

Chin J Plan Ecolo ›› 2015, Vol. 39 ›› Issue (5): 501-507.DOI: 10.17521/cjpe.2015.0048
• Orginal Article • Previous Articles Next Articles
YUAN Min, BU Zhao-Jun*( ), LIU Chao, MA Jin-Ze, WANG Sheng-Zhong
), LIU Chao, MA Jin-Ze, WANG Sheng-Zhong
Received:2015-02-02
Accepted:2015-03-31
Online:2015-05-01
Published:2015-05-26
Contact:
Zhao-Jun BU
About author:# Co-first authors
YUAN Min,BU Zhao-Jun,LIU Chao,MA Jin-Ze,WANG Sheng-Zhong. Effects of water level and light intensity on capsule production dynamics of Sphagnum capillifolium[J]. Chin J Plan Ecolo, 2015, 39(5): 501-507.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2015.0048

Fig. 1 Morphological change in Sphagnum capillifolium capsules. The capsule highlighted by the arrow is an example under low water level and weak light conditions. A, On July 21, a newborn spherical capsule is yellow, wrapped by perichaetial leaves and no seta developed. B, On August 2, a seta gradually formed and elongated and the capsule was dark brown. C, On August 6, the seta further extended and the capsule became red brown in color and thick cylindrical in shape. D, On August 8, after operculum falling off, spores released and the capsule became thin cylindrical in shape.
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 繁殖株高增长 Height increment of reproductive shoots | 16.66 | < 0.001** | 0.21 | 0.651 | 7.26 | 0.008** | ||
| 营养株高增长 Height increment of vegetative shoots | 4.44 | 0.036* | 1.21 | 0.273 | 6.08 | 0.015* | ||
| 蒴柄长度 Seta length | 10.32 | 0.002** | 0.00 | 0.966 | 0.64 | 0.426 | ||
| 孢蒴直径 Capsule diameter | 0.04 | 0.838 | 14.73 | 0.000** | 13.77 | < 0.001** | ||
Table 1 Two-way ANOVA for effects of water level and light intensity on height increment and sporophyte morphology
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 繁殖株高增长 Height increment of reproductive shoots | 16.66 | < 0.001** | 0.21 | 0.651 | 7.26 | 0.008** | ||
| 营养株高增长 Height increment of vegetative shoots | 4.44 | 0.036* | 1.21 | 0.273 | 6.08 | 0.015* | ||
| 蒴柄长度 Seta length | 10.32 | 0.002** | 0.00 | 0.966 | 0.64 | 0.426 | ||
| 孢蒴直径 Capsule diameter | 0.04 | 0.838 | 14.73 | 0.000** | 13.77 | < 0.001** | ||
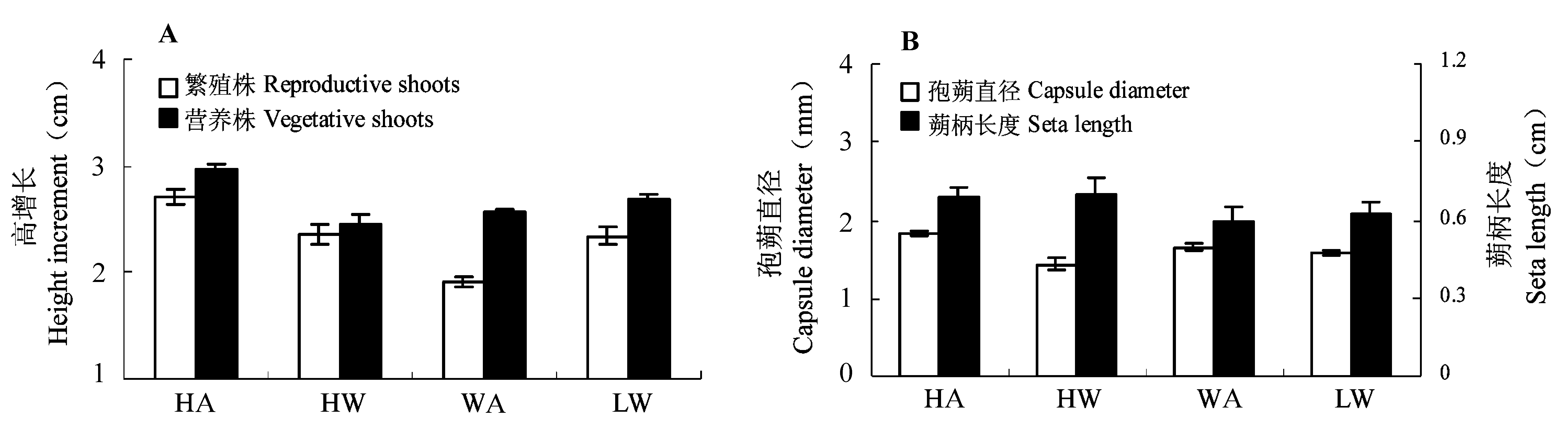
Fig. 2 Effect of water level and light intensity on shoot height increment (A) and sporophyte morphology (B) (mean ± SE). A, ambient light; H, high water level; L, low water level; W, weak light.
