

Chin J Plant Ecol ›› 2017, Vol. 41 ›› Issue (4): 489-496.DOI: 10.17521/cjpe.2016.0091
• Orginal Article • Previous Articles
Sheng YANG1,2,*, Hua-Xin ZHANG2, Qiu-Xia CHEN1, Xiu-Yan YANG2
Received:2016-03-09
Accepted:2016-09-21
Online:2017-04-10
Published:2017-05-19
Contact:
Sheng YANG
Sheng YANG, Hua-Xin ZHANG, Qiu-Xia CHEN, Xiu-Yan YANG. Responses of apical ion fluxes to NaCl stress in Elaeagnus angustifolia seedlings[J]. Chin J Plant Ecol, 2017, 41(4): 489-496.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2016.0091
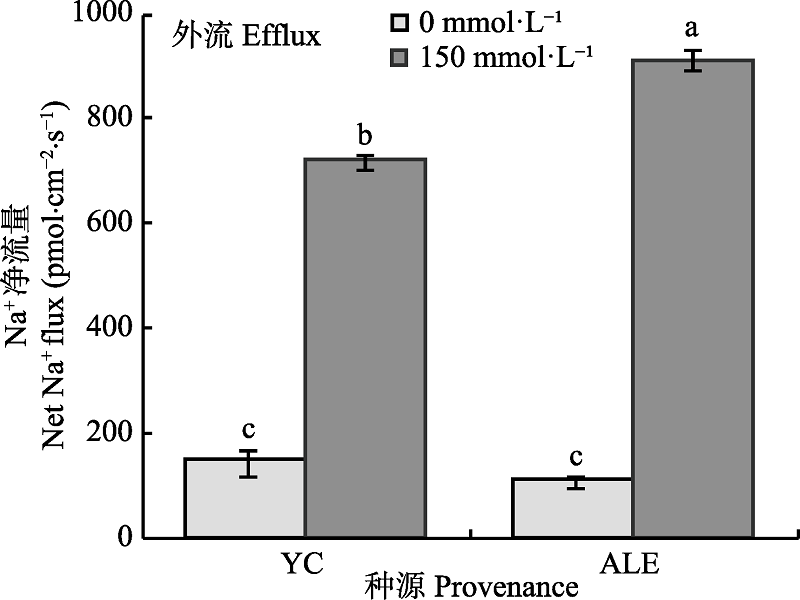
Fig. 1 Effects of NaCl stress on steady-state Na+ flux at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences between different treatments and provenances (p < 0.05).
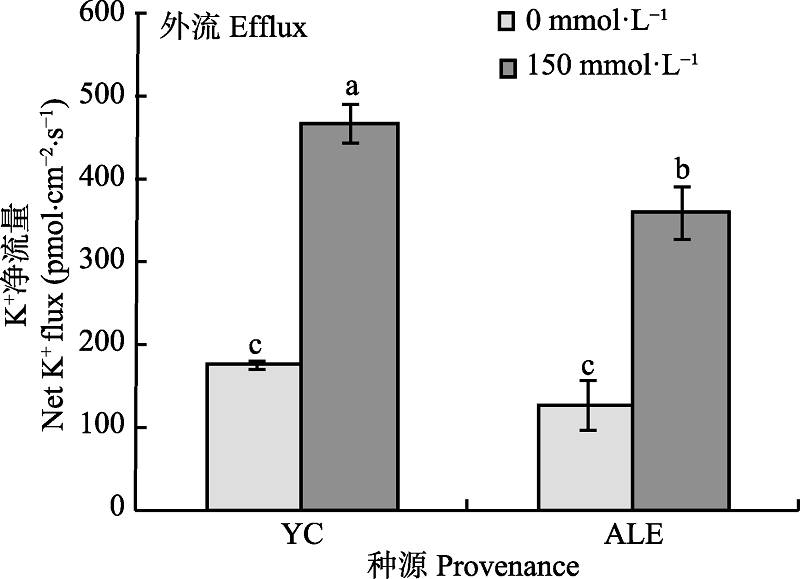
Fig. 2 Effects of NaCl stress on steady-state K+ flux at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences between different treatments and provenances (p < 0.05).
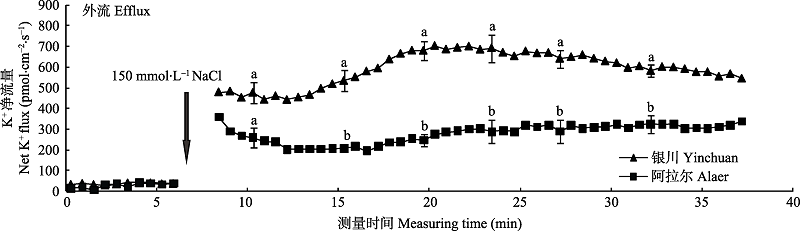
Fig. 3 Differences of NaCl stress on transient K+ kinetics at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). Different lowercase letters indicate significant differences between provenances at the same time (p < 0.05).
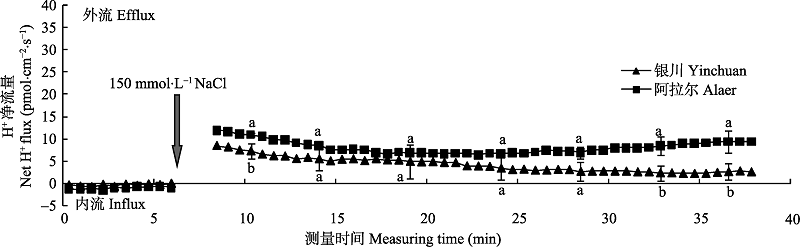
Fig. 4 Differences of NaCl stress on transient H+ kinetics at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). Different lowercase letters indicate significant differences between provenances at the same time (p < 0.05).
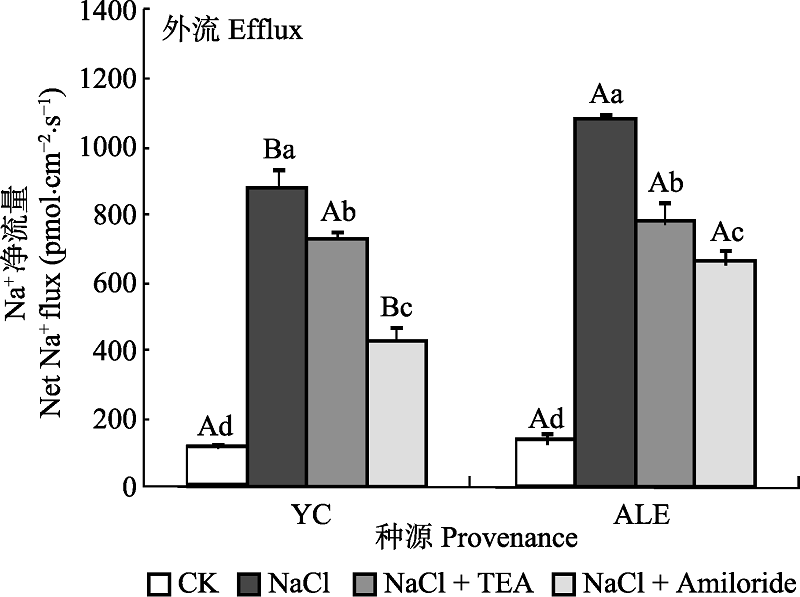
Fig. 5 Effects of two kinds of inhibitors on net Na+ flux at apical regions of two provenances of Elaeagnus angustifolia treated by NaCl stress (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences among different treatments in the same provenance (p < 0.05), While different capital letters indicate significant difference between provenances at the same treatment (p < 0.05).
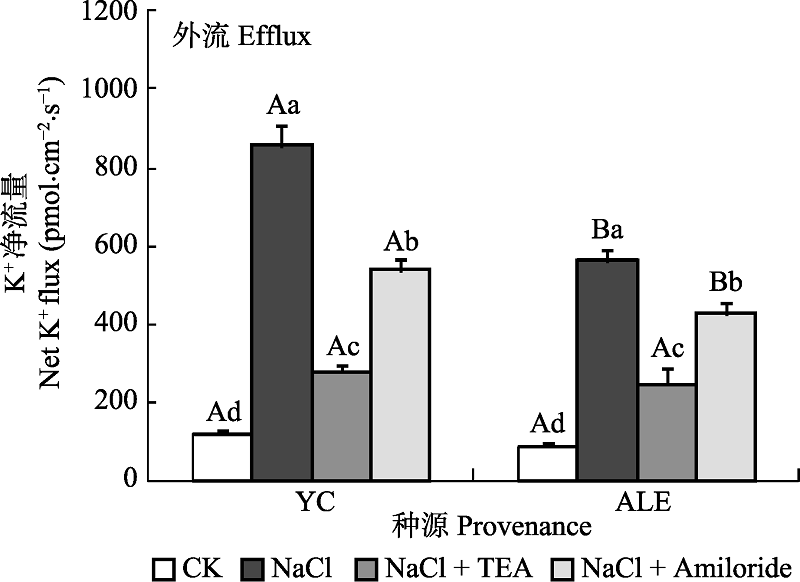
Fig. 6 Effects of two kinds of inhibitors on net K+ flux at apical regions of two provenances of Elaeagnus angustifolia treated by NaCl stress (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences among different treatments in the same provenance (p < 0.05), While different capital letters indicate significant difference between provenances at the same treatment (p < 0.05).
| [1] | Britto DT, Kronzucker HJ (2008). Cellular mechanisms of potassium transport in plants.Physiologia Plantarum, 133, 637-650. |
| [2] | Chao DY, Dilkes B, Luo H, Douqlas A, Yakubova E, Lahner B, Salt DE (2013). Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science, 341, 658-659. |
| [3] | Chen GP, Wang HZ, Shi NN, Chen SY (2006). Na+/H+ antiporter and its relationship with plant salt tolerance.China Biotechnology, 26(5), 101-106. (in Chinese with English abstract)[陈观平, 王慧中, 施农农, 陈受宜 (2006). Na+/H+逆向转运蛋白与植物耐盐性的关系研究进展. 中国生物工程杂志, 26(5), 101-106.] |
| [4] | Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005). Screening plants for salt tolerance by measuring K+ flux: A case study for barely.Plant, Cell & Environment, 28, 1230-1246. |
| [5] | Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabala S (2007a). Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley.Plant Physiology, 145, 1714-1725. |
| [6] | Chen Z, Shabala S, Mendham N, Newman I, Zhang GP, Zhou MX (2008). Combining ability of salinity tolerance on the basis of NaCl-induced K flux from roots of barley.Crop Science, 48, 1382-1388. |
| [7] | Chen Z, Zhou M, Newman IA, Mendham NJ, Zhang GP, Shabala S (2007b). Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance.Functional Plant Biology, 34, 150-162. |
| [8] | Coskun D, Britto DT, Jean YK, Kabir I, Tolay I, Torun AA, Kronzucker HJ (2013). K+ efflux and retention in response to NaCl stress do not predict salt tolerance in contrasting genotypes of rice (Oryza sativa L.). PLOS ONE, 8(2), e57767. doi: 10.1371/journal.pone.0057767. |
| [9] | Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008). A root’s ability to retain K+ correlates with salt tolerance in wheat.Journal of Experimental Botany, 59, 2697-2706. |
| [10] | Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S (2011). Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: In planta quantification methods.Plant, Cell & Environment, 34, 947-961. |
| [11] | Cuin TA, Zhou M, Parsons D, Shabala S (2012). Genetic behaviour of physiological traits conferring cytosolic K+/Na+ homeostasis in wheat.Plant Biology, 14, 438-446. |
| [12] | Demidchik V, Maathuis FJM (2007). Physiological roles of nonselective cation channels in plants: From salt stress to signaling and development.New Phytologist, 175, 384-404. |
| [13] | Feki K, Quintero FJ, Khoudi H, Leidi EO, Masmoudi K, Pardo JM, Brini F (2014). A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Reportsl Reports, 33, 277-288. |
| [14] | Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K (2012). Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Molecular Biology, 79, 137-155. |
| [15] | Guan WK, Xu N (2012). Research situation and resources utilization of Elaeagnus angustifolia. Anhui Agricultural Science Bulletin, 18(19), 119-121. (in Chinese with English abstract)[管文轲, 徐娜 (2012). 沙枣资源利用研究与开发现状述评. 安徽农学通报, 18(19), 119-121.] |
| [16] | Guo LJ, Wang YT (2008). Conservation research and prospects of Elaeagnus germplasm resources and utilization values. Chinese Wild Plant Resources, 27(5), 32-34. (in Chinese with English abstract)[郭丽君, 王玉涛 (2008). 沙枣种质资源特性及利用价值. 中国野生植物资源, 27(5), 32-34.] |
| [17] | Kong X, Luo Z, Dong H, Eneji AE, Li W (2012). Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton.Journal of Experimental Botany, 63, 2105-2116. |
| [18] | Liu BY (2007). Study on Ecophysiological Response and Ion Distribution of Elaeagnus angustifolia to Salt Stress. Master degree dissertation, Tianjin Normal University, Tianjin. 27-53. (in Chinese with English abstract)[刘宝玉 (2007). 盐胁迫下沙枣生理生态响应与离子分配研究. 硕士学位论文, 天津师范大学, 天津. 27-53.] |
| [19] | Liu ZX (2013). Physiological Mechanism of Heterogeneous Responses of Elaeagnus angustifolia to NaCl and Na2SO4 Stress. PhD dissertation, Chinese Academy of Forestry, Beijing. 100-104. (in Chinese with English abstract)[刘正祥 (2013). 沙枣对氯化钠和硫酸钠胁迫异质性响应的生理机制. 博士学位论文, 中国林业科学研究院, 北京. 100-104.] |
| [20] | Liu ZX, Zhang HX, Yang XY, Liu T, Di WB (2014). Growth, and cationic absorption, transportation and allocation of Elaeagnus angustifolia seedlings under NaCl stress. Acta Ecologica Sinica, 34, 326-336. (in Chinese with English abstract)[刘正祥, 张华新, 杨秀艳, 刘涛, 狄文彬 (2014). NaCl胁迫下沙枣幼苗生长和阳离子吸收、运输与分配特性. 生态学报, 34, 326-336.] |
| [21] | Ma Q, Bao AK, Wu GQ, Wang SM (2011). Plasma membrane Na+/H+ antiporter is involved in plant salt tolerance.Chinese Bulletin of Botany, 46, 206-215. (in Chinese with English abstract)[马清, 包爱科, 伍国强, 王锁民 (2011). 质膜Na+/H+逆向转运蛋白与植物耐盐性. 植物学报, 46, 206-215.] |
| [22] | Maathuis FJM (2006). The role of monovalent cation transporters in plant responses to salinity.Journal of Experimental Botany, 57, 1137-1147. |
| [23] | Maathuis FJM, Amtmann A (1999). K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios.Annals of Botany, 84, 123-133. |
| [24] | Olias R, Eljakaoui Z, Li JU, Morales PD, Marin-manzano MC, Pardo JM, Belver A (2009). The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs.Plant, Cell & Environment, 32, 904-916. |
| [25] | Rausch T, Kirsch M, Löw R, Lehr A, Viereck R, An ZG (1996). Salt stress responses of higher plants: The role of proton pumps and Na+/H+-antiporters.Journal of Plant Physiology, 148, 425-433. |
| [26] | Shabala S, Cuin TA (2008). Potassium transport and plant salt tolerance.Physiologia Plantarum, 133, 651-669. |
| [27] | Shi HZ, Lee B, Wu SJ, Zhu JK (2003). Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnology, 21, 81-85. |
| [28] | Shi HZ, Ishitani M, Kim C, Zhu JK (2000). The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences of the United States of America, 97, 6896-6901. |
| [29] | Sun J (2011). Signaling Network in the Perception of Salt Stress and Ionic Homeostasis Regulation in Populus euphratica. PhD dissertation, Beijing Forestry University, Beijing. 26-28. (in Chinese with English abstract)[孙健 (2011). 胡杨响应盐胁迫与离子平衡调控信号网络研究. 博士学位论文, 北京林业大学, 北京. 26-28.] |
| [30] | Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009). NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species.Plant Physiology, 149, 1141-1153. |
| [31] | Wegner LH, Stefano G, Shabala L, Rossi M, Mancuso M, Shabala S (2011). Sequential depolarization of root cortical and stelar cells induced by an acute salt shock—Implications for Na+ and K+ transport into xylem vessels. Plant, Cell & Environment, 34, 859-869. |
| [32] | Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong Z (2009). Overexpression of SOS (salt overly sensitive) genes increases salt tolerance in transgenic Arabidopsis. Molecular Plant, 2, 22-31. |
| [33] | Yang S, Liu T, Zhang HX, Li HY, Zhang L (2014). Growth and physiological characteristics of Elaeagnus angustifolia L. under salt stress. Journal of Fujian College of Forestry, 34(1), 64-70. (in Chinese with English abstract)[杨升, 刘涛, 张华新, 李焕勇, 张丽 (2014). 盐胁迫下沙枣幼苗的生长表现和生理特性. 福建林学院学报, 34(1), 64-70.] |
| [34] | Yang S, Zhang HX, Liu T, Wu HW, Yang XY, Ni JW, Chen QX (2016). Study on ion metabolism characteristics of Elaeagnus angustifolia L. seedlings under NaCl stress. Forest Research, 29(1), 140-146. (in Chinese with English abstract)[杨升, 张华新, 刘涛, 武海雯, 杨秀艳, 倪建伟, 陈秋夏 (2016). NaCl胁迫下沙枣幼苗的离子代谢特性. 林业科学研究, 29(1), 140-146.] |
| [35] | Yang S, Zhang HX, Yang XY, Chen QX, Wu HW (2015). Differential growth performance ofElaeagnus angustifolia L. provenances under NaCl stress. Scientia Silvae Sinicae, 51(9), 51-58. (in Chinese with English abstract)[杨升, 张华新, 杨秀艳, 陈秋夏, 武海雯 (2015). NaCl胁迫下不同种源沙枣的生长表现差异. 林业科学, 51(9), 51-58.] |
| [36] | Yue Y, Zhang M, Zhang J, Duan L, Li Z (2012). SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio.Journal of Plant Physiology, 169, 255-261. |
| [37] | Zhang BZ, Cao ZY, Zhao KF (1992). A study on some physiological properties ofElaeagnus angustifolia under salt stress condition. Scientia Silvae Sinicae, 28(2), 187-189. (in Chinese with English abstract)[张宝泽, 曹子谊, 赵可夫 (1992). 盐分胁迫下沙枣某些生理特性的研究. 林业科学, 28(2), 187-189.] |
| [1] | JI Ruo-Xuan, YU Xiao, CHANG Yuan, SHEN Chao, BAI Xue-Qia, XIA Xin-Li, YIN Wei-Lun, LIU Chao. Geographical provenance variation of leaf anatomical structure of Caryopteris mongholica and its significance in response to environmental changes [J]. Chin J Plant Ecol, 2020, 44(3): 277-286. |
| [2] | LÜ Jin-Hui,REN Lei,LI Yan-Feng,WANG Xuan,ZHAO Xia-Lu,ZHANG Chun-Lai. Responses to salt stress among different genotypes of tea Chrysanthemum [J]. Chin J Plant Ecol, 2013, 37(7): 656-664. |
| [3] | WANG Dian, YUAN Fang, WANG Bao-Shan, CHEN Min. Response of plant biofuel hybrid Pennisetum to NaCl stress and its salinity threshold [J]. Chin J Plant Ecol, 2012, 36(6): 572-577. |
| [4] | YUAN Lin, KARIM Ali, ZHANG Li-Quan. EFFECTS OF NACL STRESS ON ACTIVE OXYGEN METABOLISM AND MEMBRANE STABILITY IN PISTACIA VERA SEEDLINGS [J]. Chin J Plant Ecol, 2005, 29(6): 985-991. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn