际细菌优势类群的皮尔逊相关性热图。A, 健康植株。B, 非健康植株。AN, 土壤速效氮含量; AP, 土壤速效磷含量; pH, 土壤pH; SWC, 土壤含水率; TK, 土壤总钾含量; TN, 土壤总氮含量; TP, 土壤总磷含量。*, 0.01 < p ≤ 0.05, **, 0.001 < p ≤ 0.01, ***, p ≤ 0.001。Nitrospirota, 硝化螺旋菌门。其他细菌同图2。
Fig. 6 Pearson correlation heatmap of environmental factors with rhizosphere bacterial taxa in Malania oleifera. A, Healthy individuals. B, Non-healthy individuals. AN, soil available nitrogen content; AP, soil available phosphorus content; pH, soil pH; SWC, soil water content; TK, soil total potassium content; TN, soil total nitrogen content; TP, soil total phosphorus content. *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
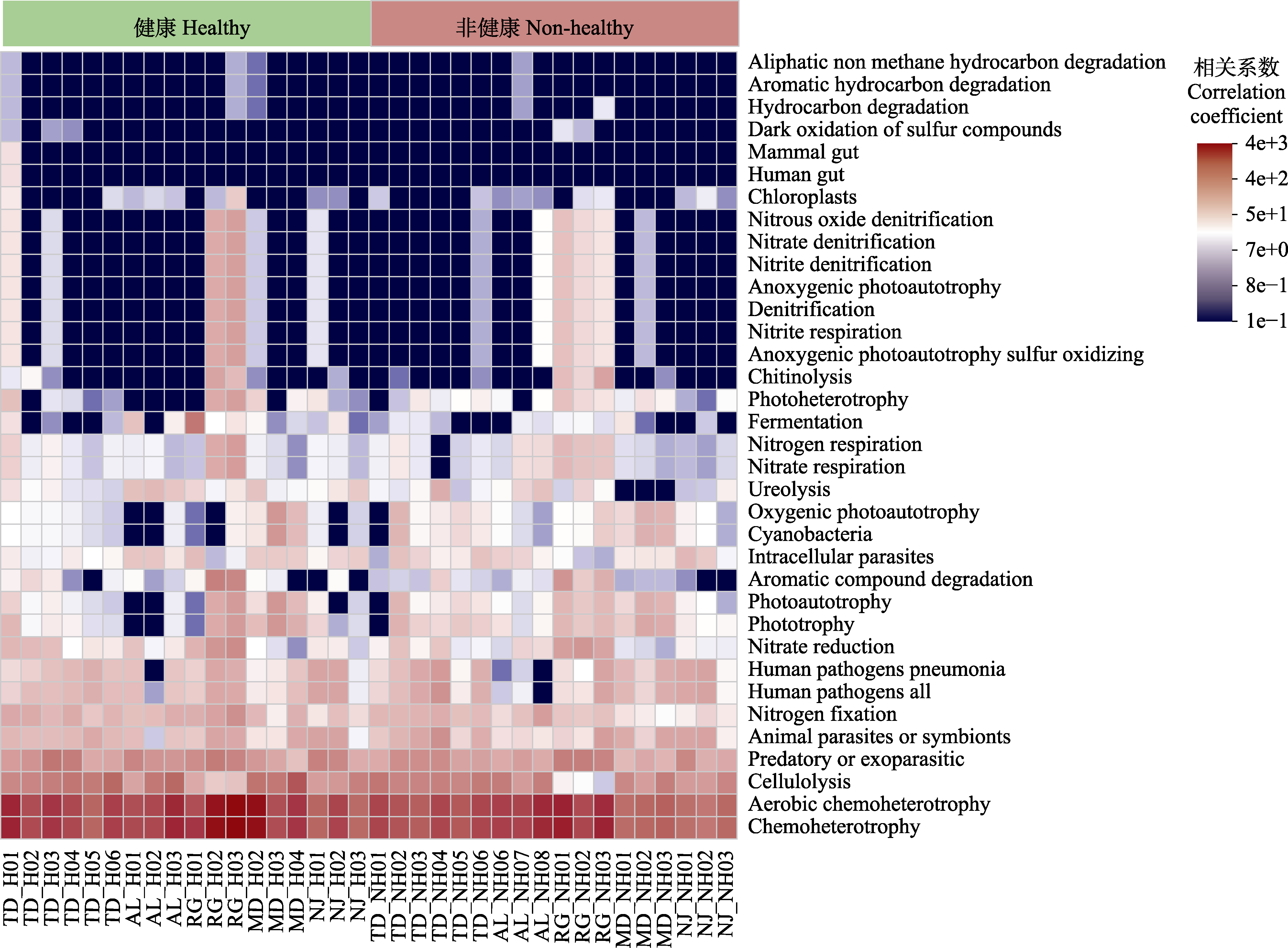
图7 蒜头果根际细菌功能预测相关性热图。Aerobic chemoheterotrophy, 有氧化能异养; Aliphatic non methane hydrocarbon degradation, 脂肪族非甲烷烃类降解; Animal parasites or symbionts, 动物寄生虫或共生体; Anoxygenic photoautotrophy, 无氧光能自养; Anoxygenic photoautotrophy sulfur oxidizing, 光合自养硫氧化; Aromatic compound degradation, 芳香族化合物降解; Aromatic hydrocarbon degradation, 芳族烃降解; Cellulolysis, 纤维素水解作用; Chemoheterotrophy, 化能异养; Chitinolysis, 几丁质分解; Chloroplasts, 叶绿体; Cyanobacteria, 蓝藻细菌; Dark oxidation of sulfur compounds, 硫化物暗氧化; Denitrification, 反硝化作用; Fermentation, 发酵; Human gut, 人类肠道; Human pathogens all, 人类病原菌; Human pathogens pneumonia, 人类病原体肺炎; Hydrocarbon degradation, 石油烃降解; Photoautotrophy, 光能自养; Photoheterotrophy, 光能异养; Phototrophy, 光营养; Mammal gut, 哺乳动物肠道菌; Nitrate denitrification, 硝化反硝化; Nitrate oxide denitrification, 氧化亚氮反硝化; Nitrate respiration, 硝酸盐呼吸; Nitrite denitrification, 亚硝酸盐脱氮; Nitrite respiration, 亚硝酸盐呼吸; Nitrogen respiration, 氮呼吸; Nitrogen fixation, 固氮作用; Oxygenic photoautotrophy, 含氧光能自养; Intracellular parasites, 胞内寄生物; Ureolysis, 尿素分解。H, 健康植株; NH, 非健康植株。AL, 岩腊村; MD, 木都村; NJ, 那见村; RG, 中就村人工基地; TD, 拖董村。
Fig. 7 Pearson correlation heatmap of predicted rhizosphere bacterial function in Malania oleifera. H, healthy individual; NH, non-healthy individual. AL, Aila village; MD, Mudu village; NJ, Najian village; RG, human planted forest of Zhongjiu village; TD, Tuodong village.