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 孢蒴增长率 Capsule growth rate | 0.74 | 0.403 | 0.62 | 0.443 | 0.23 | 0.636 | ||
| 孢蒴开裂率 Capsule cracking rate | 5.80 | 0.037* | 5.39 | 0.033* | 0.24 | 0.554 | ||
| 孢蒴遮蔽率 Rate of capsules being shaded | 11.82 | 0.003** | 0.07 | 0.792 | 2.67 | 0.122 | ||
Table 2 Repetitive measurement and analysis of variance (ANOVA) for effects of water level and light intensity on capsule production dynamics
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 孢蒴增长率 Capsule growth rate | 0.74 | 0.403 | 0.62 | 0.443 | 0.23 | 0.636 | ||
| 孢蒴开裂率 Capsule cracking rate | 5.80 | 0.037* | 5.39 | 0.033* | 0.24 | 0.554 | ||
| 孢蒴遮蔽率 Rate of capsules being shaded | 11.82 | 0.003** | 0.07 | 0.792 | 2.67 | 0.122 | ||
| 条件 Condition | 孢蒴形成→孢蒴成熟 Capsule formation→ Capsule maturation | 孢蒴成熟→蒴柄长成 Capsule maturation→ Seta maturation | 蒴柄长成→孢蒴开裂 Seta maturation→ Capsule dehiscing |
|---|---|---|---|
| 高水位 High water level | 11.0 ± 1.2 | 2.7 ± 1.2 | 2.3 ± 1.4 |
| 低水位 Low water level | 12.1 ± 2.4 | 3.8 ± 1.9 | 4.1 ± 2.4 |
| 一般光强 Ambient light | 10.4 ± 1.5 | 2.3 ± 1.4 | 2.5 ± 1.3 |
| 弱光强 Weak light | 12.7 ± 1.7 | 3.4 ± 1.9 | 3.9 ± 2.5 |
Table 3 Time needed for each stage of capsule production under different water level and light intensity (mean ± SD)
| 条件 Condition | 孢蒴形成→孢蒴成熟 Capsule formation→ Capsule maturation | 孢蒴成熟→蒴柄长成 Capsule maturation→ Seta maturation | 蒴柄长成→孢蒴开裂 Seta maturation→ Capsule dehiscing |
|---|---|---|---|
| 高水位 High water level | 11.0 ± 1.2 | 2.7 ± 1.2 | 2.3 ± 1.4 |
| 低水位 Low water level | 12.1 ± 2.4 | 3.8 ± 1.9 | 4.1 ± 2.4 |
| 一般光强 Ambient light | 10.4 ± 1.5 | 2.3 ± 1.4 | 2.5 ± 1.3 |
| 弱光强 Weak light | 12.7 ± 1.7 | 3.4 ± 1.9 | 3.9 ± 2.5 |
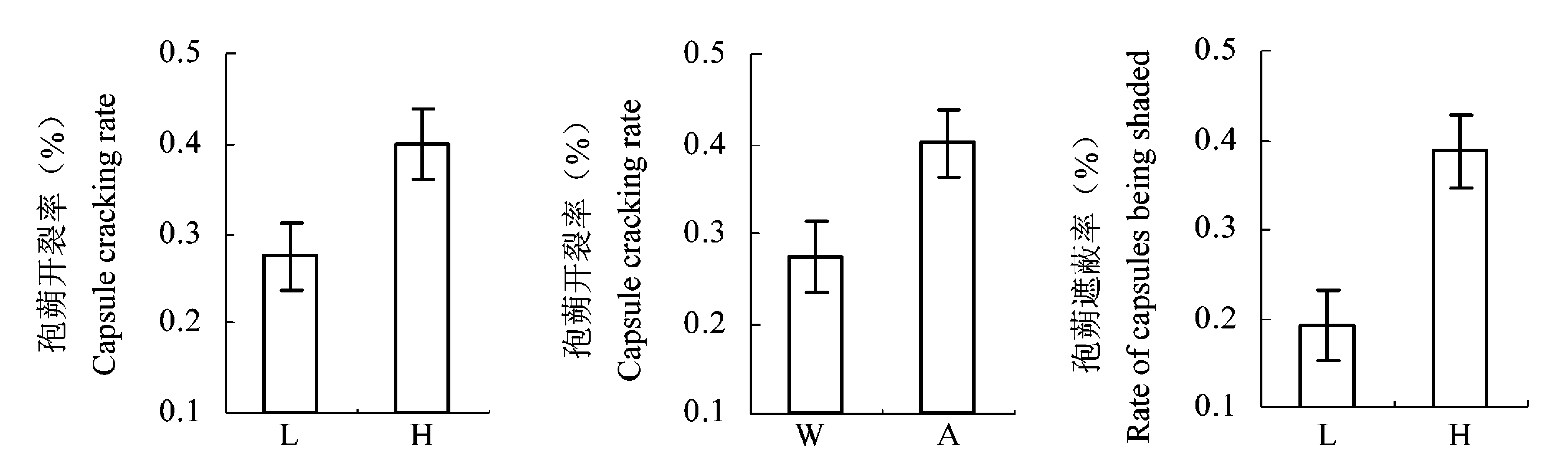
Fig. 3 Effect of water level and light intensity on capsule cracking rate and shaded rate (mean ± SE). A, ambient light; H, high water level; L, low water level; W, weak light.
| 1 | Bao WM, Cao JG (2001). Spore germination and sexual reproduction in Sphagnum.Bulletin of Biology, 36(1), 8-9(in Chinese). |
| [包文美, 曹建国 (2001). 泥炭藓及其孢子萌发和有性生殖. 生物学通报, 36(1), 8-9.] | |
| 2 | Bragazza L (2008). A climatic threshold triggers the die-off of peat mosses during an extreme heat wave.Global Change Biology, 14, 2688-2695. |
| 3 | Bragazza L, Parisod J, Buttler A, Bardgett RD (2013). Biogeochemical plant-soil microbe feedback in response to climate warming in peatlands.Nature Climate Change, 3, 273-277. |
| 4 | Bu ZJ, Rydin H, Chen X (2011). Direct and interaction- mediated effects of environmental changes on peatland bryophytes.Oecologia, 166, 555-563. |
| 5 | Bubier JL, Moore TR, Bledzki LA (2007). Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog.Global Change Biology, 13, 1168-1186. |
| 6 | Clymo RS (1998). Sphagnum, the peatland carbon economy, and climate change. In: Bates JW, Ashton NW, Duckett JG eds. Bryology for the Twenty-first Century. Maney Publishing and the British Bryological Society, Leeds, UK. 361-368. |
| 7 | Cronberg N (1993). Reproductive biology of Sphagnum.Lindbergia, 17, 69-82. |
| 8 | Crum H (1972). The geographic origins of the mosses of North America’s eastern deciduous forest.Journal of the Hattori Botanical Laboratory, 35, 269-298. |
| 9 | Ehrlén J, Bisang I, Hedenäs L (2000). Costs of sporophyte production in the moss, Dicranum polysetum.Plant Ecology, 149, 207-217. |
| 10 | Gao Q, Cao T, Fu X (2000). Types of spore dispersal of mosses in relation to evolution system.Acta Botanica Yunnanica, 22, 268-276(in Chinese with English abstract). |
| [高谦, 曹同, 付星 (2000). 藓类植物传孢类型及其系统演化关系. 云南植物研究, 22, 268-276. ] | |
| 11 | Glime JM (. Cited: Feb. 2015. |
| 12 | Gravobik SI (1986). Influence of some ecological factors on the spore productivity of Sphagnum mosses. Botanicheskii Zhurnal (in Russian), 71, 1652-1657. |
| 13 | Ingold CT (1965). Spore Liberation. Clarendon Press, Oxford. |
| 14 | Johansson V, Lönnell N, Sundberg S, Hylander K (2014). Release thresholds for moss spores: The importance of turbulence and sporophyte length.Journal of Ecology, 102, 721-729. |
| 15 | Jones EW (1986). Bryophytes in chawley brick pit, Oxford, 1948-1985.Journal of Bryology, 14, 347-358. |
| 16 | Longton RE (1997). Reproductive biology and life-history strategies.Advances of Bryology, 6, 65-101. |
| 17 | Moore TR, Roulet NT, Waddington JM (1998). Uncertainty in predicting the effect of climatic change on the carbon cycling of Candian peatlands.Climatic Change, 40, 229-245. |
| 18 | Nawaschin S (1897). Über die Sporenausschleuderung bei den Torfmoosen.Flora, 48, 151-159. |
| 19 | Rochefort L (2000). Sphagnum―A keystone genus in habitat restoration.The Bryologist, 103, 503-508. |
| 20 | Rudolph H, Kirchhoff M, Gliesmann S (1988). Sphagnum culture techniques. In: Glime JM ed. Methods in Bryology. Hattori Botanical Laboratory, Nichinan. 25-34. |
| 21 | Rydin H, Clymo RS (1989). Transport of carbon and phosphorus compounds about Sphagnum.Proceedings of the Royal Society of London Series B: Biological Sciences, 237, 63-84. |
| 22 | Söderström L, Herben T (1997). Dynamics of bryophyte metapopulations.Advances of Bryology, 6, 205-240. |
