自从1904年德国微生物学家Lorenz Hiltner首次提出了根际(rhizosphere)这一概念以来, 依赖于相关学科的发展和技术手段的不断改进, 根际研究内容与内涵不断得到丰富和发展。目前根际微区域根系-土壤-微生物界面互作过程与作用机理已成为土壤学最活跃、最敏感的研究领域。例如, 2009年, 《Plant and Soil》对地下根际研究给予专刊报道, 并指出未来根际生态研究面临的诸多挑战(Dessaux et al., 2009)。另外, 为纪念根际概念提出100周年, 同时也为了推动和交流根际研究成果的最新进展, 2004年9月在Hiltner教授的故乡, 同时也是他工作并提出根际概念的慕尼黑工业大学召开了第一届国际根际研讨会, 系统总结了根际研究的进展与未来发展方向。随后分别在法国蒙彼利埃、澳大利亚珀斯、荷兰马斯特里赫特举行了第二届(2007)、第三届(2011)、第四届(2015)国际根际大会。2016年3月, 聚焦植物科学最新研究趋势的国际学术期刊《Trends in Plant Science》发表专刊《Unravelling the Secrets of the Rhizosphere》, 指出通过破解根际的秘密, 才能更好地理解植物、微生物、土壤等非核心单元间的复杂作用, 并提升对根系发育生物学和微生物信号传导的认知。2018年1月, 国际知名学术期刊《Plant and Soil》再次对“root ecology”进行了专刊报道, 并对根系生态五大科学前沿进行了系统的总结和归纳(Erktan et al., 2018)。此外, 随着根际研究重要性的日益突出, 2016年Elsevier出版社针对植物根系-土壤相互作用研究专门创建了根际研究领域学术期刊《Rhizosphere》, 主要刊登有关植物根系、土壤生物、养分和水分之间相互作用等方面的最新研究进展, 旨在深化对植物根系与土壤相互作用与调控机理的认知。可以说, 根际研究一直以来都是国内外环境生物学、植物学、植物生理学、土壤学、微生物学、生态学、遗传学和分子生物学联合研究的热点领域, 并成为当前森林土壤学与全球气候变化研究的核心交汇与学科交叉前沿。
根系分泌物(root exudate)作为植物根系-土壤界面物质能量交换和信息传递的重要载体物质, 是构成根际微生态系统活力与功能特征的内在驱动因素, 是Hiltner教授所提出的根际概念及相关的根际生态过程存在的前提和基础(张福锁和申建波, 1999)。根系分泌物是指植物在生长过程中, 通过根系不同部位主动或被动向周围土壤持续释放大量的有机物的总称, 是一种复杂的非均一体系(吴林坤等, 2014)。根系分泌物成分众多, 数量各异, 目前对于植物根系分泌物还没有一个公认的、确定化的定义。例如, 一些研究者根据根系分泌物释放机理将其划分为分泌物(secretion, 主动释放)和渗出物(exudation, 被动扩散)(Uren, 2000; 吴林坤等, 2014)。在现代科学实践中, 考虑到现有的根系分泌物收集方法很难准确识别根系分泌物释放机理, 为了研究方便, 结合根系分泌物化学特性, 一些学者将根系分泌物组分分为三大类: 1)低分子量物质(分子量< 1000 Da)主要包括有机酸、酚酸、氨基酸、多肽、可溶性糖、可溶性蛋白、植物激素、维生素, 以及OH–、H+、Na+等离子; 2)高分子量物质(分子量˃ 1000 Da), 主要包括黏胶物质、黏液、边缘细胞、根冠细胞、未形成细胞壁的表皮细胞、聚多糖、多糖醛酸、胞外酶等。高分子量物质尽管成分比较单一, 却占据了根系分泌物中的很大比例; 3)细胞脱落物, 包括脱落的根冠细胞、根毛与细胞碎片(张豆豆等, 2014)。狭义的根系分泌物主要包括植物通过溢泌作用释放到土壤中的低分子可溶性物质, 这部分物质也是目前根系分泌物作用和功能研究主要关注的对象。根系分泌物作为植物、土壤和微生物三者间的桥梁, 在植物主动适应和被动防御外界环境变化中具有重要的作用与功能, 主要包括: (1)调控土壤生物地球化学循环关键过程(如养分循环); (2)改变土壤结构形成(如土壤团聚体); (3)释放防御性物质参与化学干扰(化感作用); (4)环境污染物修复(如缓解或者消除金属毒害和污染); (5)释放化学信号物质建立植物-微生物、微生物-微生物之间的对话与交流; (6)选择塑造根际微生物群落组成、活性与分布(Bais et al., 2006; Oburge & Jones, 2018)。
近年来, 随着研究方法和新技术的不断发展, 众多研究者在根系分泌物释放机制、收集方法、化学组分分析、根系-微生物信号分子识别与交流、养分利用效率与定向调控等方面进行了广泛的研究(van Dam & Bouwmeester et al., 2016; Warren, 2016), 极大地推进了人们对地下根际世界的认识。相关研究结果在指导生物入侵防控、化感/连作自毒作用、作物间套作模式构建、生物修复以及胁迫环境响应等方面发挥了重要作用(Sun et al., 2016)。然而, 传统的根际过程研究更突出区域特点和以实际生产问题为导向, 加之农作物易塑造模式植物, 作物生长周期较短, 根系分泌物收集方便等诸多原因, 以往对植物根系分泌物的研究主要聚焦在农业生态系统。目前有关根系分泌物在森林生态系统中的重要生态作用与调控反馈机制研究甚少, 一定程度上限制了对森林地下生态过程及其对环境变化响应的新认识。因此, 重视和提升森林生态系统根系分泌物-土壤-微生物互作过程及其生态重要性研究, 已经成为全球气候变化下土壤生态学的重要内容。本文作者结合自身研究工作进展和当前根系分泌物研究领域前沿动态, 重点综述了森林根系分泌物研究目前存在的主要问题与不足, 在此基础上展望了未来森林根系分泌物生态学研究中值得关注的重点方向和研究内容, 以期为深化森林根际生态学过程的新认识及其理论体系发展提供科学基础。
1 森林根系分泌物在土壤过程和功能中的生态重要性
随着对森林根系功能认识的不断深入和技术的发展, 根系活动在调控森林土壤功能和养分代谢过程中的重要作用已成为地下生态学研究的关注点(Cheng et al., 2014), 并使得传统的植物-土壤互作反馈模型受到了诸多挑战。过去, 传统的植物-土壤互作反馈模型主要考虑凋落物输入和分解对土壤物质循环过程的影响, 更多地把根系当作植物获取养分和水分的门户。然而, 作为连接植物与土壤的关键纽带, 根系除了吸收养分、水分和固定地上部分之外, 还可通过合成与分泌多种化合物、细根周转与菌根共生等一系列生命活动来调控土壤养分循环过程(Moore et al., 2015; Laliberté, 2017)。例如, 植物通过根系向周围土壤释放供微生物直接利用的一系列低分子含碳(C)化合物(如有机酸、糖类、酚类和氨基酸等)。这些化合物可为土壤微生物提供重要且丰富的C源和能源, 从而有效地改变土壤微生物的生物量和活性, 深刻地影响土壤有机质分解和养分代谢等微生物过程, 并在一定程度上决定了根际微生态系统C动态、能量流动以及矿质养分代谢过程 (Dijkstra et al., 2013; Wutzler & Reichstein, 2013)。Finzi等(2015)通过meta分析与模型模拟发现, 虽然根系分泌物输入仅占森林初级生产力的5%左右, 但根源C输入对温带森林土壤C-N矿化过程的贡献率却高达33%左右, 在调控土壤有机质分解与养分循环过程中发挥着与其数量和比例明显不相符的重要作用和功能。因此, 森林根系已成为地下生态过程研究的核心对象, 而根源C输入所介导的土壤生物地球化学循环过程及其生态反馈效应则是森林生态系统养分物质周转的关键环节。
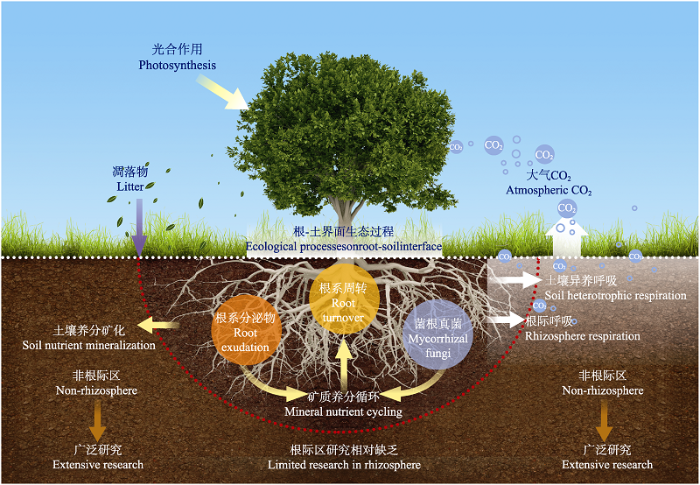
伴随着全球气候变化和土地利用方式改变, 森林类型、物种组成、根系生长以及菌根特征变化都直接影响根系C源输入通量及其介导的根际土壤生物地球化学循环过程, 进而深刻反馈于森林生态系统群落结构和生态功能。随着全球气候变化下植物根系-土壤-微生物互作过程及其生态反馈效应的重要性日益凸显, 众多研究者已在《Science》《Nature》《New Phytologist》等国际知名刊物上提出, 未来地下生态学研究应重视从植物生理视角出发(Högberg & Read, 2006; Klein et al., 2016; Laliberté, 2017), 并聚焦于以根系生命活动为核心的根际生态过程与土壤生物地球化学循环过程的耦合研究(图1), 即充分考虑以根系分泌物输入所驱动的土壤碳-养分循环过程或根际激发效应(Cheng et al., 2014; 孙悦等, 2014; Zhu et al., 2014)。相应地, 森林根系分泌物介导的根际土壤碳-养分循环过程及其对环境变化的响应已成为当前森林地下生态学研究的重要方向。
图1
图1
全球气候变化背景下森林生态系统根际生物地球化学循环过程研究框架图。
Fig. 1
Conceptual framework of rhizosphere biogeochemical processes in forests under global climate change.
2 森林根系分泌物与土壤生态过程的耦合效应及其调控机制的认知有限
纵观现有森林根系分泌物作用与功能研究, 主要集中于根系分泌物作为微生物C源所驱动的土壤碳-养分循环过程及其生态反馈效应等方面。近十多年来, 依赖于多学科的前沿交叉和新技术的广泛应用, 当前对森林根系分泌物输入特征及其诱导的土壤生物地球化学循环关键过程研究已取得较大进展和突破(Phillips et al., 2011; Yin et al., 2013; Tückmantel et al., 2017)。但受制于根际微系统复杂的时空异质性, 以及研究方法和技术的限制, 目前森林根系分泌物研究依然存在诸多挑战和不足, 主要表现在以下几方面。
2.1 缺乏有效的森林根系分泌物原位收集方法与技术
长期以来, 研究者们一直致力于探索和创新植物根系分泌物收集方法与技术。例如, 针对人工控制条件下(实验室组培、沙培或水培等)的农作物、蔬菜和草本植物等短生长周期植物, 建立了土培收集、水(溶液)培养收集、基质(蛭石、砂、琼脂等)培养收集等方法(Neumann et al., 2009), 但是这些方法都不太适用于森林多年生木本植物根系分泌物的收集(Sun et al., 2016; Warren, 2016)。此外, 目前所开展的少量森林根系分泌物收集研究大多仅停留在植株水平上, 且试验对象多为人工控制条件下的移栽幼苗(Sandnes et al., 2005; Yin et al., 2013)。相应地, 关于森林样地乃至生态系统尺度上森林根系分泌物原位收集的研究报道甚少。然而, 由于根系分泌物无论是种类或是数量均对周围环境条件(如土壤养分状况、物理损伤)十分敏感, 使得人工控制条件下获得的根系分泌物输入结果与野外实际状况存在较大差异, 限制了对森林根系分泌物输入特征、动态规律及其作用与生态功能的深入认识。基于此, 近年来一些学者构建了森林根系分泌物的原位收集方法(Phillips et al., 2008; Yin et al., 2014), 这些收集方法操作相对简便, 无需移栽植物, 能较真实地反映自然条件下植株根系分泌物的质量和数量, 但仍然存在诸多不足: (1)野外自然状态下无菌条件较难控制, 难以避免树木根系和微生物对根系分泌物中养分物质的吸收, 导致获得的根系分泌物组分与含量与实际野外状况存在一定的差异(Warren, 2016); (2)不同树种根系形态和土壤特性表现出明显的时空异质性, 现有的收集技术很难保证在不破坏根系正常生长的情况下有效收集不同形态特征根系的分泌物。因此, 根据特定的研究目标和野外实际状况, 不断创新和完善森林根系分泌物原位收集方法是该研究领域中一个长期探索和逐渐完善的过程, 也是未来森林根际生态学研究中需要着力攻关的重点技术问题(Tückmantel et al., 2017)。
2.2 忽略了森林根系分泌物不同组分及其伴随的C:N化学计量特征所驱动的生态效应研究
尽管根系分泌物输入在森林土壤生物地球化学循环过程中的重要调控作用已获得了广泛认可和极大关注, 但目前绝大多数研究只关注分泌物总C输入对土壤过程和功能的影响(Drake et al., 2011; Phillips et al., 2011; Finzi et al., 2015), 而忽略了对森林特异性根系分泌物化学组分及伴随的C:N化学计量特征对土壤生态过程与调控机理的系统性研究。这种忽略将极大地限制对森林根系-土壤-微生物互作过程及其生态重要性的深入认识, 主要体现在如下两个方面: (1)根系分泌物种类繁多, 数量差异大, 既有糖、蛋白质和氨基酸等初生代谢产物, 又有有机酸、酚类等次生代谢产物。不同根系分泌物组分由于其化学特性和能量有所差异, 进而对土壤C、N转化过程具有不同的效应(Zhu & Cheng, 2012; Keiluweit et al., 2015; Yuan et al., 2018)。(2)根系分泌物主要为一系列含C化合物, 其C:N通常高于根际土壤微生物的C:N (Cleveland & Liptzin, 2007)。植物根系和土壤微生物(如微生物合成、生长和胞外酶释放)对根际有效N的获取和激烈竞争, 导致根际区通常成为C过剩而N强烈受限制的区域(Kuzyakov, 2002)。相应地, 根际微生物利用根系分泌物生长和合成胞外酶的能力严重地受根系分泌物N含量制约, 从而反过来调控根际微生物介导的土壤生物地球化学循环过程及其对森林结构和功能的生态反馈效应。因此, 森林根系分泌物N含量或C:N化学计量特征成为驱动根际微生物群落组成和活性的重要调控因子。此外, 在叠加气候变化后, 森林根系分泌物含量、化学组分及其伴随的C:N化学计量特征变化进一步使原本知之甚少的根际生态学过程变得更为复杂。因此, 全球气候变化条件下森林根系分泌物组分C:N化学计量特征变化与土壤生态过程的偶联效应已成为一个十分重要但认知又极度缺乏的研究课题(Drake et al., 2013)。比如, 森林根系分泌物不同化学组分所驱动的土壤碳-养分通量过程与作用机理差异, 根系分泌物对土壤碳-养分微生物过程的影响效应与其C:N化学组分计量阈值范围的关联。
2.3 忽略了森林根系分泌物介导的土壤碳-养分循环过程的非生物作用机制研究
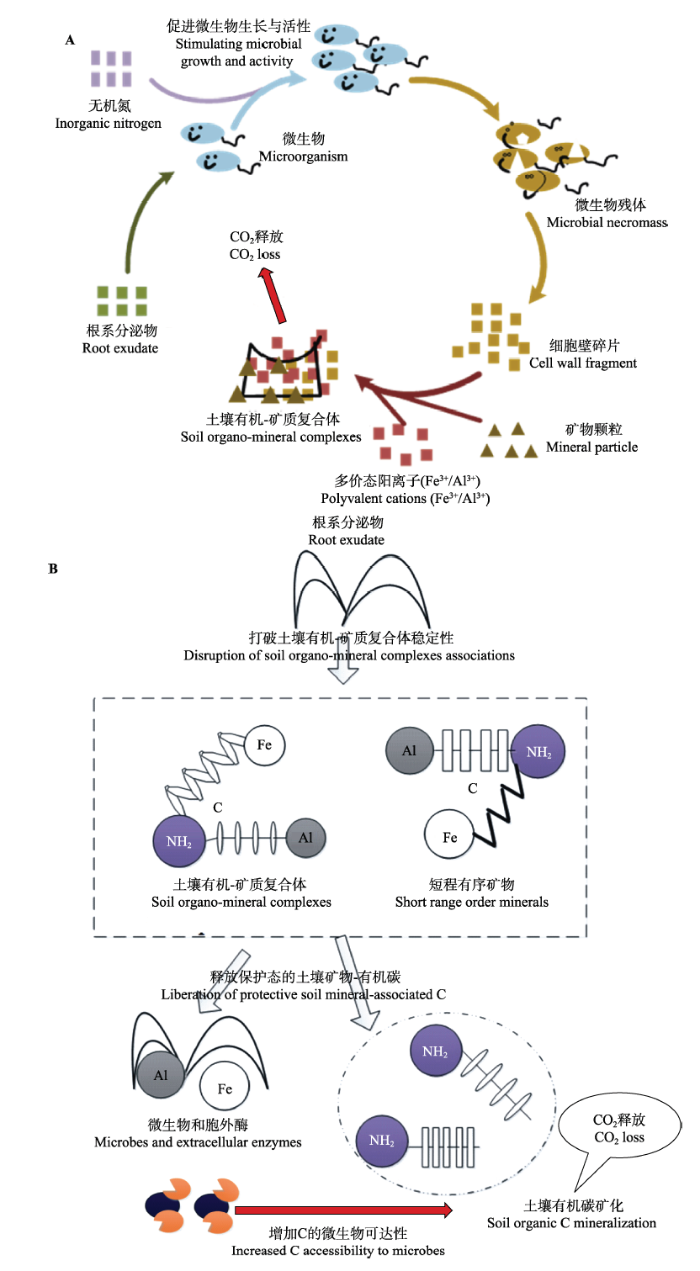
目前有关根系分泌物所驱动的土壤碳-养分矿化激发效应的研究主要聚焦于微生物作用过程, 其核心观点如下: 根系分泌物输入给土壤微生物提供有效的生物可利用能源, 并伴随着根际微生物活性和胞外酶产量的增加, 从而刺激和激发土壤有机质(SOM)分解和养分循环(即传统的“微生物共代谢”假说, 图2A) (Wutzler & Reichstein, 2013; Zhu et al., 2014; Finzi et al., 2015)。然而, 近年来一些研究表明植物根系分泌物输入也可通过间接的非生物作用过程(如保护态C活化、微生物对有机C的可接近性等)在驱动土壤碳-养分循环根际激发效应中具有重要的作用。例如, Keiluweit等(2015)发现根系分泌物(草酸)输入后, 通过配位络合作用和溶解反应等物理化学作用, 破坏或者降低了土壤有机物-矿质复合体界面的稳定性, 从而将土壤中保护态C从土壤有机-矿质复合体中释放出来供微生物分解和利用(即打破空间隔离效应而增加了保护态C的微生物可达性)(图2B)。
图2
图2
根系分泌物诱导的土壤碳(C)矿化或根际激发效应机理。A, 传统的微生物共代谢机理——根系分泌物输入后主要促进微生物生长和活性, 并伴随着土壤C矿化加快。B, 新提出的根际激发效应机理——大量土壤C由于矿物保护而不能被微生物直接利用, 根系分泌物输入后通过络合作用和溶解反应等非微生物过程打破或者降低有机-矿质复合体稳定性, 将保护态C释放出来而增加了保护态C的微生物可达性。
Fig. 2
Proposed mechanisms for the exudate-induced acceleration of the microbial mineralization of soil organic carbon in the rhizosphere (i.e., rhizosphere priming effects). A, The traditional view is that root exudate compounds stimulate microbial growth and activity via co-metabolism, and so increase the overall physiological potential of the decomposer community for carbon mineralization. B, The alternative mechanism proposed here takes into account that large quantities of soil C are inaccessible to microbes owing to associations with mineral phases. Root exudates that can act as ligands effectively liberate C through complexation and dissolution reactions with protective mineral phases, thereby promoting its accessibility to microbes and accelerating its loss from the system through microbial mineralization.
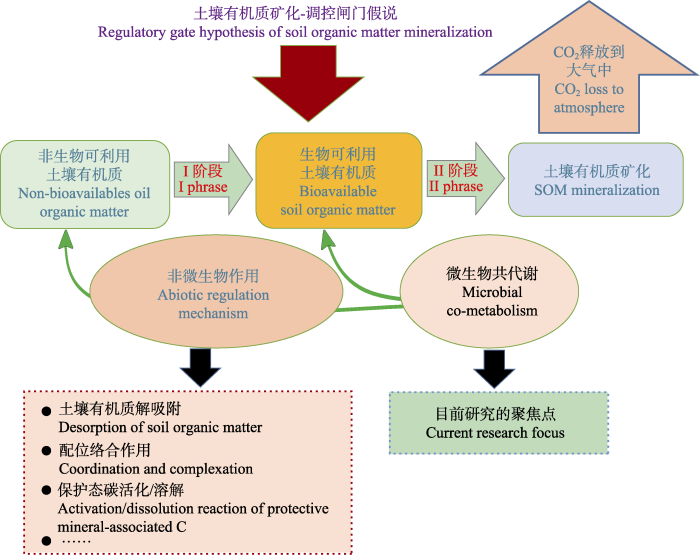
类似地, Kemmitt等(2008)开创性地提出了一种调控闸门假说(Regulatory Gate Hypothesis)来阐释SOM分解与矿化的关键调控过程。该假说将SOM分解分为两个重要阶段。I阶段: 生物不可利用SOM转化为生物可利用SOM阶段。该阶段主要受一些非生物作用过程所调控(如有机质解吸附、配位络合作用、保护态C活化/溶解、土壤孔隙水中有机C迁移扩散等), 而与微生物生物量、群落结构或特异性活性无关。II阶段: 生物可利用SOM在微生物作用下的矿化分解阶段。生物可利用SOM在土壤微生物和胞外酶的作用下进行分解和矿化, 并通过CO2释放到大气中。该阶段SOM矿化或者激发主要受微生物作用调控, 这也是目前研究的主要聚焦点(图3)。该假说暗含着SOM分解与矿化整个过程, 同时受不同的非生物与微生物作用过程调控。
图3
图3
调控闸门假说概念框架图。I阶段: 生物不利用土壤有机质(SOM)转化为生物可利用SOM; II阶段: 生物可利用SOM在微生物作用下发生的矿化分解过程。
Fig. 3
Diagrammatic representation of the Regulatory Gate Hypothesis. I phrase is the abiological transformation of non-bioavailable soil organic matter (SOM). II phrase is the biological mineralization of bioavailable SOM.
这些结果表明根系分泌物并非仅通过给土壤微生物生长提供能源和C源这一途径来调控土壤生态过程, 非生物作用过程在根系分泌物所驱动的土壤碳-养分循环过程中也起着至关重要的作用, 并对传统的“微生物共代谢”理论提出了巨大的挑战。最新的观点认为: 根系分泌物介导的土壤碳-养分循环过程应同时受生物与非生物两种作用机制共同驱动, 但两种作用机制的相对贡献大小可能受根系分泌物输入、供试土壤特性以及环境条件等因素影响(Yin et al., 2016; Jilling et al., 2018)。然而, 目前有关微生物/非生物作用机制对根系分泌物诱导的土壤碳-养分循环过程影响效应缺乏更多直接的试验证据(Tan et al., 2017; Yuan et al., 2018)。因此, 求证和量化微生物/非生物作用过程对根系分泌物驱动的土壤生物地球化学循环过程的相对贡献与调控机理已成为一个十分重要但认知又极度缺乏的研究课题。
2.4 菌根真菌共生增强了森林根系-土壤-微生物互作过程的复杂性和不可预知性
菌根是由土壤真菌与植物根系形成的一种互惠共生体, 广泛分布于不同的陆地生态系统中, 目前已发现80%以上的陆生植物与真菌形成菌根, 主导了根际区土壤复杂有机物合成、根系C输入-矿质养分转运和交换等关键生态过程(Klein et al., 2016; Luginbuehl et al., 2017)。丛枝菌根(AM)和外生菌根(ECM)是森林生态系统中普遍存在的两种菌根类型, 对于温带和北方森林而言, 几乎所有的植物根系表面都有真菌附着, 形成所谓的外生菌根, 并通过产生大量的外延菌丝(以下简称菌丝)在土壤中形成庞大、功能多样的菌丝网络系统(Smith & Read, 2008)。
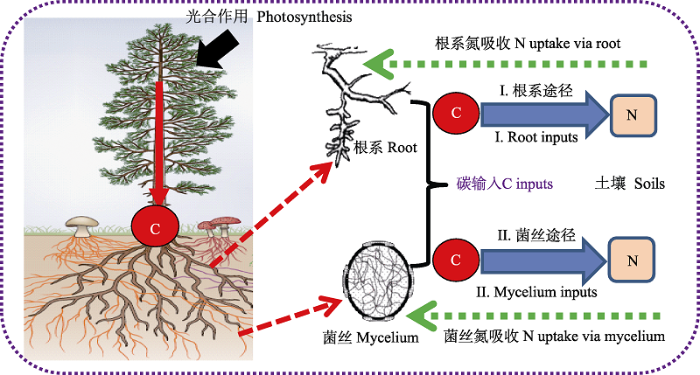
森林植物将大量光合作用固定的C分配到地下根系并转移到土壤(即根源C), 其转移途径除了通过根系分泌物和细根周转进入到周围土壤中(即根系途径), 还可通过将光合产物或合成的含C化合物转移到菌丝中, 然后由菌丝输入到土壤中(即菌丝途径)(Treseder & Holden, 2013; Terrer et al., 2018) (图4)。此外, 菌丝也可作为土壤动物和微生物的一种重要的食物来源, 进而调控土壤微生物活性与微生物群落特征(Wallander et al., 2011)。因此, 菌丝被认为是森林生态系统中除根系途径之外C源进入土壤的另外一个重要通道(Wallander et al., 2011)。由于森林根系和菌丝C源具有截然不同的生物化学特性和周转速率, 因而深刻地诱导差异化的土壤C动态变化与养分循环过程。因此, ECM共生使得原本认知有限的森林地下根系-土壤-微生物互作过程及其生态反馈效应变得更为复杂和不可预知。然而, 现有的试验研究和理论模型大都将根系和菌丝生长视为一个整体进行考虑(Wallander et al., 2013), 而缺乏进一步区分和精准辨识森林根系/菌丝C源输入途径对土壤生态过程和功能的差异化影响, 导致对森林根系-土壤-微生物互作过程及其作用机理依然缺乏足够的认知和理解。
图4
图4
森林生态系统地下根源碳(C)输入到土壤中两种途径的示意图。
Fig. 4
Two pathways of root-derived carbon (C) input (i.e., root- and mycelium-derived C) to soils in forest ecosystems. N, nitrogen.
3 研究展望
3.1 森林根系分泌物研究的原位技术体系构建与提升
根系分泌物的研究方法与体系构建始终是森林根际生态学研究的前沿和难点之一。精确的仪器、先进的技术和严谨科学的方法为森林根系分泌物生态学研究带来了新的发展与机遇。近年来, 尽管根系分泌物在研究方法与技术上取得了长足的进步, 但研究过程中仍然面临着诸多挑战和难点。展望未来, 创新和发展新的研究技术体系依然是森林根际生态学未来研究的重点工作内容。
3.1.1 构建和完善根系分泌物的原位收集研究方法
现有的森林根系分泌物收集与生态学效应研究的主要研究对象为控制条件下的单一移栽幼苗, 而有关森林生态系统根系分泌物原位收集的研究甚少。然而, 正如前文所述, 除了生长环境会影响植物根系分泌物输入的数量与质量之外, 野外自然条件下树种种内/种间互作(竞争、协作)也可能深刻地影响森林根系分泌物的分泌模式与动态变化(Yin et al., 2018), 导致无法将室内或纯人工控制系统条件下的单一树种根系分泌物试验结果外推于野外自然条件, 限制了对森林根系分泌物输入特征和动态规律的深入认识。近年来, 相关学者逐渐构建了一些森林根系分泌物原位收集技术体系, 一定程度上解决了野外收集的困难, 但现有原位收集技术还是存在诸多不足, 如仍无法避免树木根系和微生物对根系分泌物成分的吸收, 且无法实现根系分泌物的原位动态收集(Warren, 2016)。因此, 根据特定的研究目的和实际情况, 研发可操作性强且能尽可能地准确真实反映森林树种根系分泌物状况的原位、动态、实时收集方法与技术, 将是该研究领域未来的一项重要工作。
3.1.2 完善根系分泌物组分的现代分析方法
精确分析根系分泌物化学组分和含量是深入开展根系分泌物作用与功能研究的前提, 也是森林根系分泌物研究的重点内容(Haichar et al., 2014)。根系分泌物组分繁多, 各组分含量较低, 且极易被微生物降解, 这对根系分泌物化学组分定性和定量精准分析提出了较大的挑战, 迫切需要建立可行、高效、稳定的根系分泌物分离纯化和鉴定方法, 为森林根际生态学研究提供技术支撑。目前对于根系分泌物的分析通常采用有机溶剂萃取并结合高效液相色谱法、气相质谱法等进行定性定量测定(Xia et al., 2012; Strehmel et al., 2014; Li et al., 2018)。虽然这些方法的广泛运用很大程度上促进了根系分泌物的研究, 但这些技术本身还是存在诸多局限性。例如, 这些方法不但耗时, 而且所鉴定出的根系分泌物组分仅占根系分泌物种类中的一小部分, 而造成很大一部分化学组分信息缺失。同时, 已有分析方法也主要关注根系分泌物中含量较高或者具有重要功能的一些化学组分(如有机酸), 而忽略了根系分泌物中含量较低但可能具有同等重要作用的一些特异性组分(如根系-微生物界面的信号化学物质)。此外, 现有的根系分泌物组分分析方法无法实现野外原位实时动态检测, 导致收集的根系分泌物样品在带回实验室等待测试的过程中极易遭受微生物污染, 使得测定的样品组分和含量与实际野外状况存在较大的差异。因此, 亟需结合代谢组学、蛋白质组学、生物传感器、比率荧光化学传感器等新技术, 针对不同根系分泌物化学组分建立高效、完善的现代分析方法和产品, 以便及时准确地获取森林根系分泌物输入的定性和定量的关键信息(Fuhrer & Zamboni, 2015; Martinière et al., 2018)。
3.2 加强森林根系分泌物输入时空分布格局与根际土壤微生物特征的关联研究
森林地下根际是土壤生物地球化学循环过程的热点区域, 并受土壤特性、环境条件(如温度)、根系分泌物组分特性、根系特征等多种因素影响, 使得根际区土壤物理、化学和生物学特征表现出高度的时空异质性(Hinsinger et al., 2005)。然而, 受研究方法和手段的限制, 现有根际模型和试验研究大多将根际区域简单视为一个均一体, 很少考虑根系分泌物输入数量与质量的时空分布格局变化及其介导的土壤异质性差异, 极大地限制了在细微尺度上对森林根系活动-土壤界面过程与调控机理的认识与理解(Preece et al., 2018)。结合森林根际生态过程目前的研究进展和国际前沿动态, 如下几个研究方向和内容在未来森林根系分泌物生态学研究中值得重点关注。
3.2.1 重视根际区水平方向上根系分泌物输入异质性研究
目前众多研究将森林根系分泌物在根际区的输入视为均一体, 几乎都未考虑根系分泌物数量与质量在根际区沿距离梯度上的异质性。实际上, 受树种根系(菌根)特征和不同分泌物组分释放距离远近的影响, 根表不同距离所释放的根系分泌物组分和含量存在较大差异, 相应地微生物群落结构、数量与分布也随距离远近表现出高度的异质性(Darrah, 1991; Holz et al., 2018)。然而, 目前森林根际土壤取样通常采用抖落法或者取离根表面一定范围的土壤统一作为根际土, 这种传统的取样方法很大程度上掩盖了根际土壤特征在距根系不同距离所呈现出的高度异质性, 限制了森林根际生态学研究及其理论体系的发展。因此, 未来研究应结合先进的技术手段(如C同位素标记)和完善的根际土壤取样方法, 加强森林根系-土壤界面中不同距离梯度上根系分泌物输入特征差异及其与根际土壤特征的关联性原位研究。
3.2.2 加强根际区垂直方向上根系分泌物输入异质性研究
目前森林根系分泌物输入主要考虑表层土壤(0-15 cm), 而有关深层土壤中的根系分泌物输入信息几乎一片空白。然而, 由于不同深度土壤层物理、化学和生物学特性存在较大差异, 导致森林树种在不同土壤层可能采取差异化的根系C投入-养分收益策略, 进而根系分泌物输入特征在不同土壤层深度表现出差异化的垂直分布格局(Tückmantel et al., 2017; Shahzad et al., 2018), 并进一步深刻调控土壤有机质分解与养分矿化过程。因此, 未来研究应加强森林根系分泌物在土壤层垂直方向上的输入特征及其主要影响因素研究, 以丰富森林根系分泌物输入对深层土壤C根际激发效应与土壤C库动态的认识。
3.2.3 深化根系分泌物输入特征季节动态变化规律研究
受森林根系分泌物野外原位收集方法、操作可行性等诸多因素限制, 目前森林根系分泌物输入速率及其通量的研究主要集中在生长季节, 而非生长季的研究甚少。这类研究的缺乏导致对森林根系分泌物输入通量动态规律缺乏基本的了解, 并影响生态系统尺度上对森林根系分泌物输入通量的准确估算。考虑到森林根系分泌物主要源自植物地上光合C产物的分配, 而不同季节环境因子(如温度、光强等)深刻地控制着森林根系分泌物的种类、含量与通量等(Yin et al., 2014; Nakayama & Tateno, 2018)。因此, 未来研究应结合野外长期试验与同位素标记等技术手段, 加强森林根系分泌物输入动态特征(包括日动态、季节动态等)与树种物候、生理、外界环境条件的偶联关系研究, 以丰富对森林根系分泌物季节动态变化规律及其主导因素的认识。比如, 森林根系分泌物生长季与非生长季输入通量大小及其相对贡献如何?控制树种根系分泌物释放通量大小的主要生物与非生物因素有哪些?地上叶片光合产物产生与地下根系分泌物释放时间动态是否同步?如果不同步, 二者相差时间多长?目前对上述基本信息的了解几乎一片空白, 未来研究应加强对森林根系分泌物输入动态规律及其调控因素的研究, 这些信息对于森林生态系统地下C通量估算和根际土壤碳-养分循环过程模型构建均具有重要作用。
3.2.4 强化不同根级/功能模块与根系分泌物输入特征关联性研究
受研究方法和野外条件限制, 目前森林根系分泌物收集通常将一定直径范围内的细根视为一个整体考虑, 而很少关注根系不同形态和区域根系分泌物输入数量与质量的差异(Proctor & He, 2017)。然而, 近年来人们逐渐认识到, 由于树木细根具有高度的结构和功能异质性, 采用传统简单的基于某一直径阈值(即直径法)的研究方法很难全面准确地揭示细根功能特征变化规律。相应地, 根系分级以及功能模块方法近年来在细根结构与功能研究中逐渐引起重视并得到广泛应用(Pregitzer et al., 2002; Guo et al., 2008)。研究者按照根的分枝等级将传统意义的细根(直径2 mm以下)划分出不同等级, 并进一步根据根级之间的差异性以及相似性将细根划分为不同功能模块, 通常1-2级细根由于直径较细, 组织N含量较高, 新陈代谢旺盛, 皮层较厚, 易被菌根真菌侵染而主要承担养分和水分吸收功能(即吸收根); 3-5级细根由于直径较粗, 组织N含量低, 真菌侵染率低, 有明显的次生结构而主要承担养分和水分运输功能(即运输根)(McCormack et al., 2015)。不同根级/功能模块其解剖结构、形态构型、化学组成和生理功能等特征差异势必会深刻影响和调控其根系分泌物的数量与质量。然而, 目前关于森林不同根系功能模块/根级与根系分泌物输入特征的关联目前未见报道, 尤其在叠加菌根侵染这一要素后, 一定程度上限制了对森林根系生态功能的认识与根际生态学理论的拓展。相应地, 从细微尺度上加强森林不同根级/功能模块根系分泌物输入特征差异与土壤过程的偶联研究是未来森林根际生态学优先研究的重要领域。
3.3 加强森林根系分泌物介导的土壤生物地球化学循环过程作用机理研究
由于森林根系分泌物输入在土壤有机质分解和养分循环过程中具有十分重要的调控作用, 根系分泌物所介导的土壤碳-养分循环过程激发效应一直是土壤生态学研究的核心内容。但受制于根际微系统复杂的时空异质性以及研究方法和技术的限制, 目前该领域相关研究主要处于生态效应现象探究阶段, 而相对缺乏细微尺度上根系分泌物输入对土壤碳-养分循环过程作用机理的深入揭示, 尤其在全球气候变化背景下。目前人们对森林根系-土壤-微生物互作过程及其对环境变化的响应机理依然缺乏足够的认知和理解, 未来应从以下几个方面加强森林根系分泌物对土壤碳-养分循环过程影响的作用机制的研究。
3.3.1 深化森林根系分泌物对土壤碳-养分循环影响的微生物与非生物作用过程研究
土壤碳-养分循环过程一直是土壤微观机理研究始终关注的重要问题和热点, 而根际又是土壤微观机理研究的重要区域。目前大部分关于森林根系分泌物对土壤碳-养分通量所诱导的生态效应研究主要聚焦于微生物共代谢过程。然而, 近年来相关研究证实了根系分泌物也可通过间接的非生物作用过程影响土壤有机-矿质复合体稳定性, 并深刻地调控土壤碳-养分循环过程, 这对传统的“微生物共代谢”理论提出了巨大的挑战。因此, 非生物作用过程近年来已经成为根际土壤碳-养分循环过程研究的热点方向(Keiluweit et al., 2015; Jilling et al., 2018; Yuan et al., 2018), 未来该领域研究应加强与土壤学、微生物学、土壤界面化学等学科的交叉, 并利用当前先进技术(如同步辐射近边精细吸收光谱(NEXAFS)、扫描透射X射线显微技术(STXM)等), 求证和量化微生物/非生物作用过程对根系分泌物驱动的土壤生物地球化学循环过程的相对贡献与主导因素。比如, 什么情况下微生物作用过程起主导作用, 而又在什么情况下非生物作用过程反而贡献更大。
3.3.2 重视森林根系/菌根C输入对土壤碳-养分循环的影响差异与作用机理研究
ECM和AM是森林生态系统中广泛存在的两种菌根类型, 它们在森林生态过程和功能中的重要调控作用已得到了广泛的认知和关注(Laliberté, 2017), 但由于受根际微系统复杂的时空异质性以及研究方法和技术的限制, 现有的试验研究和理论模型大都将根系和菌根视为一个整体进行考虑(Wallander et al., 2013)。大量研究结果已表明, 森林菌根通过外延菌丝可向土壤输入不同于根系途径的含C化合物和胞外水解酶, 并诱导激发特异化的土壤碳-养分微生物代谢过程, 该过程被称为菌丝际激发效应(Meier et al., 2015)。例如, Zhang等(2018b)采用不同孔径的内生长管(从物理上原位区分根系和外延菌丝各自的作用), 研究了西南亚高山针叶林根系/菌丝C输入对土壤C库动态的影响效应差异。结果发现, 外延菌丝C输入途径对土壤中新C的贡献(~65%)远高于根系C输入途径(~35%)。进一步分析发现, 虽然来源于菌丝/根系的新C输入诱导了相似的激发效应方向, 但外延菌丝C输入诱导了更大的激发效应强度, 约为根系C输入激发效应强度的2倍; 相似地, 亚高山针叶林菌丝C输入对土壤N矿化的促进作用贡献约80%, 而根系C输入的相对贡献仅为20%左右(Zhang et al., 2018a)。这些结果表明, 菌丝C在森林土壤碳-养分循环过程中具有非常重要的作用, 未来森林土壤碳-养分循环过程及土壤生物地球化学循环模型构建应充分重视和考虑菌根真菌外延菌丝所介导的生态学作用。然而, 现有研究仅停留在菌丝/根系C输入对土壤生物地球化学循环过程影响的现象探究阶段, 而有关不同C源输入对土壤过程的作用机理研究目前还未见报道。因此, 全球气候变化条件下森林细根/菌根不同途径C输入对土壤生物地球化学循环过程的生态学效应与作用机理差异已成为根际生态学研究领域一个十分重要但认知又极为缺乏的研究课题, 势必将成为森林根际生态学过程研究的核心界面和新热点。
3.3.3 关注森林根系分泌物对土壤磷(P)循环过程的影响效应与作用机制研究
由于土壤颗粒对P的吸附和固定, P在土壤中的移动性很差, 只能通过扩散方式到达根系表面, 导致植物对土壤有机P的利用非常有限。相应地, 土壤P对植物生长的限制是森林生态系统中较为普遍的一种现象。如何促进P循环并提高P的有效性对维持森林生态系统结构和功能的稳定性具有重要的意义, 也是森林土壤生态学研究的一个重要内容(Finzi et al., 2015)。然而, 现有森林根系分泌物对土壤生物地球化学循环过程的研究主要集中在土壤C、N转化过程, 而对P循环的生态学效应与作用机制研究和直接试验证据甚少。事实上, 根系分泌物对土壤P循环具有重要的调控效应。一方面, 根系分泌物中低分子化合物输入(如有机酸)可通过螯合和pH促进的P可溶性而提高土壤P有效性; 另一方面, 根系分泌物可通过释放磷酸酶而直接裂解束缚在SOM中的有机P供植物利用, 从而使得根际范围区表现出明显的P耗竭现象(Cleveland et al., 2013)。此外, 由于森林菌根外延菌丝通过形成庞大的菌丝网络, 有效地扩展了根系的可达范围, 外延菌丝所占据的土壤空间远远大于根系所占据的土壤空间, 使得外延菌丝活化难溶性磷酸盐的量可能高于根系的活化量。同时, 森林菌根外延菌丝是磷酸酶释放的重要位置, 进而把难溶性的P转化为可直接被植物根吸收利用的可溶性P, 对土壤有机P活化具有重要的调控作用(Cairney, 2012)。因此, 未来研究还需要重点关注全球气候变化背景下森林菌根/外延菌丝分泌物变化对土壤P循环所诱导的激发效应与作用机理研究。
3.4 加强森林根系分泌物介导的物种间互作关系研究
3.4.1 深化以根系分泌物为介质的根际化学信号识别与通讯研究
根系分泌物除了释放大量的初级代谢物质为微生物提供营养源而驱动相关的土壤碳-养分微生物共代谢过程, 还可释放一些具有生物活性的大分子和小分子次生代谢物质, 这些纷繁复杂的次生代谢物质可通过直接(产生毒素、化感物质)或者间接(改变土壤化学、微生物特征和营养吸收、营养级联关系)的方式, 与其他土壤生物形成复杂且强烈的地下化学通讯与根际交流(种间作用、化感作用、病虫害抗性、根系识别、病原菌抑制、菌根真菌共生关联等), 在很大程度上调控了植物与其他有机生命体的相互关系, 并对生态系统结构和功能产生重要的影响(van Dam & Bouwmeester, 2016; Hu et al., 2018)。虽然根际信号交流与分子互作已经成为近年来根系生态学研究的热点方向, 但对根系分泌物中调控根际行为的分子信号物质精准识别的了解甚为有限, 且目前开展的有限研究也主要集中在农业生态系统中(Kong et al., 2006; Sun et al., 2016)。
根系分泌的化学信号物质在土壤介质中可以扩散和迁移, 因而其传播不需要根系的直接接触, 但其浓度往往较低, 且在传输过程中易被土壤其他微生物利用降解, 因此, 这些化学信号到达目标对象后是否仍保持有效的作用浓度让人质疑, 这也是限制森林根系分泌物介导的地下化学通讯研究进展缓慢的原因之一。然而, 近年来, 随着对森林地下菌根网络C交换和化感物质迁移研究的不断深入, 人们发现森林地下庞大的菌根网络可有效地提高化学信号物质在土壤中的传输距离和转移能力, 进而可能从大尺度上调控植物根系-微生物间的相互作用(Klein et al., 2016)。目前有关森林菌根网络的研究主要集中在水分相互传递、矿质元素、营养物质运输, 而很少研究区分和识别菌根真菌网络所转运的信号物质及其介导的根际对话形式与机理。因此, 未来研究应加强森林根系(菌根)分泌的化学信号物质识别, 并阐释其转运和调控分子机制, 尤其是在野外原位条件下的研究。这将为从根系分泌物途径揭秘植物间的根系互作过程与识别机理提供新的研究途径与视野。例如, 森林根系(菌根)释放的具有重要作用与功能的化学信号物质的准确识别, 森林地下菌根真菌网络对于化学信号物质的传递选择是否具有特异性等。
3.4.2 加强根系分泌物-微生物-动物营养级联关系及其生态重要性研究
现有有关植物根系分泌物对土壤生物影响的研究主要聚焦于微生物群落结构与功能特性变化, 而很少关注根系分泌物输入对土壤动物区系及其关联的多重营养级联效应的影响。事实上, 土壤动物、微生物作为森林生态系统物质循环中的重要消费者, 二者在生态系统的作用过程中与根系分泌物输入关系密切、相互影响(van Dam & Bouwmeester, 2016)。一方面, 根系分泌物为土壤微生物生长和繁殖提供所需的C源和能源, 改变土壤微生物区系, 反过来, 改变的微生物区系通过调控土壤有机质分解和养分矿化过程为植物提供所需的养分; 另一方面, 根系分泌物释放可促进土壤细菌、真菌的生长繁殖, 从而有利于以这些微生物为食的原生动物的生长。与此同时, 土壤动物通过食用植物残体碎屑、土壤有机质, 捕食细菌、真菌等, 也可改善土壤理化性质而影响土壤物质循环。此外, 很多土壤动物(如原生动物、蚯蚓等)还能分泌一些激素类物质促进植物生长(Singh et al., 2004)。因此, 森林根系分泌物-微生物-土壤动物之间存在着错综复杂的捕食关系, 构成了不同层级的食物网, 从而驱动和调控土壤物质传递和循环、资源再分配。鉴于根系分泌物-微生物-动物营养级联在土壤生物地球化学循环过程中的重要作用, 森林根系分泌物-微生物-土壤动物营养级联关系及其生态反馈效应将成为根际生态学研究领域未来重点关注的一个课题。
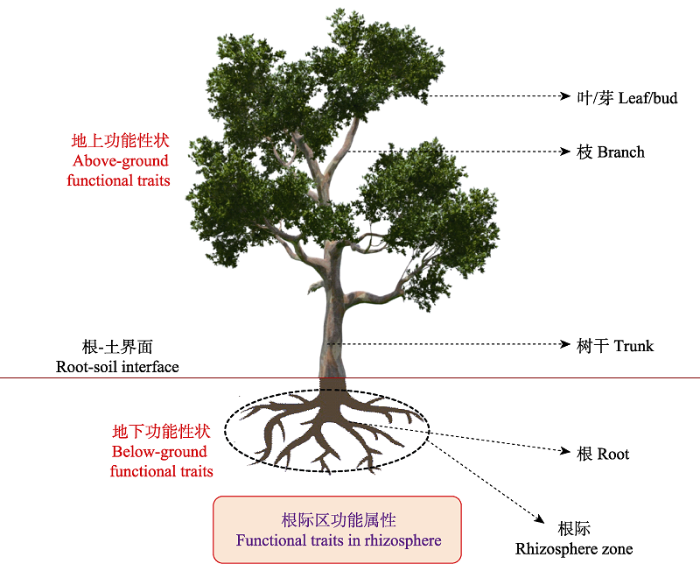
3.5 强化森林根系分泌物介导的根际功能属性特征及其进化生态学意义研究
植物功能性状是指植物体具有的与其定植、存活、生长和死亡紧密相关的一系列核心植物属性, 它是植物与环境长期相互作用结果的体现, 因而植物功能性状具有重要的生态功能与进化学意义(Shipley et al., 2016)。相应地, 植物功能性状研究已成为解决重要生态学问题的可靠途径。然而长期以来, 关于森林植物功能性状和进化理论框架主要聚焦于根、茎、叶、芽等地上部分, 但对地下部分功能性状和组织方式研究相对较少(Bardgett et al., 2014)(图5)。因此, 为更好地理解和预测植物功能性状以及功能多样性与生态系统功能的关系和生物进化, 近年来不少学者已聚焦于森林植物地下功能性状及其与地上功能性状的关联性研究, 并获得了一系列重要成果。例如, 中国科学院地理科学与资源研究所郭大立团队经过14年的潜心积累, 对全球7个生物群区的369种植物一级根功能形状进行了深入分析, 首次在全球尺度上揭示了根系功能性状的生物地理格局, 提出了一个全新的植物进化理论: 植物在长达4亿年的进化过程中, 地下吸收根朝更加高效、独立的方向进化, 为不同植物“制定”了独特的养分、水分吸收策略,从而推动了植物的传播和进化历程。该工作揭秘了根系在植物进化和适应过程中的推动作用, 对于生物多样性保护具有重要意义。该成果于2018年3月以简报(Letters)形式发表在《Nature》杂志, 这也是《Nature》杂志首次发表大尺度根系生态学研究领域的成果(Ma et al., 2018)。
图5
图5
拓展和丰富以根际区功能属性为代表的地下功能性状指标体系。
Fig. 5
Broadening functional traits of root and microbe in rhizosphere and enriching the suite of belowground functional traits.
目前植物功能性状手册中地上功能性状指标数量超过20种, 而地下功能性状指标数量非常有限(Pérez-Hargunideguy et al., 2013)。同时, 现有少量的地下根系功能性状研究主要集中在根系本身的形态和化学特征, 而关于根系生理特征及其介导的根系-土壤-微生物界面属性特征在定义地下植物资源获取策略及进化生态学意义时并未得到充分考虑(Roumet et al., 2016; Guyonnet et al., 2018), 迫切需要将其纳入植物功能性状的研究中, 以拓展和丰富森林生态系统地下功能性状指标体系(图5)。这其中, 根系分泌物作为一种C源/能源投入, 是一个耗能的过程, 植物通过整合多元信息(环境变化、土壤养分有效性、微生物特征、植物生长发育等)来调整植物根系分泌物释放的种类和含量, 并最终决定植物选择激进、平衡或者保守策略以使植物生长收益最大化。因此, 植物根系分泌物种类与含量是植物根部长期进化和对环境条件适应的结果, 并在森林根际微区域诱导了一系列独特的根系和土壤微生物功能性状特征, 即根际功能属性特征, 它涵括了根际微系统中的根系、菌根(菌丝)与根际土壤等核心单元, 具有重要的生态学功能和进化学意义。相应地, 在区域大尺度上深化森林根际属性(根系、菌根真菌、根际土壤与微生物属性)的空间分异规律及其与环境因子(如温度、降水、海拔等)的关联性研究, 不但可以丰富和补充地下功能性状, 完善全球和区域植物属性数据库建设; 同时对于解密地下功能属性在植物群落进化、种群分布格局、适应过程与生物多样性维持机制等方面的作用将提供新的研究视野(Laliberté, 2017)。
综上所述, 伴随着全球气候变化和土地利用方式的变化, 森林类型、物种组成、地下C分配格局以及菌根侵染变化都直接影响根系分泌物C输入的数量与质量, 从而深刻地影响森林根际区域根系-土壤互作过程及其生态反馈效应。展望未来, 为了能更深入、全面地揭示全球气候变化下植物-土壤-微生物互作机理及其生态反馈效应, 未来森林根际生态学研究应重视从植物生理视角出发, 不断完善和改进根系分泌物研究技术体系与方法, 并通过多学科交叉与先进技术手段的运用(如微生物基因组学、各种宏组学技术、同位素技术、显微成像技术、土壤原位酶谱技术、质谱分析等), 从细微尺度上深化森林根系分泌物时空分布格局(如季节/日动态、水平/垂直分布格局、根级/根功能模块分布)特征研究; 在此基础上, 加强森林根系(菌根)分泌物输入介导的土壤碳-养分循环过程、根际信号交流与对话机理及其与环境之间的关联等研究; 从大尺度上加强森林根系分泌物介导的根际功能属性的空间分异规律与环境因子的关联性研究, 最终为深入揭示森林地下根际王国这一“黑箱”所蕴含的诸多奥秘提供新的视角和有效途径。

参考文献
The role of root exudates in rhizosphere interactions with plants and other organisms
Going underground: Root traits as drivers of ecosystem processes
DOI:10.1016/j.tree.2014.10.006
URL
PMID:25459399
[本文引用: 1]

Ecologists are increasingly adopting trait-based approaches to understand how community change influences ecosystem processes. However, most of this research has focussed on aboveground plant traits, whereas it is becoming clear that root traits are important drivers of many ecosystem processes, such as carbon (C) and nutrient cycling, and the formation and structural stability of soil. Here, we synthesise emerging evidence that illustrates how root traits impact ecosystem processes, and propose a pathway to unravel the complex roles of root traits in driving ecosystem processes and their response to global change. Finally, we identify research challenges and novel technologies to address them.
Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil
DOI:10.1016/j.soilbio.2011.12.029
URL
[本文引用: 1]

Extramatrical mycelia (EMM) of ectomycorrhizal (ECM) fungi are potentially extensive in soil and receive significant allocations of plant-derived carbon. Although losses from living EMM occur via respiration and exudation, EMM represents a considerable biomass component and potential carbon sink in many forest soils. ECM root tips and rhizomorphs may persist in soil for many months, but interactions between grazing arthropods and decomposers probably facilitate more rapid turnover of diffuse EMM. Elevated atmospheric CO2 concentration [CO2] is likely to increase carbon allocation to ECM fungi by their tree hosts. This will probably increase root colonization by ECM fungi and drive changes in their communities in soil. The likely effects of elevated [CO2] and other climate change factors on the production and turnover of EMM production are difficult to predict from current evidence, and this hampers our understanding of their potential value as future carbon sinks. Responses of grazing soil arthropods to future climate change will have a strong influence on EMM turnover, along with the abilities of ECM fungi to store carbon in below-ground, and this should be seen as a priority area for future research.
Synthesis and modeling perspectives of rhizosphere priming
DOI:10.1111/nph.12440
URL
PMID:23952258
[本文引用: 2]

I.II.III.IV.V.VI.VII. SummaryThe rhizosphere priming effect (RPE) is a mechanism by which plants interact with soil functions. The large impact of the RPE on soil organic matter decomposition rates (from 50% reduction to 380% increase) warrants similar attention to that being paid to climatic controls on ecosystem functions. Furthermore, global increases in atmospheric CO2 concentration and surface temperature can significantly alter the RPE. Our analysis using a game theoretic model suggests that the RPE may have resulted from an evolutionarily stable mutualistic association between plants and rhizosphere microbes. Through model simulations based on microbial physiology, we demonstrate that a shift in microbial metabolic response to different substrate inputs from plants is a plausible mechanism leading to positive or negative RPEs. In a case study of the Duke Free-Air CO2 Enrichment experiment, performance of the PhotoCent model was significantly improved by including an RPE-induced 40% increase in soil organic matter decomposition rate for the elevated CO2 treatment demonstrating the value of incorporating the RPE into future ecosystem models. Overall, the RPE is emerging as a crucial mechanism in terrestrial ecosystems, which awaits substantial research and model development.
Patterns of new versus recycled primary production in the terrestrial biosphere
DOI:10.1073/pnas.1302768110
URL
PMID:23861492
[本文引用: 1]

Nitrogen (N) and phosphorus (P) availability regulate plant productivity throughout the terrestrial biosphere, influencing the patterns and magnitude of net primary production (NPP) by land plants both now and into the future. These nutrients enter ecosystems via geologic and atmospheric pathways and are recycled to varying degrees through the plant-soil-microbe system via organic matter decay processes. However, the proportion of global NPP that can be attributed to new nutrient inputs versus recycled nutrients is unresolved, as are the large-scale patterns of variation across terrestrial ecosystems. Here, we combined satellite imagery, biogeochemical modeling, and empirical observations to identify previously unrecognized patterns of new versus recycled nutrient (N and P) productivity on land. Our analysis points to tropical forests as a hotspot of new NPP fueled by new N (accounting for 45% of total new NPP globally), much higher than previous estimates from temperate and high-latitude regions. The large fraction of tropical forest NPP resulting from new N is driven by the high capacity for N fixation, although this varies considerably within this diverse biome; N deposition explains a much smaller proportion of new NPP. By contrast, the contribution of new N to primary productivity is lower outside the tropics, and worldwide, new P inputs are uniformly low relative to plant demands. These results imply that new N inputs have the greatest capacity to fuel additional NPP by terrestrial plants, whereas low P availability may ultimately constrain NPP across much of the terrestrial biosphere.
C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass?
Measuring the diffusion coefficient of rhizosphere exudates in soil. I. The diffusion of non-sorbing compounds
DOI:10.1111/j.1365-2389.1991.tb00419.x
URL
[本文引用: 1]

A quick,convenient and robust method is presented for measuring the effective diffusion coefficients of non-sorbing solutes in soil. The method estimates the effective diffusion coefficient from a measured diffusion profile by optimizing the solution of a numerical simulation model describing the experimental system. The method was used to measure the effective diffusion coefficients of compounds found in root exudates.
Rhizosphere: So many achievements and even more challenges
DOI:10.1007/s11104-009-0063-5
URL
[本文引用: 1]

The story of this “Rhizosphere book project” startedabout 3 years ago, as the three of us were discussingthe organization of the “International Rhizosphere 2Conference” held in Montpellier in 2007 (Hartmannet al. 2008a; Jones and Hinsinger 2008).
Rhizosphere priming: A nutrient perspective
DOI:10.3389/fmicb.2013.00216
URL
PMID:23908649
[本文引用: 1]

Rhizosphere priming is the change in decomposition of soil organic matter (SOM) caused by root activity. Rhizosphere priming plays a crucial role in soil carbon (C) dynamics and their response to global climate change. Rhizosphere priming may be affected by soil nutrient availability, but rhizosphere priming itself can also affect nutrient supply to plants. These interactive effects may be of particular relevance in understanding the sustained increase in plant growth and nutrient supply in response to a rise in atmospheric CO2concentration. We examined how these interactions were affected by elevated CO2in two similar semiarid grassland field studies. We found that an increase in rhizosphere priming enhanced the release of nitrogen (N) through decomposition of a larger fraction of SOM in one study, but not in the other. We postulate that rhizosphere priming may enhance N supply to plants in systems that are N limited, but that rhizosphere priming may not occur in systems that are phosphorus (P) limited. Under P limitation, rhizodeposition may be used for mobilization of P, rather than for decomposition of SOM. Therefore, with increasing atmospheric CO2concentrations, rhizosphere priming may play a larger role in affecting C sequestration in N poor than in P poor soils.
Stoichiometry constrains microbial response to root exudation-insights from a model and a field experiment in a temperate forest
DOI:10.5194/bg-10-821-2013
URL
[本文引用: 1]

Plant roots release a wide range of chemicals into soils. This process, termed root exudation, is thought to increase the activity of microbes and the exoenzymes they synthesize, leading to accelerated rates of carbon (C) mineralization and nutrient cycling in rhizosphere soils relative to bulk soils. The nitrogen (N) content of microbial biomass and exoenzymes may introduce a stoichiometric constraint on the ability of microbes to effectively utilize the root exudates, particularly if the exudates are rich in C but low in N. We combined a theoretical model of microbial activity with an exudation experiment to test the hypothesis that the ability of soil microbes to utilize root exudates for the synthesis of additional biomass and exoenzymes is constrained by N availability. The field experiment simulated exudation by automatically pumping solutions of chemicals often found in root exudates ("exudate mimics") containing C alone or C in combination with N (C : N ratio of 10) through microlysimeter "root simulators" into intact forest soils in two 50-day experiments. The delivery of C-only exudate mimics increased microbial respiration but had no effect on microbial biomass or exoenzyme activities. By contrast, experimental delivery of exudate mimics containing both C and N significantly increased microbial respiration, microbial biomass, and the activity of exoenzymes that decompose low molecular weight components of soil organic matter (SOM, e.g., cellulose, amino sugars), while decreasing the activity of exoenzymes that degrade high molecular weight SOM (e.g., polyphenols, lignin). The modeling results were consistent with the experiments; simulated delivery of C-only exudates induced microbial N-limitation, which constrained the synthesis of microbial biomass and exoenzymes. Exuding N as well as C alleviated this stoichiometric constraint in the model, allowing for increased exoenzyme production, the priming of decomposition, and a net release of N from SOM (i.e., mineralization). The quantity of N released from SOM in the model simulations was, under most circumstances, in excess of the N in the exudate pulse, suggesting that the exudation of N-containing compounds can be a viable strategy for plant-N acquisition via a priming effect. The experimental and modeling results were consistent with our hypothesis that N-containing compounds in root exudates affect rhizosphere processes by providing substrates for the synthesis of N-rich microbial biomass and exoenzymes. This study suggests that exudate stoichiometry is an important and underappreciated driver of microbial activity in rhizosphere soils.
Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2
Frontiers in root ecology: Recent advances and future challenges
DOI:10.1007/s11104-018-3618-5
URL
[本文引用: 1]

Background Roots play a pivotal role in defining plant ecological success and mediating terrestrial ecosystem functioning. However, roots are difficult to study as they are hidden in the soil matrix...
Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles
DOI:10.1111/gcb.12816
URL
PMID:25421798
[本文引用: 4]

Abstract While there is an emerging view that roots and their associated microbes actively alter resource availability and soil organic matter (SOM) decomposition, the ecosystem consequences of such rhizosphere effects have rarely been quantified. Using a meta-analysis, we show that multiple indices of microbially mediated C and nitrogen (N) cycling, including SOM decomposition, are significantly enhanced in the rhizospheres of diverse vegetation types. Then, using a numerical model that combines rhizosphere effect sizes with fine root morphology and depth distributions, we show that root-accelerated mineralization and priming can account for up to one-third of the total C and N mineralized in temperate forest soils. Finally, using a stoichiometrically constrained microbial decomposition model, we show that these effects can be induced by relatively modest fluxes of root-derived C, on the order of 4% and 6% of gross and net primary production, respectively. Collectively, our results indicate that rhizosphere processes are a widespread, quantitatively important driver of SOM decomposition and nutrient release at the ecosystem scale, with potential consequences for global C stocks and vegetation feedbacks to climate.
High-throughput discovery metabolomics
Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods
DOI:10.1111/j.1469-8137.2007.02242.x
URL
PMID:17944827
[本文引用: 1]

090004 Fine roots constitute a large and dynamic component of the carbon cycles of terrestrial ecosystems. The reported fivefold discrepancy in turnover estimates between median longevity (ML) from minirhizotrons and mean residence time (MRT) using carbon isotopes may have global consequences. 090004 Here, a root branch order-based model and a simulated factorial experiment were used to examine four sources of error. 090004 Inherent differences between ML, a number-based measure, and MRT, a mass-based measure, and the inability of the MRT method to account for multiple replacements of rapidly cycling roots were the two sources of error that contributed more to the disparity than did the improper choice of root age distribution models and sampling bias. Sensitivity analysis showed that the rate at which root longevity increases as order increases was the most important factor influencing the disparity between ML and MRT. 090004 Assessing root populations for each branch order may substantially reduce the errors in longevity estimates of the fine root guild. Our results point to the need to acquire longevity estimates of different orders, particularly those of higher orders.
Root exudation rate as functional trait involved in plant nutrient-use strategy classification
DOI:10.1002/ece3.4383
URL
[本文引用: 1]

Plant–microbe interactions actively control nitrogen (N) cycling in the ecosystem. We hypothesize that the investment into exudation and the coupling of plant–microbe N cycling will be larger in competitive plants compared to the more conservative species. Root exudation of competitive (Glyceria maxima) and conservative (Carex acuta) plants was estimated by 13C-CO2 labeling. Seasonal changes... [Show full abstract]
Root exudates mediated interactions belowground
DOI:10.1016/j.soilbio.2014.06.017
URL
[本文引用: 1]

61Plant–microbe interactions are mediated by root exudation of different secondary metabolites.61Flavonoides and strigolactones exudation serve as signal molecules for symbiosis establishment.61Root exudates contain antimicrobial compounds to cope with pathogens.61Border cells protect root apex from pathogens and parasitic nematodes.61Nutrients from root exudates are assimilated by root associated bacteria.
Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes
DOI:10.1111/j.1469-8137.2005.01512.x
URL
PMID:16219069
[本文引用: 1]

The rhizosphere differs from the bulk soil in a range of biochemical, chemical and physical processes that occur as a consequence of root growth, water and nutrient uptake, respiration and rhizodeposition. These processes also affect microbial ecology and plant physiology to a considerable extent. This review concentrates on two features of this unique environment: rhizosphere geometry and heterogeneity in both space and time. Although it is often depicted as a soil cylinder of a given radius around the root, drawing a boundary between the rhizosphere and bulk soil is an impossible task because rhizosphere processes result in gradients of different sizes. For instance, because of diffusional constraints, root uptake can result in a depletion zone extending <1 mm for phosphate to several centimetres for nitrate, while respiration may affect the bulk of the soil. Rhizosphere processes are responsible for spatial and temporal heterogeneities in the soil, although these are sometimes difficult to distinguish from intrinsic soil heterogeneity. A further complexity is that these processes are regulated by plants, microbial communities and soil constituents, and their many interactions. Novel in situ techniques and modelling will help in providing a holistic view of rhizosphere functioning, which is a prerequisite for its management and manipulation.
Towards a more plant physiological perspective on soil ecology
Root hairs increase rhizosphere extension and carbon input to soil
DOI:10.1093/aob/mcx127
URL
PMID:29267846
[本文引用: 1]

Background: Recent advances in imaging techniques now make it possible to visualize the biogeochemical and physical environment around the roots, the rhizosphere. Detailed images of pore space geometry and water content dynamics around roots have demonstrated the heterogeneity of the rhizosphere compared with the soil far from the roots. These findings have inspired new models of root water... [Show full abstract]
Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota
DOI:10.1038/s41467-018-05122-7
URL
[本文引用: 1]

Plants associate—analogous to animals or us humans—with a multitude of microorganisms, which collectively function as a microbiome. A major discovery of the last decade is that numerous organisms of a microbiome (aka microbiota) are not unpretentious background actors. Instead, some microbiota members influence host processes including behavior, appetite, and health in animals (1) and... [Show full abstract]
Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes
DOI:10.1007/s10533-018-0459-5
URL
[本文引用: 2]

Despite decades of research progress, ecologists are still debating which pools and fluxes provide nitrogen (N) to plants and soil microbes across different ecosystems. Depolymerization of soil...
Mineral protection of soil carbon counteracted by root exudates
DOI:10.1038/NCLIMATE2580
URL
[本文引用: 3]

Multiple lines of existing evidence suggest that climate change enhances root exudation of organic compounds into soils. Recent experimental studies show that increased exudate inputs may cause a net loss of soil carbon. This stimulation of microbial carbon mineralization (`priming’) is commonly rationalized by the assumption that exudates provide a readily bioavailable supply of energy for the decomposition of native soil carbon (co-metabolism). Here we show that an alternate mechanism can cause carbon loss of equal or greater magnitude. We find that a common root exudate, oxalic acid, promotes carbon loss by liberating organic compounds from protective associations with minerals. By enhancing microbial access to previously mineral-protected compounds, this indirect mechanism accelerated carbon loss more than simply increasing the supply of energetically more favourable substrates. Our results provide insights into the coupled biotic-abiotic mechanisms underlying the `priming’ phenomenon and challenge the assumption that mineral-associated carbon is protected from microbial cycling over millennial timescales.
Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass a new perspective
DOI:10.1016/j.soilbio.2007.06.021
URL
[本文引用: 1]

Soil organic matter is extensively humified; some fractions existing for more than 1000 years. The soil microbial biomass is surrounded by about 50 times its mass of soil organic matter, but can only metabolize it very slowly. Paradoxically, even if more than 90% of the soil microbial biomass is killed, the mineralization of soil organic matter proceeds at the same rate as in an unperturbed soil. Here we show that soil organic matter mineralization is independent of microbial biomass size, community structure or specific activity. We suggest that the rate limiting step is governed by abiological processes (which we term the Regulatory Gate hypothesis), which convert non-bioavailable soil organic matter into bioavailable soil organic matter, and cannot be affected by the microbial population. This work challenges one of the long held theories in soil microbiology proposed by Winogradsky, of the existence of autochthonous and zymogenous microbial populations. This has significant implications for our understanding of carbon mineralization in soils and the role of soil micro-organisms in the global carbon cycle. Here we describe experiments designed to determine if the Regulatory Gate operates. We conclude that there is sufficient experimental evidence for it to be offered as a working hypothesis.
Belowground carbon trade among tall trees in a temperate forest
DOI:10.1126/science.aad6188
URL
PMID:27081070
[本文引用: 3]

Forest trees compete for light and soil resources, but photoassimilates, once produced in the foliage, are not considered to be exchanged between individuals. Applying stable carbon isotope labeling at the canopy scale, we show that carbon assimilated by 40-meter-tall spruce is traded over to neighboring beech, larch, and pine via overlapping root spheres. Isotope mixing signals indicate that the interspecific, bidirectional transfer, assisted by common ectomycorrhiza networks, accounted for 40% of the fine root carbon (about 280 kilograms per hectare per year tree-to-tree transfer). Although competition for resources is commonly considered as the dominant tree-to-tree interaction in forests, trees may interact in more complex ways, including substantial carbon exchange.
Allelochemicals released by rice roots and residues in soil
Review: Factors affecting rhizosphere priming effects
DOI:10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-# URL [本文引用: 1]
Below-ground frontiers in trait-based plant ecology
DOI:10.1111/nph.14247
URL
PMID:27735077
[本文引用: 4]

Abstract Contents 1597 I. 1597 II. 1597 III. 1598 IV. 1598 V. 1600 VI. 1601 VII. 1601 VIII. 1601 1602 References 1602 SUMMARY: Trait-based approaches have led to significant advances in plant ecology, but are currently biased toward above-ground traits. It is becoming clear that a stronger emphasis on below-ground traits is needed to better predict future changes in plant biodiversity and their consequences for ecosystem functioning. Here I propose six 'below-ground frontiers' in trait-based plant ecology, with an emphasis on traits governing soil nutrient acquisition: redefining fine roots; quantifying root trait dimensionality; integrating mycorrhizas; broadening the suite of root traits; determining linkages between root traits and abiotic and biotic factors; and understanding ecosystem-level consequences of root traits. Focusing research efforts along these frontiers should help to fulfil the promise of trait-based ecology: enhanced predictive capacity across ecological scales. 0008 2016 The Authors. New Phytologist 0008 2016 New Phytologist Trust.
The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations
DOI:10.1007/s11104-017-3555-8
URL
[本文引用: 1]

Aims Nitrogen supply and atmospheric CO2 concentration ([CO2]) could influence root exudates directly by altering compound concentrations in roots and indirectly by regulating root morphology. This...
Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant
DOI:10.1126/science.aan0081
URL
PMID:28596311
[本文引用: 1]

Plants form beneficial associations with arbuscular mycorrhizal fungi, which facilitate nutrient acquisition from the soil. In return, the fungi receive organic carbon from the plants. The transcription factor RAM1 (REQUIRED FOR ARBUSCULAR MYCORRHIZATION 1) is crucial for this symbiosis, and we demonstrate that it is required and sufficient for the induction of a lipid biosynthetic pathway that is expressed in plant cells accommodating fungal arbuscules. Lipids are transferred from the plant to mycorrhizal fungi, which are fatty acid auxotrophs, and this lipid export requires the glycerol-3-phosphate acyltransferase RAM2, a direct target of RAM1. Our work shows that in addition to sugars, lipids are a major source of organic carbon delivered to the fungus, and this is necessary for the production of fungal lipids.
Evolutionary history resolves global organization of root functional traits
DOI:10.1038/nature25783
URL
PMID:29620725
[本文引用: 1]

Abstract This corrects the article DOI: 10.1038/nature25783.
Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging
DOI:10.1073/pnas.1721769115
URL
[本文引用: 1]

plants expressing stable membrane-anchored ratiometric fluorescent sensors based on pHluorin. These sensors enabled noninvasive pH-specific measurements in mature root cells from the medium-epidermis interface up to the inner cell layers that lie beyond the Casparian strip. The membrane-associated apoplastic pH was much more alkaline than the overall apoplastic space pH. Proton concentration associated with the plasma membrane was very stable, even when the growth medium pH was altered. This is in apparent contradiction with the direct connection between root intercellular space and the external medium. The plasma membrane-associated pH in the stele was the most preserved and displayed the lowest apoplastic pH (6.0 to 6.1) and the highest transmembrane delta pH (1.5 to 2.2). Both pH values also correlated well with optimal activities of channels and transporters involved in ion uptake and redistribution from the root to the aerial part. In growth medium where ionic content is minimized, the root plasma membrane-associated pH was more affected by environmental proton changes, especially for the most external cell layers. Calcium concentration appears to play a major role in apoplastic pH under these restrictive conditions, supporting a role for the cell wall in pH homeostasis of the unstirred surface layer of plasma membrane in mature roots.
Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes
DOI:10.1111/nph.13363
URL
PMID:25756288
[本文引用: 1]

Abstract Fine roots acquire essential soil resources and mediate biogeochemical cycling in terrestrial ecosystems. Estimates of carbon and nutrient allocation to build and maintain these structures remain uncertain because of the challenges of consistently measuring and interpreting fine-root systems. Traditionally, fine roots have been defined as all roots 0909¤ 2 mm in diameter, yet it is now recognized that this approach fails to capture the diversity of form and function observed among fine-root orders. Here, we demonstrate how order-based and functional classification frameworks improve our understanding of dynamic root processes in ecosystems dominated by perennial plants. In these frameworks, fine roots are either separated into individual root orders or functionally defined into a shorter-lived absorptive pool and a longer-lived transport fine-root pool. Using these frameworks, we estimate that fine-root production and turnover represent 22% of terrestrial net primary production globally - a c. 30% reduction from previous estimates assuming a single fine-root pool. Future work developing tools to rapidly differentiate functional fine-root classes, explicit incorporation of mycorrhizal fungi into fine-root studies, and wider adoption of a two-pool approach to model fine roots provide opportunities to better understand below-ground processes in the terrestrial biosphere. 0008 2015 The Authors. New Phytologist 0008 2015 New Phytologist Trust.
The rhizosphere and hyphosphere differ in their impacts on carbon and nitrogen cycling in forests exposed to elevated CO2
DOI:10.1111/nph.13122
URL
PMID:25348688
[本文引用: 1]

Summary While multiple experiments have demonstrated that trees exposed to elevated CO2 can stimulate microbes to release nutrients from soil organic matter, the importance of root- versus mycorrhizal-induced changes in soil processes are presently unknown. We analyzed the contribution of roots and mycorrhizal activities to carbon (C) and nitrogen (N) turnover in a loblolly pine ( Pinus taeda ) forest exposed to elevated CO2 by measuring extracellular enzyme activities at soil microsites accessed via root windows. Specifically, we quantified enzyme activity from soil adjacent to root tips (rhizosphere), soil adjacent to hyphal tips (hyphosphere), and bulk soil. During the peak growing season, CO2 enrichment induced a greater increase of N-releasing enzymes in the rhizosphere (215% increase) than in the hyphosphere (36% increase), but a greater increase of recalcitrant C-degrading enzymes in the hyphosphere (118%) than in the rhizosphere (19%). Nitrogen fertilization influenced the magnitude of CO2 effects on enzyme activities in the rhizosphere only. At the ecosystem scale, the rhizosphere accounted for c . 50% and 40% of the total activity of N- and C-releasing enzymes, respectively. Collectively, our results suggest that root exudates may contribute more to accelerated N cycling under elevated CO2 at this site, while mycorrhizal fungi may contribute more to soil C degradation.
Interactions among roots, mycorrhizas and free-living microbial communities differentially impact soil carbon processes
DOI:10.1111/1365-2745.12484
URL
[本文引用: 1]

Summary Plant roots, their associated microbial community and free-living soil microbes interact to regulate the movement of carbon from the soil to the atmosphere, one of the most important and least understood fluxes of terrestrial carbon. Our inadequate understanding of how plant–microbial interactions alter soil carbon decomposition may lead to poor model predictions of terrestrial carbon feedbacks to the atmosphere. Roots, mycorrhizal fungi and free-living soil microbes can alter soil carbon decomposition through exudation of carbon into soil. Exudates of simple carbon compounds can increase microbial activity because microbes are typically carbon limited. When both roots and mycorrhizal fungi are present in the soil, they may additively increase carbon decomposition. However, when mycorrhizas are isolated from roots, they may limit soil carbon decomposition by competing with free-living decomposers for resources. We manipulated the access of roots and mycorrhizal fungi to soil in02situ in a temperate mixed deciduous forest. We added 13C-labelled substrate to trace metabolized carbon in respiration and measured carbon-degrading microbial extracellular enzyme activity and soil carbon pools. We used our data in a mechanistic soil carbon decomposition model to simulate and compare the effects of root and mycorrhizal fungal presence on soil carbon dynamics over longer time periods. Contrary to what we predicted, root and mycorrhizal biomass did not interact to additively increase microbial activity and soil carbon degradation. The metabolism of 13C-labelled starch was highest when root biomass was high and mycorrhizal biomass was low. These results suggest that mycorrhizas may negatively interact with the free-living microbial community to influence soil carbon dynamics, a hypothesis supported by our enzyme results. Our steady-state model simulations suggested that root presence increased mineral-associated and particulate organic carbon pools, while mycorrhizal fungal presence had a greater influence on particulate than mineral-associated organic carbon pools. Synthesis . Our results suggest that the activity of enzymes involved in organic matter decomposition was contingent upon root–mycorrhizal–microbial interactions. Using our experimental data in a decomposition simulation model, we show that root–mycorrhizal–microbial interactions may have longer-term legacy effects on soil carbon sequestration. Overall, our study suggests that roots stimulate microbial activity in the short term, but contribute to soil carbon storage over longer periods of time.
Solar radiation strongly influences the quantity of forest tree root exudates
DOI:10.1007/s00468-018-1685-0
URL
[本文引用: 1]

Key message The quantity of root exudate carbon produced by Quercus crispula Blume was strongly influenced by the amount of solar radiation 102day before collection.
Strategies and methods for studying the rhizosphere—The plant science toolbox
DOI:10.1007/s11104-009-9953-9
URL
[本文引用: 1]

This review summarizes and discusses methodological approaches for studies on the impact of plant roots on the surrounding rhizosphere and for elucidation of the related mechanisms, covering a range from simple model experiments up to the field scale. A section on rhizosphere sampling describes tools and culture systems employed for analysis of root growth, root morphology, vitality testing and for monitoring of root activity with respect to nutrient uptake, water, ion and carbon flows in the rhizosphere. The second section on rhizosphere probing covers techniques to detect physicochemical changes in the rhizosphere as a consequence of root activity. This comprises compartment systems to obtain rhizosphere samples, visualisation techniques, reporter gene approaches and remote sensing technologies for monitoring the conditions in the rhizosphere. Approaches for the experimental manipulation of the rhizosphere by use of molecular and genetic methods as tools to study rhizosphere processes are discussed in a third section. Finally it is concluded that in spite of a wide array of methodological approaches developed in the recent past for studying processes and interactions in the rhizosphere mainly under simplified conditions in model experiments, there is still an obvious lack of methods to test the relevance of these findings under real field conditions or even on the scale of ecosystems. This also limits reliable data input and validation in current rhizosphere modelling approaches. Possible interactions between different environmental factors or plantmicrobial interactions (e.g. mycorrhizae) are frequently not considered in model experiments. Moreover, most of the available knowledge arises from investigations with a very limited number of plant species, mainly crops and studies considering also intraspecific genotypic differences or the variability within wild plant species are just emerging.
Sampling root exudates—Mission impossible?
DOI:10.1016/j.rhisph.2018.06.004
URL
[本文引用: 1]

Accurate information about the quantity, quality and spatiotemporal dynamics of metabolite release from plant roots is vital to understanding the functional significance of root exudates in biogeochemical processes occurring at the root-microbe-soil-interface. Significant progress in analytical techniques nowadays allows us to gain a much better picture of the rich diversity of compounds that are present in root exudates, but ultimately the choice of exudation sampling strategy will determine the ecological significance of obtained exudation results. Unfortunately, in the past, little consideration has been given to the experimental strategy used to sample root exudates. To date, our knowledge on root exudation is mainly based on plants grown and sampled in nutrient solution culture (hydroponics). Despite the operational benefit of hydroponic systems, the question remains as to how ecologically relevant exudation results obtained under these artificial conditions are compared to soil environments, particularly in the context of exudate driven rhizosphere processes. The quantitative and qualitative measurement of root exudation in soil, however, is fraught with problems due to: (i) continual removal of exudates from solution by the microbial community; (ii) loss of exudates from solution due to their sorption to the solid phase; and (iii) simultaneous release of compounds from soil organic matter breakdown. While a perfect method for sampling root exudates does not exist, soil based approaches, if appropriately applied and interpreted, may still provide more realistic insights into exudation dynamics in natural soil environments. This review aims to provide an overview of different root exudation sampling approaches and their advantages and limitations to support the selection of the most suitable experimental procedure for any specific research question. We address critical methodological aspects that need to be considered in the choice of experimental approach, like growth and sampling medium (soil, hydroponic), sterility, sampling location (whole root system, individual root segments) as well as plant age, daytime, re-uptake of metabolites affecting duration and timing of the sampling event and data presentation. In addition, we summarize the main analytical approaches to analyze root exudates, ranging from liquid sample analysis to isotope tracking and imaging techniques.
New handbook for standardised measurement of plant functional traits worldwide
DOI:10.1071/BT12225
URL
[本文引用: 1]

Plant functional traits are the features (morphological, physiological, phenological) that represent ecological strategies and determine how plants respond to environmental factors, affect other trophic levels and influence ecosystem properties. Variation in plant functional traits, and trait syndromes, has proven useful for tackling many important ecological questions at a range of scales, giving rise to a demand for standardised ways to measure ecologically meaningful plant traits. This line of research has been among the most fruitful avenues for understanding ecological and evolutionary patterns and processes. It also has the potential both to build a predictive set of local, regional and global relationships between plants and environment and to quantify a wide range of natural and human-driven processes, including changes in biodiversity, the impacts of species invasions, alterations in biogeochemical processes and vegetation-atmosphere interactions. The importance of these topics dictates the urgent need for more and better data, and increases the value of standardised protocols for quantifying trait variation of different species, in particular for traits with power to predict plant-and ecosystem-level processes, and for traits that can be measured relatively easily. Updated and expanded from the widely used previous version, this handbook retains the focus on clearly presented, widely applicable, step-by-step recipes, with a minimum of text on theory, and not only includes updated methods for the traits previously covered, but also introduces many new protocols for further traits. This new handbook has a better balance between whole-plant traits, leaf traits, root and stem traits and regenerative traits, and puts particular emphasis on traits important for predicting species' effects on key ecosystem properties. We hope this new handbook becomes a standard companion in local and global efforts to learn about the responses and impacts of different plant species with respect to environmental changes in the present, past and future.
A new approach for capturing soluble root exudates in forest soils
DOI:10.1111/j.1365-2435.2008.01495.x
URL
[本文引用: 1]

1. Soluble root exudates are notoriously difficult to collect in non-hydroponic systems because they are released in a narrow zone around roots and are rapidly assimilated by rhizosphere microbes. This has substantially limited our understanding of their rates of release and chemical composition in situ , and by extension, their ecological significance. 2. Here we describe the advantages and limitations of several commonly employed methods for measuring exudation with respect to their potential adaptability for field use in forest ecosystems. Then, we introduce a novel in situ method for measuring exudation in forest soils, and present preliminary results of the spatial and temporal dynamics of loblolly pine ( Pinus taeda L.) exudation at the Duke Forest FACTS-1 site, North Carolina, USA from April 2007 to July 2008. 3. Exudation rates varied by an order of magnitude, with the highest rates occurring in late-June 2007 and mid-July 2008, and the lowest rates occurring during late-August 2007. On an annual basis, we estimate pine roots in the upper 15 cm of soil release c. 9 g C m 0908082 year 0908081 via this flux, which represents 10900092% of net primary productivity at the site. 4. The magnitude of exudation rates did not differ across an N availability gradient but did track general patterns of below-ground C allocation at the site. Exudation was well-predicted by root morphological characteristics such as surface area and the number of root and mycorrhizal tips, further supporting a possible link between root C allocation and exudation. 5. Because all methods for estimating exudates introduce experimental artefacts, we suggest that only a limited amount of ecologically relevant information is probably gleaned from a single method. Thus, a complementary suite of experimental approaches will best enable researchers to understand consequences of changing patterns of exudation in the wake of global environmental change.
Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation
DOI:10.1111/j.1461-0248.2010.01570.x
URL
PMID:21176050
[本文引用: 2]

The degree to which rising atmospheric CO(2) will be offset by carbon (C) sequestration in forests depends in part on the capacity of trees and soil microbes to make physiological adjustments that can alleviate resource limitation. Here, we show for the first time that mature trees exposed to CO(2) enrichment increase the release of soluble C from roots to soil, and that such increases are coupled to the accelerated turnover of nitrogen (N) pools in the rhizosphere. Over the course of 3 years, we measured in situ rates of root exudation from 420 intact loblolly pine (Pinus taeda L.) roots. Trees fumigated with elevated CO(2) (200 p.p.m.v. over background) increased exudation rates ( g C cm(-1) root h(-1) ) by 55% during the primary growing season, leading to a 50% annual increase in dissolved organic inputs to fumigated forest soils. These increases in root-derived C were positively correlated with microbial release of extracellular enzymes involved in breakdown of organic N (R(2) = 0.66; P = 0.006) in the rhizosphere, indicating that exudation stimulated microbial activity and accelerated the rate of soil organic matter (SOM) turnover. In support of this conclusion, trees exposed to both elevated CO(2) and N fertilization did not increase exudation rates and had reduced enzyme activities in the rhizosphere. Collectively, our results provide field-based empirical support suggesting that sustained growth responses of forests to elevated CO(2) in low fertility soils are maintained by enhanced rates of microbial activity and N cycling fuelled by inputs of root-derived C. To the extent that increases in exudation also stimulate SOM decomposition, such changes may prevent soil C accumulation in forest ecosystems.
Thirsty tree roots exude more carbon
DOI:10.1093/treephys/tpx163
URL
PMID:29304257
[本文引用: 1]

The relationships of monoterpene emission with temperature, light, photosynthesis and stomatal conductance (gs) were studied in Quercus ilex L. trees throughout the four annual seasons under field conditions. The highest monoterpene emission was measured in spring and summer (midday average of 11 g [g DW]611 h611), whereas the lowest rates were found in autumn and winter (midday averages of... [Show full abstract]
Fine root architecture of nine North American trees
DOI:10.2307/3100029
URL
[本文引用: 1]

The fine roots of trees are concentrated on lateral branches that arise from perennial roots. They are important in the acquisition of water and essential nutrients, and at the ecosystem level, they make a significant contribution to biogeochemical cycling. Fine roots have often been studied according to arbitrary size classes, e.g., all roots less than 1 or 2 mm in diameter. Because of the size class approach, the position of an individual root on the complex lateral branching system has often been ignored, and relationships between the form of the branching root system and its function are poorly understood. The fine roots of both gymnosperms and angiosperms, which formed ectomycorrhizae (EM) and arbuscular mycorrhizae (AM) fungal associations, were sampled in 1998 and 1999. Study sites were chosen to encompass a wide variety of environments in four regions of North America. Intact lateral branches were collected from each species and 18561 individual roots were dissected by order, with distal roots numbered as first-order roots. This scheme is similar to the one commonly used to number the order of streams. Fine root diameter, length, specific root length (SRL; m/g), and nitrogen (N) concentration of nine North American tree species (Acer saccharum, Juniperus monosperma, Liriodendron tulipifera, Picea glauca, Pinus edulis, Pinus elliottii, Pinus resinosa, Populus balsamifera, and Quercus alba) were then compared and contrasted. Lateral roots <0.5 mm in diameter accounted for >75% of the total number and length of individual roots sampled in all species except Liriodendron tulipifera. Both SRL and N concentration decreased with increasing root order in all nine species, and this pattern appears to be universal in all temperate and boreal trees. Nitrogen concentrations ranged from 8.5 to 30.9 g/kg and were highest in the first-order "root tips." On a mass basis, first-order roots are expensive to maintain per unit time (high tissue N concentration). Tissue N appears to be a key factor in understanding the C cost of maintaining first- and second-order roots, which dominate the display of absorbing root length. There were many significant differences among species in diameter, length, SRL, and N concentration. For example, two different species can have similar SRL but very different tissue N concentrations. Our findings run contrary to the common idea that all roots of a given size class function the same way and that a common size class for fine roots works well for all species. Interestingly, fine root lateral branches are apparently deciduous, with a distinct lateral branch scar. The position of an individual root on the branching root system appears to be important in understanding the function of fine roots.
Quantifying root extracts and exudates of sedge and shrub in relation to root morphology
Root structure-function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy
DOI:10.1111/nph.13828
URL
PMID:26765311
[本文引用: 1]

Summary Although fine roots are important components of the global carbon cycle, there is limited understanding of root structure–function relationships among species. We determined whether root respiration rate and decomposability, two key processes driving carbon cycling but always studied separately, varied with root morphological and chemical traits, in a coordinated way that would demonstrate the existence of a root economics spectrum (RES). Twelve traits were measured on fine roots (diameter ≤02202mm) of 74 species (31 graminoids and 43 herbaceous and dwarf shrub eudicots) collected in three biomes. The findings of this study support the existence of a RES representing an axis of trait variation in which root respiration was positively correlated to nitrogen concentration and specific root length and negatively correlated to the root dry matter content, lignin02:02nitrogen ratio and the remaining mass after decomposition. This pattern of traits was highly consistent within graminoids but less consistent within eudicots, as a result of an uncoupling between decomposability and morphology, and of heterogeneity of individual roots of eudicots within the fine-root pool. The positive relationship found between root respiration and decomposability is essential for a better understanding of vegetation–soil feedbacks and for improving terrestrial biosphere models predicting the consequences of plant community changes for carbon cycling.
Organic acids in root exudates and soil solution of Norway spruce and silver birch
DOI:10.1016/j.soilbio.2004.07.036
URL
[本文引用: 1]

Here we report on low molecular weight organic acids in root exudates and soil solutions of Norway spruce and silver birch grown in rhizoboxes, sterile microcosms and the field. Monocarboxylic acids dominated in all three experimental systems. Formic, shikimic and oxalic acids were found in both spruce and birch microcosms. Fumaric acid was exclusive for spruce, while lactic, malonic, butyric and phthalic acids were only found in the birch microcosms. In spruce rhizoboxes oxalic, lactic, formic, butyric and pthalic acids were found. In addition, citric, adipic, propionic, succinic and acetic acids were observed in the rhizosphere of birch. Behind root windows in the field, only oxalic and lactic acids were found in the rhizosphere of spruce fine roots, whereas also formic and phthalic were observed close to birch fine roots, all at low concentrations. The rhizosphere of mycorrhizal short roots of birch contained butyric acid along with the acids observed for birch fine roots. Our results emphasise that characteristics of both the trees e.g. species, developmental stage, root density, mycorrhizal status, and the experimental system, i.e. growth conditions are important for the composition and the amount of organic acids. We conclude that the rhizosphere of birch contains more organic acids at higher concentrations than spruce.
Root penetration in deep soil layers stimulates mineralization of millennia old organic carbon
DOI:10.1016/j.soilbio.2018.06.010
URL
[本文引用: 1]

Climate and land-use changes modify plant rooting depth, signifying that organic matter with long residence times in deep soil layers can be exposed to rhizospheres and associated microbial activities. The presence of roots in soils stimulates mineralization of native soil C, via a process termed the rhizosphere priming effect (RPE), which may in consequence lead to loss of soil C. By growing a deep rooting grass, Festuca arundinacea, on soil columns and under continuous dual labelling (13C- & 14C-CO2), we show that root penetration up to 8062cm into a soil profile stimulated mineralization of 6515,000 year-old soil C. The RPE, after normalization with root biomass, was similar along the soil profile indicating that deep C is as vulnerable to priming as surface C. The RPE was strongly correlated with respiration of plant-derived C, and a PLFA marker representative of saprophytic fungi (18:2046c) across all soil layers. Moreover, experimental disruption of soil structure further stimulated soil C mineralization. These findings suggest that the slow soil C mineralization in deep layers results from an impoverishment of energy-rich plant C for microorganisms (especially for saprophytic fungi), combined with a physical disconnection between soil C and microorganisms. Based on our results, we anticipate higher mineralization rates of deep millennia-old SOM in response to deeper root penetration which could be induced by changes in agricultural practices and climate.
Reinforcing loose foundation stones in trait-based plant ecology
DOI:10.1007/s00442-016-3549-x URL [本文引用: 1]
Unravelling rhizosphere-microbial interactions: Opportunities and limitations
DOI:10.1016/j.tim.2004.06.008
URL
PMID:15276615
[本文引用: 1]

The rhizosphere is a biologically active zone of the soil around plant roots that contains soil-borne microbes including bacteria and fungi. Plant–microbe interactions in the rhizosphere can be beneficial to the plant, the microbes or to neither of them. One of the major difficulties that plant biologists and microbiologists face when studying these interactions is that many groups of microbes that inhabit this zone are not cultivable in the laboratory. Recent developments in molecular biology methods are shedding some light on rhizospheric microbial diversity. This review discusses recent findings and future challenges in the study of plant–microbe interactions in the rhizosphere.
Profiling of secondary metabolites in root exudates of Arabidopsis thaliana
Mechanisms of rhizosphere priming effects and their ecological significance
DOI:10.3724/SP.J.1258.2014.00007
URL
[本文引用: 1]

土壤激发效应是指由各种有机物质添加等处理所引起的土壤有机质周转强烈的短期改变.根际是激发效应最主要也是最重要的发生部位.根际激发效应能够反映生态系统土壤碳氮周转的速度,并影响植物、土壤微生物等对养分的获取和竞争,维持生态系统各组分间的养分平衡.虽然对根际激发效应的产生机制已取得一定程度的认知,但是对根际激发效应在土壤碳氮转化过程中的作用机理及其生态重要性依然缺乏足够的理解.该文在论述激发效应的研究历史和主要发生部位的基础上对最新研究进展进行了综合分析,提出了一个具体的根际激发效应的发生机制,深入剖析了影响根际激发效应的生物与非生物因素,并阐释了根际激发效应的生态重要性,对未来根际激发效应的研究方向进行了展望.
根际激发效应的发生机制及其生态重要性
DOI:10.3724/SP.J.1258.2014.00007
URL
[本文引用: 1]

土壤激发效应是指由各种有机物质添加等处理所引起的土壤有机质周转强烈的短期改变.根际是激发效应最主要也是最重要的发生部位.根际激发效应能够反映生态系统土壤碳氮周转的速度,并影响植物、土壤微生物等对养分的获取和竞争,维持生态系统各组分间的养分平衡.虽然对根际激发效应的产生机制已取得一定程度的认知,但是对根际激发效应在土壤碳氮转化过程中的作用机理及其生态重要性依然缺乏足够的理解.该文在论述激发效应的研究历史和主要发生部位的基础上对最新研究进展进行了综合分析,提出了一个具体的根际激发效应的发生机制,深入剖析了影响根际激发效应的生物与非生物因素,并阐释了根际激发效应的生态重要性,对未来根际激发效应的研究方向进行了展望.
Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency
DOI:10.1111/nph.14057
URL
PMID:27292630
[本文引用: 3]

Summary Microbial nitrification in soils is a major contributor to nitrogen (N) loss in agricultural systems. Some plants can secrete organic substances that act as biological nitrification inhibitors (BNIs), and a small number of BNIs have been identified and characterized. However, virtually no research has focused on the important food crop, rice ( Oryza sativa ). Here, 19 rice varieties were explored for BNI potential on the key nitrifying bacterium Nitrosomonas europaea . Exudates from both indica and japonica genotypes were found to possess strong BNI potential. Older seedlings had higher BNI abilities than younger ones; Zhongjiu25 (ZJ25) and Wuyunjing7 (WYJ7) were the most effective genotypes among indica and japonica varieties, respectively. A new nitrification inhibitor, 1,9-decanediol, was identified, shown to block the ammonia monooxygenase (AMO) pathway of ammonia oxidation and to possess an 80% effective dose (ED80) of 9002ng02μl611. Plant N-use efficiency (NUE) was determined using a 15N-labeling method. Correlation analyses indicated that both BNI abilities and 1,9-decanediol amounts of root exudates were positively correlated with plant ammonium-use efficiency and ammonium preference. These findings provide important new insights into the plant–bacterial interactions involved in the soil N cycle, and improve our understanding of the BNI capacity of rice in the context of02NUE.
Physico-chemical protection, rather than biochemical composition, governs the responses of soil organic carbon decomposition to nitrogen addition in a temperate agroecosystem
DOI:10.1016/j.scitotenv.2017.04.143
URL
PMID:28445825
[本文引用: 1]

Abstract The heterogeneous responses of soil organic carbon (SOC) decomposition in different soil fractions to nitrogen (N) addition remain elusive. In this study, turnover rates of SOC in different aggregate fractions were quantified based on changes in 13 C following the conversion of C 3 to C 4 vegetation in a temperate agroecosystem. The turnover of both total organic matter and specific organic compound classes within each aggregate fraction was inhibited by N addition. Moreover, the intensity of inhibition increases with decreasing aggregate size and increasing N addition level, but does not vary among chemical compound classes within each aggregate fraction. Overall, the response of SOC decomposition to N addition is dependent on the physico-chemical protection of SOC by aggregates and minerals, rather than the biochemical composition of organic substrates. The results of this study could help to understand the fate of SOC in the context of increasing N deposition. Copyright 2017 Elsevier B.V. All rights reserved.
Ecosystem responses to elevated CO2 governed by plant-soil interactions and the cost of nitrogen acquisition
DOI:10.1111/nph.14872
URL
PMID:29105765
[本文引用: 1]

Abstract Contents Summary I. 'Introduction' II. 'The return on investment approach' III. 'CO 2 response spectrum' IV. 'Discussion' 'Acknowledgements' References Summary Land ecosystems sequester on average about a quarter of anthropogenic CO 2 emissions. It has been proposed that nitrogen (N) availability will exert an increasingly limiting effect on plants ability to store additional carbon (C) under rising CO 2 , but these mechanisms are not well understood. Here, we review findings from elevated CO 2 experiments using a plant economics framework, highlighting how ecosystem responses to elevated CO 2 may depend on the costs and benefits of plant interactions with mycorrhizal fungi and symbiotic N-fixing microbes. We found that N-acquisition efficiency is positively correlated with leaf-level photosynthetic capacity and plant growth, and negatively with soil C storage. Plants that associate with ectomycorrhizal fungi and N-fixers may acquire N at a lower cost than plants associated with arbuscular mycorrhizal fungi. However, the additional growth in ectomycorrhizal plants is partly offset by decreases in soil C pools via priming. Collectively, our results indicate that predictive models aimed at quantifying C cycle feedbacks to global change may be improved by treating N as a resource that can be acquired by plants in exchange for energy, with different costs depending on plant interactions with microbial symbionts.
Root exudation patterns in a beech forest: Dependence on soil depth, root morphology, and environment
DOI:10.1016/j.soilbio.2017.01.006
URL
[本文引用: 3]

61Specific root exudation decreased in the subsoil to less than a fifth.61Root morphology changed from fibrous-type roots in the topsoil to pioneer-type roots in the subsoil.61Root exudation rate was positively related to EOC and ETN in the topsoil.61Exudation was particularly low in subsoil poor in SOM where positive priming effects are unlikely.
Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants
In: Pinto R, Varanini Z, Nannipieri P eds.
Metabolomics in the rhizosphere: Tapping into belowground chemical communication
DOI:10.1016/j.tplants.2016.01.008
URL
PMID:26832948
[本文引用: 3]

Metabolomics is becoming accepted as a tool for the (untargeted) analysis of metabolites in root exudates. The importance of the rhizosphere microbiome for the functioning of plants is becoming increasingly clear. Statistical analysis and genetic mapping are used to establish relations between metabolites and (other) traits. The increased understanding of metabolic engineering in plants will allow the critical assessment of the role of individual compounds in plant hizosphere communication. Novel chemical-analytical platforms, such as laser-assisted electrospray ionisation (LAESI), will allow for spatial metabolomics on the scale of roots and interacting organisms.
Growth and carbon sequestration by ectomycorrhizal fungi in intensively fertilized Norway spruce forests
DOI:10.1016/j.foreco.2011.05.035
URL
[本文引用: 2]

A substantial portion of the carbon (C) fixed by the trees is allocated belowground to ectomycorrhizal (EM) symbionts, but this fraction usually declines after fertilization. The aim of the present study was to estimate the effect of optimal fertilization (including all the necessary nutrients) on the growth of EM fungi in young Norway spruce forests over a three year period. In addition, the amount of carbon sequestered by EM mycelia was estimated using a method based on the difference in δ 13C between C 3 and C 4 plants. Sand-filled ingrowth mesh bags were used to estimate EM growth, and similar bags amended with compost made from maize leaves (a C 4 plant) were used to estimate C sequestration. Fertilizers had been applied either every year or every second year since 2002 and the estimates of EM growth started in 2007. The application of fertilizer reduced EM growth to between 0% and 40% of the growth in the control plots at one site (Ebbeg01rde), while no significant effect was found at the other three sites studied. The effect of the fertilizer was similar in sand-filled and maize-compost-amended mesh bags, but the total production of EM fungi was 3–4 times higher in maize-compost-amended mesh bags. The fertilizer tended to reduce EM growth more when applied every year than when applied every second year. The amount of C sequestered in maize-compost-amended mesh bags collected from unfertilized treatments was estimated to be between 0.2 and 0.7 mg C g sand 611 at Ebbeg01rde and between 0.2 and 0.5 mg C g sand 611 at Gr01ngshammar. This corresponds to between 300 and 1100 kg C per ha, assuming a similar production in the soil as in the mesh bags. Fertilization at the Ebbeg01rde site reduced carbon sequestration, which confirmed the results based on estimates of fungal growth (ergosterol levels). A correlation was found between fungal biomass and δ 13C in mesh bags amended with maize compost. Based on this, it was estimated that a fungal production of 1 μg ergosterol corresponded to 0.33 mg of sequestered carbon. In conclusion, the effect of the fertilizer on EM growth seemed to be dependent on the effect of the fertilizer on tree growth. Thus, at Ebbeg01rde, were tree growth was less stimulated by the fertilizer, EM growth was reduced upon fertilization. At other sites, where tree growth was more stimulated, the fertilizer did not influence EM growth. The large amounts of carbon sequestered during the experiment may be a result of fungal residues remaining in the soil after the death of the hyphae.
Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils—A review
DOI:10.1016/j.soilbio.2012.08.027
URL
[本文引用: 2]

Mycorrhizal fungi constitute a considerable sink for carbon in most ecosystems. This carbon is used for building extensive mycelial networks in the soil as well as for metabolic activity related to nutrient uptake. A number of methods have been developed recently to quantify production, standing biomass and turnover of extramatrical mycorrhizal mycelia (EMM) in the field. These methods include minirhizotrons, in-growth mesh bags and cores, and indirect measurements of EMM based on classification of ectomycorrhizal fungi into exploration types. Here we review the state of the art of this methodology and discuss how it can be developed and applied most effectively in the field, Furthermore, we also discuss different ways to quantify fungal biomass based on biomarkers such as chitin, ergosterol and PLFAs, as well as molecular methods, such as qPCR. The evidence thus far indicates that mycorrhizal fungi are key components of microbial biomass in many ecosystems. We highlight the need to extend the application of current methods to focus on a greater range of habitats and mycorrhizal types enabling incorporation of mycorrhizal fungal biomass and turnover into biogeochemical cycling models. (C) 2012 Elsevier Ltd. All rights reserved.
Simultaneous efflux and uptake of metabolites by roots of wheat
DOI:10.1007/s11104-016-2892-3
URL
[本文引用: 4]

Abstract Background & aims Some metabolites (e.g. amino acids) present in root exudates can be taken up by roots, but we do not know if this ability extends to the broader suite of metabolites found in exudates. The aim of this study was to examine the ability of wheat (Triticum aestivum L.) to efflux and take up a broad suite of small metabolites. Methods Four-week-old plants were placed into an uptake solution that contained a broad suite of 13C-labelled metabolites. Uptake was estimated from the decrease in concentration of the 13C isotopologue in the solution; and from the appearance of 13C isotopologues within roots. Efflux was estimated from appearance of 12C isotopologues in solution. Results When wheat plants were placed in 13C-metabolite solutions the concentration of U-13C isotopologues of 37 metabolites decreased as would be expected if plants were taking up the U-13C metabolites. After 4 h immersion in 13C metabolite solution, roots contained detectable amounts of U-13C isotopologues of 55 metabolites. U-13C isotopologues of organic acids were not detected within roots. Conclusions These findings indicate that wheat can take up a broad suite of N-containing metabolites and some sugars, but there was no evidence for uptake of organic acids.
Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates
DOI:10.3724/SP.J.1258.2014.00027
URL
[本文引用: 2]

根系分泌物是植物与土壤进行物质交换和信息传递的重要载体物质,是植物响应外界胁迫的重要途径,是构成植物不同根际微生态特征的关键因素,也是根际对话的主要调控者。根系分泌物对于生物地球化学循环、根际生态过程调控、植物生长发育等均具有重要功能,尤其是在调控根际微生态系统结构与功能方面发挥着重要作用,调节着植物-植物、植物-微生物、微生物-微生物间复杂的互作过程。植物化感作用、作物间套作、生物修复、生物入侵等都是现代农业生态学的研究热点,它们都涉及十分复杂的根际生物学过程。越来越多的研究表明,不论是同种植物还是不同种植物之间相互作用的正效应或是负效应,都是由根系分泌物介导下的植物与特异微生物共同作用的结果。近年来,随着现代生物技术的不断完善,有关土壤这一"黑箱"的研究方法与技术取得了长足的进步,尤其是各种宏组学技术(meta-omics technology),如环境宏基因组学、宏转录组学、宏蛋白组学、宏代谢组学等的问世,极大地推进了人们对土壤生物世界的认知,尤其是对植物地下部生物多样性和功能多样性的深层次剖析,根际生物学特性的研究成果被广泛运用于指导生产实践。深入系统地研究根系分泌物介导下的植物-土壤-微生物的相互作用方式与机理,对揭示土壤微生态系统功能、定向调控植物根际生物学过程、促进农业生产可持续发展等具有重要的指导意义。该文综述了根系分泌物的概念、组成及功能,论述了根系分泌物介导下植物与细菌、真菌、土壤动物群之间的密切关系,总结了探索根际生物学特性的各种研究技术及其优缺点,并对该领域未来的研究方向进行了展望。
根系分泌物介导下植物-土壤-微生物互作关系研究进展与展望
DOI:10.3724/SP.J.1258.2014.00027
URL
[本文引用: 2]

根系分泌物是植物与土壤进行物质交换和信息传递的重要载体物质,是植物响应外界胁迫的重要途径,是构成植物不同根际微生态特征的关键因素,也是根际对话的主要调控者。根系分泌物对于生物地球化学循环、根际生态过程调控、植物生长发育等均具有重要功能,尤其是在调控根际微生态系统结构与功能方面发挥着重要作用,调节着植物-植物、植物-微生物、微生物-微生物间复杂的互作过程。植物化感作用、作物间套作、生物修复、生物入侵等都是现代农业生态学的研究热点,它们都涉及十分复杂的根际生物学过程。越来越多的研究表明,不论是同种植物还是不同种植物之间相互作用的正效应或是负效应,都是由根系分泌物介导下的植物与特异微生物共同作用的结果。近年来,随着现代生物技术的不断完善,有关土壤这一"黑箱"的研究方法与技术取得了长足的进步,尤其是各种宏组学技术(meta-omics technology),如环境宏基因组学、宏转录组学、宏蛋白组学、宏代谢组学等的问世,极大地推进了人们对土壤生物世界的认知,尤其是对植物地下部生物多样性和功能多样性的深层次剖析,根际生物学特性的研究成果被广泛运用于指导生产实践。深入系统地研究根系分泌物介导下的植物-土壤-微生物的相互作用方式与机理,对揭示土壤微生态系统功能、定向调控植物根际生物学过程、促进农业生产可持续发展等具有重要的指导意义。该文综述了根系分泌物的概念、组成及功能,论述了根系分泌物介导下植物与细菌、真菌、土壤动物群之间的密切关系,总结了探索根际生物学特性的各种研究技术及其优缺点,并对该领域未来的研究方向进行了展望。
Priming and substrate quality interactions in soil organic matter models
DOI:10.5194/bg-10-2089-2013 URL [本文引用: 2]
Use of electrospray ionization ion-trap tandem mass spectrometry and principal component analysis to directly distinguish monosaccharides
DOI:10.1002/rcm.6219
URL
PMID:22555919
[本文引用: 1]

Carbohydrates are good source of drugs and play important roles in metabolism processes and cellular interactions in organisms. Distinguishing monosaccharide isomers in saccharide derivates is an...
Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition
DOI:10.1111/nph.15074
URL
PMID:29512165
[本文引用: 1]

Understanding soil organic matter (SOM) decomposition and its interaction with rhizosphere processes is a crucial topic in soil biology and ecology. Using a natural 13C tracer method to separately measure SOM-derived CO2 from root-derived CO2, this study aims to connect the level of rhizosphere-dependent SOM decomposition with the C and N balance of the whole plant–soil system, and to... [Show full abstract]
Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming
DOI:10.1111/gcb.12161
URL
PMID:23504744
[本文引用: 2]

Despite the perceived importance of exudation to forest ecosystem function, few studies have attempted to examine the effects of elevated temperature and nutrition availability on the rates of root exudation and associated microbial processes. In this study, we performed an experiment in which in situ exudates were collected from Picea asperata seedlings that were transplanted in disturbed soils exposed to two levels of temperature (ambient temperature and infrared heater warming) and two nitrogen levels (unfertilized and 25 g N m612 a611). Here, we show that the trees exposed to an elevated temperature increased their exudation rates I (μg C g611 root biomass h611), II (μg C cm611 root length h611) and III (μg C cm612 root area h611) in the unfertilized plots. The altered morphological and physiological traits of the roots exposed to experimental warming could be responsible for this variation in root exudation. Moreover, these increases in root-derived C were positively correlated with the microbial release of extracellular enzymes involved in the breakdown of organic N (R2 = 0.790; P = 0.038), which was coupled with stimulated microbial activity and accelerated N transformations in the unfertilized soils. In contrast, the trees exposed to both experimental warming and N fertilization did not show increased exudation rates or soil enzyme activity, indicating that the stimulatory effects of experimental warming on root exudation depend on soil fertility. Collectively, our results provide preliminary evidence that an increase in the release of root exudates into the soil may be an important physiological adjustment by which the sustained growth responses of plants to experimental warming may be maintained via enhanced soil microbial activity and soil N transformation. Accordingly, the underlying mechanisms by which plant root-microbe interactions influence soil organic matter decomposition and N cycling should be incorporated into climate-carbon cycle models to determine reliable estimates of long-term C storage in forests.
Resource stoichiometry mediates soil C loss and nutrient transformations in forest soils
DOI:10.1016/j.apsoil.2016.09.001
URL
[本文引用: 1]

Root exudation is increasingly being recognized as an important driver of ecosystem processes; however, few studies have examined the degree to which variations in exudate stoichiometry and soil resources affect microbial controls of nutrient availability and decomposition. We added root exudate mimics of varying chemical quality to soils collected from two adjacent forest stands (one a 70 year-old spruce plantation, the other a 200 year-old spruce-fir forest) that differ strongly in N availability. The exudate treatments were added for 50 consecutive days, and included water (control), C alone, N alone, and three combinations of C and N that varied stoichiometrically (i.e., C:N ratio of 10, 50 and 100). Exudate additions containing little or no N promoted the greatest losses of soil C in two soils, with the greatest losses occurring in the moderately labile (i.e., acid-extractable) fraction of the low N plantation soils. However, despite the uniformity of priming effects between sites ( 7% loss of soil C for both), there was little congruence in exudate-induced effects on microbial biomass and activity. In the plantation soils, exudates generally increased microbial biomass (especially fungi), accelerated N cycling and increased lignin-degrading enzyme activities relative to controls. In contrast, exudate additions to spruce-fir soils mostly decreased microbial biomass, decelerated N cycling, and had variable impacts on lignin-degrading enzyme activities (decreased phenol oxidase but increased peroxidase). Collectively, this study suggests that while root exudates with low C and N have the potential to accelerate soil C losses by stimulating microbes to mine N from soil organic matter, the consequences of exudate inputs on nutrient fluxes are less predictable, and may hinge on the recalcitrance of (soil organic matter) SOM, N availability and microbial communities.
Root-induced changes in nutrient cycling in forests depend on exudation rates
DOI:10.1016/j.soilbio.2014.07.022
URL
[本文引用: 2]

61Mycorrhizal type influences annual exudation rates in hardwood forests.61The magnitude of rhizosphere effects is positively correlated to exudation rates.61Modest fluxes of carbon can disproportionately affect ecosystem nitrogen cycling.
Impacts of oxalic acid and glucose additions on N transformation in microcosms via artificial roots
DOI:10.1016/j.soilbio.2018.03.002
URL
[本文引用: 3]

Root exudates can accelerate nitrogen (N) cycling by stimulating the decomposition of soil organic matter (SOM); however, it remains unclear how inputs of individual exudate components affect the biotic and abiotic processes that drive the transformation and release of inorganic N. In a well-controlled rhizosphere system, we added two exudate chemicals (i.e., glucose and oxalic acid) through artificial roots to soils collected from two forests (an 70-year-old spruce plantation and an 200-year-old spruce-fir forest) over a period of 50 days. The results showed that oxalic acid significantly accelerated both N mineralization and availability, which are mechanisms involved in abiotic process that disrupt previously protected mineral-organic associations (e.g., metal-organic complexes (MOCs) and short-range order phases (SROs)) and biotic process that accelerate microbial processes. Glucose also enhanced N decomposition, but this enhancement was significantly smaller than that obtained with oxalic acid, presumably because glucose addition increased the formation of mineral-organic associations of MOCs and SROs, thereby limiting microbial access. In addition, the preferential utilization of glucose by microbes can also lead to lower N decomposition. Our results also showed that the component-induced changes in N mineralization were lower in the established spruce plantation (e.g., from 45% to 89%) than in the spruce-fir forest (e.g., from 105% to 150%), potentially due to the decresed biotic processes observed after exudate additions in the spruce plantation compared with the spruce-fir forest. The decreased changes in N mineralization in the spruce plantation were also related to component-induced abiotic processes. These observations suggested that oxalic acid and glucose differentially impact rhizosphere N transformation in forest soils, but the impacts are mediated by soil type.
A review of plant root exudates
DOI:10.3969/j.issn.1002-2724.2005.05.041
URL
[本文引用: 1]

简要介绍了根系分泌物的种类、数量;综合论述了根系分泌物的分泌 特性、生理及分子生物学基础和根系特定分泌物对土壤养分有效性的影响;讨论了根系分泌物的影响因素和根系分泌的部位及根系分泌物在土壤中扩散的范围.并阐 明了根系分泌物与根际养分的活化及吸收、异株克生的关系,以及与土壤固氮微生物及连作障碍的关系.
植物根系分泌物研究综述
DOI:10.3969/j.issn.1002-2724.2005.05.041
URL
[本文引用: 1]

简要介绍了根系分泌物的种类、数量;综合论述了根系分泌物的分泌 特性、生理及分子生物学基础和根系特定分泌物对土壤养分有效性的影响;讨论了根系分泌物的影响因素和根系分泌的部位及根系分泌物在土壤中扩散的范围.并阐 明了根系分泌物与根际养分的活化及吸收、异株克生的关系,以及与土壤固氮微生物及连作障碍的关系.
Preliminary development of the theoretical concept on rhizosphere micro-ecosystem
DOI:10.3969/j.issn.1008-0864.1999.04.004
URL
[本文引用: 1]

本文简述了根际研究的历史进程及根际微生态学的发展概况,讨论了根际微生态系统的基本概念和理论模型,初步构建了其理论框架,明确了其结构、边界、特征、研究范畴及内容,提出了令后个体、群体水平根际微生态系统理论研究的思路和重点.
根际微生态系统理论框架的初步构建
DOI:10.3969/j.issn.1008-0864.1999.04.004
URL
[本文引用: 1]

本文简述了根际研究的历史进程及根际微生态学的发展概况,讨论了根际微生态系统的基本概念和理论模型,初步构建了其理论框架,明确了其结构、边界、特征、研究范畴及内容,提出了令后个体、群体水平根际微生态系统理论研究的思路和重点.
a). Mycelia-derived C contributes more to nitrogen cycling than root-derived C in ectomycorrhizal alpine forests
DOI:10.1111/1365-2435.13236 URL [本文引用: 1]
Mycelium- and root-derived C inputs differ in their impacts on soil organic C pools and decomposition in forests
DOI:10.1016/j.soilbio.2018.05.015
URL
[本文引用: 1]

While multiple lines of evidence suggests that root carbon (C) can significantly impact the soil organic C (SOC) pool and subsequent C cycles via rhizosphere priming effects, the relative magnitude of the effects that root- and fungal-derived C inputs have in driving the priming of SOC decomposition are currently unknown. In this study, we used ingrowth cores and stable C isotope analyses to quantify root- and mycelium-derived C sequestered in soil labile and recalcitrant C pools and their relative contributions to the decomposition of native SOC in a spruce-fir-dominated coniferous forest on the eastern Tibetan Plateau, China. The results showed that new C sequestered in the soil labile C pool was primarily (77%) from mycelium-derived C, while 60% of the root-derived C sequestered in the soil was incorporated into the recalcitrant pool. Furthermore, although the total native SOC pool was not significantly influenced by new C derived from both roots and mycelia, mycelium-derived C induced a remarkably greater negative priming effect ( 12.0%) on the native labile C pool than did root-derived C ( 5.8%); in contrast, mycelium-derived C induced a greater positive priming effect (13.8%) than root-derived C (7.1%) on the native recalcitrant C pool. Collectively, our findings suggest that mycelium-derived C make a greater contribution to the newly sequestered C in the soil labile C pool than root-derived C, thereby inducing a remarkably greater positive priming effect on the decomposition of native soil recalcitrant C. Therefore, mycelium-derived C inputs may play a dominant role in soil C dynamics and long-term C storage, at least in alpine forest ecosystems where ectomycorrhizal mutualisms dominate.
Nodulated soybean enhances rhizosphere priming effects on soil organic matter decomposition more than non-nodulated soybean
DOI:10.1016/j.soilbio.2012.04.016
URL
[本文引用: 1]

The phenomenon that rhizosphere processes significantly control soil organic matter (SOM) decomposition, also termed rhizosphere priming effect (RPE), is now increasingly recognized as significant as the effects of soil temperature and moisture on SOM decomposition. However, the exact mechanisms responsible for RPE remain largely unknown. Particularly, some reports have suggested that the quality of rhizodeposits may play a significant role in causing different levels of RPE among various plant species. However, direct evidence for the “rhizodeposit quality hypothesis” has been lacking. Here we tested the hypothesis by investigating RPE on soil carbon (C) and nitrogen (N) mineralization of two soybean (Glycine max L. Merr.) isolines differing only in their ability to form nodules and to fix N2, and thus differing in tissue N concentration and rhizodeposit quality. We used a continuous 13C-labeling method for measuring RPE on soil organic C decomposition, and employed an N-budgeting method for quantifying RPE on soil net N mineralization. We found that the rhizodeposits from nodulated soybean produced a stronger RPE (53% vs. 26%) on soil organic C decomposition than the rhizodeposits from non-nodulated soybean at the maturity stage when nodulated soybean had significantly higher plant tissue N concentration but similar plant biomass, while both soybean isolines produced a similar RPE (33–34%) at the vegetative stage when there was no difference in plant tissue N concentration or plant biomass. The levels of RPE on soil net N mineralization were similar between the two isolines, ranging from 25% at the vegetative stage to 38–46% at the maturity stage. Moreover, RPE on soil organic C decomposition was not linearly proportional to RPE on soil net N mineralization. These results indicate that higher rhizodeposit quality is one of the most likely causes to the higher RPE of the nodulated soybean compared to the non-nodulated soybean. Further investigations of rhizodeposit quality and quantity between the two soybean isolines are warranted to further test this rhizodeposit quality hypothesis.
Rhizosphere priming effects on soil carbon and nitrogen mineralization
DOI:10.1016/j.soilbio.2014.04.033
URL
[本文引用: 2]

61Living roots increased soil C decomposition by 27–245%.61Living roots enhanced soil gross N mineralization by up to 62%.61Living roots led to higher microbial biomass and extracellular enzyme activity.61Results supported the microbial activation hypothesis for rhizosphere priming effect.61Rhizosphere priming was correlated with root biomass and rhizosphere respiration.