| [1] |
Agler MT, Ruhe J, Kroll S, Morhenn C, Kim ST, Weigel D, Kemen EM (2016). Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biology, 14, e1002352. DOI: 10.1371/journal.pbio.1002352.
DOI URL |
| [2] |
Andreote FD, de Cássia Pereira e Silva M (2017). Microbial communities associated with plants: learning from nature to apply it in agriculture. Current Opinion in Microbiology, 37, 29-34.
DOI PMID |
| [3] |
Astudillo-García C, Bell JJ, Webster NS, Glasl B, Jompa J, Montoya JM, Taylor MW (2017). Evaluating the core microbiota in complex communities: a systematic investigation. Environmental Microbiology, 19, 1450-1462.
DOI PMID |
| [4] |
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17, 478-486.
DOI PMID |
| [5] |
Bouffaud ML, Poirier MA, Muller D, Moënne-Loccoz Y (2014). Root microbiome relates to plant host evolution in maize and other Poaceae. Environmental Microbiology, 16, 2804-2814.
DOI URL |
| [6] |
Chaya A, Kurosawa N, Kawamata A, Kosugi M, Imura S (2019). Community structures of bacteria, archaea, and eukaryotic microbes in the freshwater glacier lake Yukidori-Ike in Langhovde, east Antarctica. Diversity, 11, 105. DOI: 10.3390/d11070105.
DOI |
| [7] |
Chen QL, Ding J, Zhu YG, He JZ, Hu HW (2020a). Soil bacterial taxonomic diversity is critical to maintaining the plant productivity. Environment International, 140, 105766. DOI: 10.1016/j.envint.2020.105766.
DOI URL |
| [8] |
Chen ZM, Wang Q, Ma JW, Chapman S, Zou P, Ye J, Yu QG, Sun WC, Lin H, Jiang LN (2020b). Soil microbial activity and community composition as influenced by application of pig biogas slurry in paddy field in southeast China. Paddy and Water Environment, 18, 15-25.
DOI |
| [9] |
Coleman-Derr D, Desgarennes D, Fonseca-Garcia C, Gross S, Clingenpeel S, Woyke T, North G, Visel A, Partida- Martinez LP, Tringe SG (2016). Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytologist, 209, 798-811.
DOI PMID |
| [10] |
Crossley MN, Dennison WC, Williams RR, Wearing AH (2002). The interaction of water flow and nutrients on aquatic plant growth. Hydrobiologia, 489, 63-70.
DOI URL |
| [11] |
Escudero-Martinez C, Bulgarelli D (2019). Tracing the evolutionary routes of plant-microbiota interactions. Current Opinion in Microbiology, 49, 34-40.
DOI PMID |
| [12] |
Fierer N, Jackson RB (2006). The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences of the United States of America, 103, 626-631.
DOI PMID |
| [13] | Fierer N, Leff Jonathan W, Adams Byron J, Nielsen Uffe N, Bates Scott T, Lauber Christian L, Owens S, Gilbert Jack A, Wall Diana H, Caporaso JG (2012). Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proceedings of the National Academy of Sciences of the United States of America, 109, 21390-21395. |
| [14] | Garrido-Oter R, Nakano RT, Dombrowski N, Ma KW, Team A, McHardy AC, Schulze-Lefert P (2018). Modular traits of the rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host & Microbe, 24, 155-167. |
| [15] |
Gschwendtner S, Esperschütz J, Buegger F, Reichmann M, Müller M, Munch JC, Schloter M (2011). Effects of genetically modified starch metabolism in potato plants on photosynthate fluxes into the rhizosphere and on microbial degraders of root exudates. FEMS Microbiology Ecology, 76, 564-575.
DOI PMID |
| [16] | Guo FB, Wang SH, Wang J, Zhu F, Chen ZH, Yuan XL (2018). Fruit yield and characters of wild Malania oleifera, a rare plant species in southwest China. Guihaia, 38, 57-64. |
| [郭方斌, 王四海, 王娟, 朱枫, 陈中华, 原晓龙 (2018). 珍稀植物蒜头果野生植株结实量及果实特征研究. 广西植物, 38, 57-64.] | |
| [17] |
Hamonts K, Trivedi P, Garg A, Janitz C, Grinyer J, Holford P, Botha FC, Anderson IC, Singh BK (2018). Field study reveals core plant microbiota and relative importance of their drivers. Environmental Microbiology, 20, 124-140.
DOI PMID |
| [18] |
Hossain Z, Sugiyama SI (2011). Geographical structure of soil microbial communities in northern Japan: effects of distance, land use type and soil properties. European Journal of Soil Biology, 47, 88-94.
DOI URL |
| [19] |
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017). The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Frontiers in Plant Science, 8, 1617. DOI: 10.3389/fpls.2017.01617.
DOI URL |
| [20] | Lai JY (2006). Study on Conservation Biology of Rare and Precious Plant Malania oleifera. PhD dissertation, Sichuang Univerisity, Chengdu. 73-85. |
| [赖家业 (2006). 珍稀植物蒜头果保护生物学研究. 博士学位论文, 四川大学, 成都. 73-85.] | |
| [21] |
Levy A, Salas Gonzalez I, Mittelviefhaus M, Clingenpeel S, Herrera Paredes S, Miao JM, Wang KR, Devescovi G, Stillman K, Monteiro F, Rangel Alvarez B, Lundberg DS, Lu TY, Lebeis S, Jin Z, et al. (2018). Genomic features of bacterial adaptation toplants. Nature Genetics, 50, 138-150.
DOI |
| [22] |
Lewis WH, Tahon G, Geesink P, Sousa DZ, Ettema TJG (2021). Innovations to culturing the uncultured microbial majority. Nature Reviews Microbiology, 19, 225-240.
DOI PMID |
| [23] |
Liu LM, Wang SS, Chen JF (2021a). Anthropogenic activities change the relationship between microbial community taxonomic composition and functional attributes. Environmental Microbiology, 23, 6663-6675.
DOI URL |
| [24] |
Liu ML, Li XR, Zhu RQ, Chen N, Ding L, Chen CY (2021b). Vegetation richness, species identity and soil nutrients drive the shifts in soil bacterial communities during restoration process. Environmental Microbiology Reports, 13, 411-424.
DOI URL |
| [25] | Louca S, Parfrey LW, Doebeli M (2016). Decoupling function and taxonomy in the global ocean microbiome. Science, 35, 1272-1277. |
| [26] |
Lu SP, Gischkat S, Reiche M, Akob DM, Hallberg KB, Küsel K (2010). Ecophysiology of Fe-cycling bacteria in acidic sediments. Applied and Environmental Microbiology, 76, 8174-8183.
DOI PMID |
| [27] |
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL (2012). Defining the core Arabidopsis thaliana root microbiome. Nature, 488, 86-90.
DOI |
| [28] |
Mendes R, Kruijt M, Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM (2011). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science, 332, 1097-1100.
DOI PMID |
| [29] |
Pankratov TA, Kirsanova LA, Kaparullina EN, Kevbrin VV, Dedysh SN (2012). Telmatobacter bradus Gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria, and emended description of Acidobacterium capsulatum Kishimoto et al. 1991. International Journal of Systematic and Evolutionary Microbiology, 62, 430-437.
DOI PMID |
| [30] |
Peters NK, Frost JW, Long SR (1986). A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science, 233, 977-980.
DOI PMID |
| [31] |
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013). Going back to the roots, the microbial ecology of the rhizosphere. Nature Reviews Microbiology, 11, 789-799.
DOI PMID |
| [32] |
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, van Wees SCM, Bakker PAHM (2014). Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology, 52, 347-375.
DOI PMID |
| [33] |
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009). The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant and Soil, 321, 341-361.
DOI URL |
| [34] |
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil, 321, 305-339.
DOI URL |
| [35] |
Rovira AD (1965). Interactions between plant roots and soil microorganisms. Annual Review of Microbiology, 19, 241-266.
PMID |
| [36] |
Saeed Q, Wang XK, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, Naseem M, Kintl A, Ejaz M, Naveed M, Brtnicky M, Mustafa A (2021). Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: a comprehensive review of effects and mechanisms. International Journal of Molecular Sciences, 22, 10529. DOI: 10.3390/ijms221910529.
DOI URL |
| [37] |
Schulz S, Giebler J, Chatzinotas A, Wick LY, Fetzer I, Welzl G, Harms H, Schloter M (2012). Plant litter and soil type drive abundance, activity and community structure of alkB harbouring microbes in different soil compartments. The ISME Journal, 6, 1763-1774.
DOI |
| [38] |
Trivedi P, Trivedi C, Grinyer J, Anderson IC, Singh BK (2016). Harnessing host-vector microbiome for sustainable plant disease management of phloem-limited bacteria. Frontiers in Plant Science, 7, 1423. DOI: 10.3389/fpls.2016.01423.
DOI PMID |
| [39] |
Vetterlein D, Carminati A, Kögel-Knabner I, Bienert GP, Smalla K, Oburger E, Schnepf A, Banitz T, Tarkka MT, Schlüter S (2020). Rhizosphere spatiotemporal organization—A key to rhizosphere functions. Frontiers in Agronomy, 2, 8. DOI: 10.3389/fagro.2020.00008.
DOI URL |
| [40] |
Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016). Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiological Research, 184, 13-24.
DOI PMID |
| [41] |
Wu LK, Lin XM, Lin WX (2014). Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chinese Journal of Plant Ecology, 38, 298-310.
DOI |
|
[吴林坤, 林向民, 林文雄 (2014). 根系分泌物介导下植物-土壤-微生物互作关系研究进展与展望. 植物生态学报, 38, 298-310.]
DOI |
|
| [42] |
Xue PP, Carrillo Y, Pino V, Minasny B, McBratney AB (2018). Soil properties drive microbial community structure in a large scale transect in south eastern Australia. Scientific Reports, 8, 11725. DOI: 10.1038/s41598-018-30005-8.
DOI |
| [43] |
Yang T, Tedersoo L, Soltis PS, Soltis DE, Gilbert JA, Sun M, Shi Y, Wang HF, Li YT, Zhang J, Chen ZD, Lin HY, Zhao YP, Fu CX, Chu HY (2019). Phylogenetic imprint of woody plants on the soil mycobiome in natural mountain forests of eastern China. The ISME Journal, 13, 686-697.
DOI |
| [44] |
York LM, Carminati A, Mooney SJ, Ritz K, Bennett MJ (2016). The holistic rhizosphere, integrating zones, processes, and semantics in the soil influenced by roots. Journal of Experimental Botany, 67, 3629-3643.
DOI URL |
| [45] |
Zhou SB, Peng SC, Li ZQ, Zhang DJ, Zhu YT, Li XQ, Hong MY, Li WC, Lu PL (2022). Characterization of microbial communities and functions in shale gas wastewaters and sludge: implications for pretreatment. Journal of Hazardous Materials, 424, 127649. DOI: 10.1016/j.jhazmat.2021.127649.
DOI URL |
| [1] | 肖文宏, 周青松, 朱朝东, 吴东辉, 肖治术. 野生动物监测技术和方法应用进展与展望[J]. 植物生态学报, 2020, 44(4): 409-417. |
| [2] | 路颖, 李坤, 倪瑞强, 梁强, 李传荣, 张彩虹. 泰山4种优势造林树种细根分解对细菌群落结构的影响[J]. 植物生态学报, 2018, 42(12): 1200-1210. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19