| 23 | Soro A, Sundberg S, Rydin H (1999). Species diversity, niche metrics and species associations in harvested and undisturbed bogs.Journal of Vegetation Science, 10, 549-560. |
| 24 | Stark LR, Mishler BD, McLetchie DN (2000). The cost of realized sexual reproduction: Assessing patterns of reproductive allocation and sporophyte abortion in a desert moss.American Journal of Botany, 87, 1599-1608. |
| 25 | Sundberg S (2000). The ecological significance of sexual reproduction in peat mosses (Sphagnum).Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology, 581, 1-37. |
| 26 | Sundberg S (2002). Sporophyte production and spore dispersal phenology in Sphagnum: The importance of summer moisture and patch characteristics.Canadian Journal of Botany, 80, 543-556. |
| 27 | Sundberg S (2005). Larger capsules enhance short-range spore dispersal in Sphagnum, but what happens further away?Oikos, 108, 115-124. |
| 28 | Sundberg S (2010). Size matters for violent discharge height and settling speed of Sphagnum spores: Important attributes for dispersal potential.Annals of Botany, 105, 291-300. |
| 29 | Sundberg S (2013). Spore rain in relation to regional sources and beyond.Ecography, 36, 364-373. |
| 30 | Sundberg S, Rydin H (1998). Spore number in Sphagnum and its dependence on spore and capsule size.Journal of Bryology, 20, 1-16. |
| 31 | Sundberg S, Rydin H (2000). Experimental evidence for a persistent spore bank in Sphagnum.New Phytologist, 148, 105-116. |
| 32 | Whitaker DL, Edwards J (2010). Sphagnum moss disperses spores with vortex rings.Science, 329, 406. |
| [1] | LI Xiao-Ling, ZHU Dao-Ming, YU Yu-Rong, WU Hao, MOU Li, HONG Liu, LIU Xue- Fei, BU Gui-Jun, XUE Dan, WU Lin. Effects of simulated nitrogen deposition on growth and decomposition of two bryophytes in ombrotrophic peatland, southwestern Hubei, China [J]. Chin J Plant Ecol, 2023, 47(5): 644-659. |
| [2] | LI Xue-Ying, ZHU Wen-Quan, LI Pei-Xian, XIE Zhi-Ying, ZHAO Cen-Liang. Predicting phenology shifts of herbaceous plants on the Qinghai-Xizang Plateau under climate warming with the space-for-time method [J]. Chin J Plant Ecol, 2020, 44(7): 742-751. |
| [3] | LIU Xue-Fei, WU Lin, WANG Han, HONG Liu, XIONG Li-Jun. Growth and decomposition characteristics of Sphagnum in a subalpine wetland, southwestern Hubei, China [J]. Chin J Plant Ecol, 2020, 44(3): 228-235. |
| [4] | FENG Lu, BU Zhao-Jun, WU Yu-Huan, LIU Sha-Sha, LIU Chao. Characteristic environmental factors in peatlands facilitate the formation of persistent Sphagnum spore banks [J]. Chin J Plant Ecol, 2019, 43(6): 512-520. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn