

Chin J Plant Ecol ›› 2017, Vol. 41 ›› Issue (4): 480-488.DOI: 10.17521/cjpe.2016.0210
• Orginal Article • Previous Articles Next Articles
Liang-Hua CHEN, Juan LAI, Xiang-Wei HU, Wan-Qin YANG, Jian ZHANG*( ), Xiao-Jun WANG, Ling-Jie TAN
), Xiao-Jun WANG, Ling-Jie TAN
Received:2016-06-27
Accepted:2016-12-25
Online:2017-04-10
Published:2017-05-19
Contact:
Jian ZHANG
Liang-Hua CHEN, Juan LAI, Xiang-Wei HU, Wan-Qin YANG, Jian ZHANG, Xiao-Jun WANG, Ling-Jie TAN. Effects of inoculation with arbuscular mycorrhizal fungi on photosynthetic physiology in females and males of Populus deltoides exposed to cadmium pollution[J]. Chin J Plant Ecol, 2017, 41(4): 480-488.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2016.0210
| 处理 Treatment | 丛枝菌根 AM | 性别 Sex | 净光合速率 Pn (µmol·m-2·s-1) | 气孔导度 Gs (mol·m-2·s-1) | 胞间CO2浓度 Ci (µmol·mol-1) | 蒸腾速率 Tr (mmol·m-2·s-1) |
|---|---|---|---|---|---|---|
| 对照 | - | 雄株 Male | 11.91 ± 0.26b | 0.37 ± 0.04bcd | 318.40 ± 10.05a | 4.37 ± 0.27ab |
| Control | - | 雌株 Female | 13.68 ± 0.79ab | 0.51 ± 0.02ab | 310.85 ± 7.71ab | 5.50 ± 0.27a |
| + | 雄株 Male | 12.34 ± 0.34b | 0.41 ± 0.03abc | 313.96 ± 7.72ab | 5.11 ± 0.20a | |
| + | 雌株 Female | 14.43 ± 0.42a | 0.52 ± 0.05a | 313.68 ± 10.97ab | 5.37 ± 0.44a | |
| Cd污染 | - | 雄株 Male | 9.70 ± 0.46c | 0.25 ± 0.02de | 270.87 ± 6.51bc | 3.48 ± 0.26bc |
| Cd pollution | - | 雌株 Female | 6.58 ± 0.27d | 0.13 ± 0.02e | 243.14 ± 9.73c | 2.10 ± 0.33d |
| + | 雄株 Male | 8.86 ± 0.35c | 0.27 ± 0.02d | 273.86 ± 9.00bc | 2.95 ± 0.26cd | |
| + | 雌株 Female | 9.59 ± 0.28c | 0.30 ± 0.01cd | 288.10 ± 4.52ab | 3.26 ± 0.11bcd | |
| Fs | ns | ns | ns | ns | ||
| Fcd | *** | *** | *** | *** | ||
| FAMF | * | ** | ns | ns | ||
| Fs×cd | *** | *** | ns | ** | ||
| Fs×AMF | ** | ns | * | ns | ||
| Fcd×AMF | ns | ns | ns | ns | ||
| Fs×cd×AMF | ** | * | ns | ** |
Table 1 Effects of arbuscular mycorrhizae fungi (AMF) inoculation on gas exchange rate in females and males of Populus deltoides exposed to Cd pollution (mean ± SE)
| 处理 Treatment | 丛枝菌根 AM | 性别 Sex | 净光合速率 Pn (µmol·m-2·s-1) | 气孔导度 Gs (mol·m-2·s-1) | 胞间CO2浓度 Ci (µmol·mol-1) | 蒸腾速率 Tr (mmol·m-2·s-1) |
|---|---|---|---|---|---|---|
| 对照 | - | 雄株 Male | 11.91 ± 0.26b | 0.37 ± 0.04bcd | 318.40 ± 10.05a | 4.37 ± 0.27ab |
| Control | - | 雌株 Female | 13.68 ± 0.79ab | 0.51 ± 0.02ab | 310.85 ± 7.71ab | 5.50 ± 0.27a |
| + | 雄株 Male | 12.34 ± 0.34b | 0.41 ± 0.03abc | 313.96 ± 7.72ab | 5.11 ± 0.20a | |
| + | 雌株 Female | 14.43 ± 0.42a | 0.52 ± 0.05a | 313.68 ± 10.97ab | 5.37 ± 0.44a | |
| Cd污染 | - | 雄株 Male | 9.70 ± 0.46c | 0.25 ± 0.02de | 270.87 ± 6.51bc | 3.48 ± 0.26bc |
| Cd pollution | - | 雌株 Female | 6.58 ± 0.27d | 0.13 ± 0.02e | 243.14 ± 9.73c | 2.10 ± 0.33d |
| + | 雄株 Male | 8.86 ± 0.35c | 0.27 ± 0.02d | 273.86 ± 9.00bc | 2.95 ± 0.26cd | |
| + | 雌株 Female | 9.59 ± 0.28c | 0.30 ± 0.01cd | 288.10 ± 4.52ab | 3.26 ± 0.11bcd | |
| Fs | ns | ns | ns | ns | ||
| Fcd | *** | *** | *** | *** | ||
| FAMF | * | ** | ns | ns | ||
| Fs×cd | *** | *** | ns | ** | ||
| Fs×AMF | ** | ns | * | ns | ||
| Fcd×AMF | ns | ns | ns | ns | ||
| Fs×cd×AMF | ** | * | ns | ** |
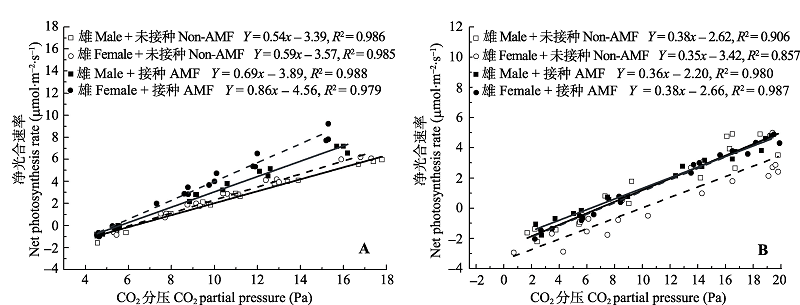
Fig. 1 Effects of arbuscular mycorrhizae fungi (AMF) inoculation on Pn-Ci curves of females and males of Populus deltoides under control (A) and Cd-stressed (B) conditions.
| 处理 Treatment | 丛枝菌根 AM | 性别 Sex | 最大羧化速率 Vcmax (µmol·m-2·s-1) | 最大电子传递速率 Jmax (µmol·m-2·s-1) | 磷酸丙糖利用率 TPU (µmol·m-2·s-1) | CO2补偿点 Г (µmol·mol-1) |
|---|---|---|---|---|---|---|
| 对照 | - | 雄株 Male | 34.17 ± 1.14bc | 88.20 ± 1.80bc | 6.26 ± 0.14abcd | 6.20 ± 0.36b |
| Control | - | 雌株 Female | 37.80 ± 0.17b | 93.60 ± 3.38b | 7.91 ± 0.64abc | 6.10 ± 0.21b |
| + | 雄株 Male | 42.57 ± 1.34b | 117.33 ± 6.77a | 8.88 ± 0.95a | 5.57 ± 0.08b | |
| + | 雌株 Female | 52.30 ± 3.13a | 137.33 ± 5.78a | 8.24 ± 0.63ab | 5.34 ± 0.12b | |
| Cd 污染 | - | 雄株 Male | 25.87 ± 2.39cd | 71.20 ± 2.72cd | 4.17 ± 0.27d | 6.87 ± 0.28b |
| Cd pollution | - | 雌株 Female | 22.67 ± 1.36d | 59.50 ± 3.50d | 5.19 ± 0.41bcd | 9.91 ± 0.46a |
| + | 雄株 Male | 24.17 ± 2.81d | 68.97 ± 4.34cd | 4.68 ± 0.45cd | 6.08 ± 0.20b | |
| + | 雌株 Female | 25.30 ± 1.03cd | 73.23 ± 4.52bcd | 6.54 ± 1.22abcd | 7.02 ± 0.38b | |
| Fs | ns | ns | ns | ns | ||
| Fcd | *** | *** | *** | *** | ||
| FAMF | *** | *** | * | ns | ||
| Fs×cd | ** | * | * | ns | ||
| Fs×AMF | ns | * | ns | ** | ||
| Fcd×AMF | *** | *** | ns | ** | ||
| Fs×cd×AMF | ns | ns | ns | ** |
Table 2 Effects of arbuscular mycorrhizae fungi (AMF) inoculation on parameters derived from the fitted Pn-Ci curves in females and males of Populus deltoides exposed to Cd pollution (mean ± SE)
| 处理 Treatment | 丛枝菌根 AM | 性别 Sex | 最大羧化速率 Vcmax (µmol·m-2·s-1) | 最大电子传递速率 Jmax (µmol·m-2·s-1) | 磷酸丙糖利用率 TPU (µmol·m-2·s-1) | CO2补偿点 Г (µmol·mol-1) |
|---|---|---|---|---|---|---|
| 对照 | - | 雄株 Male | 34.17 ± 1.14bc | 88.20 ± 1.80bc | 6.26 ± 0.14abcd | 6.20 ± 0.36b |
| Control | - | 雌株 Female | 37.80 ± 0.17b | 93.60 ± 3.38b | 7.91 ± 0.64abc | 6.10 ± 0.21b |
| + | 雄株 Male | 42.57 ± 1.34b | 117.33 ± 6.77a | 8.88 ± 0.95a | 5.57 ± 0.08b | |
| + | 雌株 Female | 52.30 ± 3.13a | 137.33 ± 5.78a | 8.24 ± 0.63ab | 5.34 ± 0.12b | |
| Cd 污染 | - | 雄株 Male | 25.87 ± 2.39cd | 71.20 ± 2.72cd | 4.17 ± 0.27d | 6.87 ± 0.28b |
| Cd pollution | - | 雌株 Female | 22.67 ± 1.36d | 59.50 ± 3.50d | 5.19 ± 0.41bcd | 9.91 ± 0.46a |
| + | 雄株 Male | 24.17 ± 2.81d | 68.97 ± 4.34cd | 4.68 ± 0.45cd | 6.08 ± 0.20b | |
| + | 雌株 Female | 25.30 ± 1.03cd | 73.23 ± 4.52bcd | 6.54 ± 1.22abcd | 7.02 ± 0.38b | |
| Fs | ns | ns | ns | ns | ||
| Fcd | *** | *** | *** | *** | ||
| FAMF | *** | *** | * | ns | ||
| Fs×cd | ** | * | * | ns | ||
| Fs×AMF | ns | * | ns | ** | ||
| Fcd×AMF | *** | *** | ns | ** | ||
| Fs×cd×AMF | ns | ns | ns | ** |
| 处理 Treatment | 丛枝菌根 AM | 性别 Sex | Fv/Fm | ΦPSII | qP | qN |
|---|---|---|---|---|---|---|
| 对照 | - | 雄株 Male | 0.80 ± 0.01ab | 0.72 ± 0.01a | 0.94 ± 0.01a | 0.19 ± 0.01cd |
| Control | - | 雌株 Female | 0.81 ± 0.01ab | 0.73 ± 0.00a | 0.96 ± 0.00a | 0.19 ± 0.01cd |
| + | 雄株 Male | 0.81 ± 0.00ab | 0.73 ± 0.00a | 0.94 ± 0.01a | 0.24 ± 0.01bc | |
| + | 雌株 Female | 0.81 ± 0.00ab | 0.73 ± 0.00a | 0.93 ± 0.01a | 0.17 ± 0.01d | |
| Cd 污染 | - | 雄株 Male | 0.78 ± 0.00bc | 0.70 ± 0.01a | 0.93 ± 0.01a | 0.21 ± 0.01cd |
| Cd pollution | - | 雌株 Female | 0.69 ± 0.01e | 0.60 ± 0.02b | 0.83 ± 0.01b | 0.33 ± 0.01a |
| + | 雄株 Male | 0.76 ± 0.00cd | 0.70 ± 0.01a | 0.92 ± 0.01a | 0.26 ± 0.01b | |
| + | 雌株 Female | 0.76 ± 0.01d | 0.70 ± 0.02a | 0.93 ± 0.02a | 0.26 ± 0.01b | |
| Fs | *** | ** | ** | ns | ||
| Fcd | *** | *** | *** | *** | ||
| FAMF | *** | ** | ns | ns | ||
| Fs×cd | *** | *** | ** | *** | ||
| Fs×AMF | *** | * | ** | *** | ||
| Fcd×AMF | ns | * | *** | ns | ||
| Fs×cd×AMF | *** | ** | *** | ns |
Table 3 Effects of arbuscular mycorrhizae fungi (AMF) inoculation on chlorophyll a fluorescence parameters in females and males of Populus deltoides exposed to Cd pollution (mean ± SE)
| 处理 Treatment | 丛枝菌根 AM | 性别 Sex | Fv/Fm | ΦPSII | qP | qN |
|---|---|---|---|---|---|---|
| 对照 | - | 雄株 Male | 0.80 ± 0.01ab | 0.72 ± 0.01a | 0.94 ± 0.01a | 0.19 ± 0.01cd |
| Control | - | 雌株 Female | 0.81 ± 0.01ab | 0.73 ± 0.00a | 0.96 ± 0.00a | 0.19 ± 0.01cd |
| + | 雄株 Male | 0.81 ± 0.00ab | 0.73 ± 0.00a | 0.94 ± 0.01a | 0.24 ± 0.01bc | |
| + | 雌株 Female | 0.81 ± 0.00ab | 0.73 ± 0.00a | 0.93 ± 0.01a | 0.17 ± 0.01d | |
| Cd 污染 | - | 雄株 Male | 0.78 ± 0.00bc | 0.70 ± 0.01a | 0.93 ± 0.01a | 0.21 ± 0.01cd |
| Cd pollution | - | 雌株 Female | 0.69 ± 0.01e | 0.60 ± 0.02b | 0.83 ± 0.01b | 0.33 ± 0.01a |
| + | 雄株 Male | 0.76 ± 0.00cd | 0.70 ± 0.01a | 0.92 ± 0.01a | 0.26 ± 0.01b | |
| + | 雌株 Female | 0.76 ± 0.01d | 0.70 ± 0.02a | 0.93 ± 0.02a | 0.26 ± 0.01b | |
| Fs | *** | ** | ** | ns | ||
| Fcd | *** | *** | *** | *** | ||
| FAMF | *** | ** | ns | ns | ||
| Fs×cd | *** | *** | ** | *** | ||
| Fs×AMF | *** | * | ** | *** | ||
| Fcd×AMF | ns | * | *** | ns | ||
| Fs×cd×AMF | *** | ** | *** | ns |
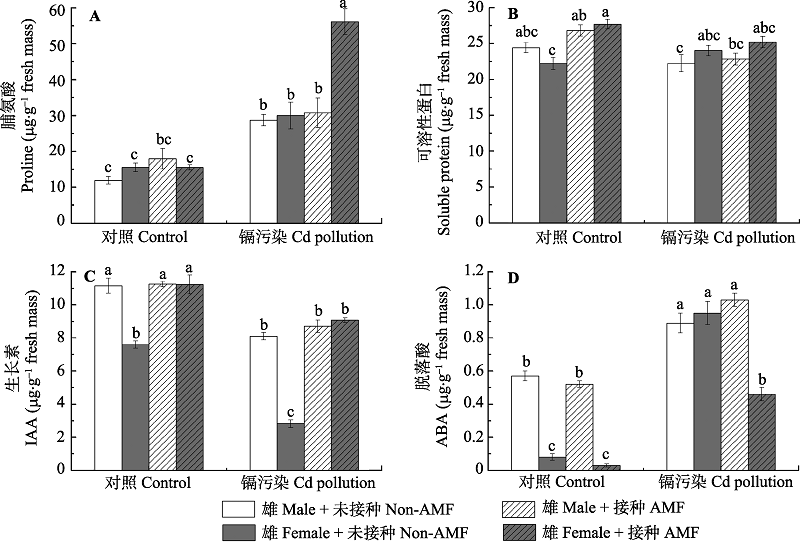
Fig. 2 Effects of arbuscular mycorrhizae fungi (AMF) inoculation on osmotic solutes and phytohormones in females and males of Populus deltoides exposed to Cd pollution (mean ± SE). Different letters indicate significant differences between treatments (p < 0.05) according to Tukey test.
| 参数 Parameter | 因子 Factor | ||||||
|---|---|---|---|---|---|---|---|
| Fs | Fcd | FAMF | Fs×cd | Fs×AMF | Fcd×AMF | Fs×cd×AMF | |
| 脯氨酸 Proline | ** | *** | *** | ** | * | ** | *** |
| 可溶性蛋白 Soluble protein | ns | ** | *** | * | ns | * | ns |
| 生长素 Auxin | *** | *** | *** | *** | *** | ** | *** |
| 脱落酸 Abscisic acid | *** | *** | *** | *** | *** | * | *** |
Table 4 Statistical significance of the single and interactive effects of sex, Cd and arbuscular mycorrhizae fungi (AMF) on osmotic solutes and phytohormones based on univariate analyses of variance.
| 参数 Parameter | 因子 Factor | ||||||
|---|---|---|---|---|---|---|---|
| Fs | Fcd | FAMF | Fs×cd | Fs×AMF | Fcd×AMF | Fs×cd×AMF | |
| 脯氨酸 Proline | ** | *** | *** | ** | * | ** | *** |
| 可溶性蛋白 Soluble protein | ns | ** | *** | * | ns | * | ns |
| 生长素 Auxin | *** | *** | *** | *** | *** | ** | *** |
| 脱落酸 Abscisic acid | *** | *** | *** | *** | *** | * | *** |
| [1] | Ali B, Qian P, Jin R, Ali S, Khan M, Aziz R, Tian T, Zhou W (2014). Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biologia Plantarum, 58, 131-138. |
| [2] | Benavides MP, Gallego SM, Tomaro ML (2005). Cadmium toxicity in plants.Brazilian Journal of Plant Physiology, 17, 21-34. |
| [3] | Björkman O, Demmig B (1987). Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins.Planta, 170, 489-504. |
| [4] | Burzyński M, Kłobus G (2004). Changes of photosynthetic parameters in cucumber leaves under Cu, Cd, and Pb stress.Photosynthetica, 42, 505-510. |
| [5] | Cao L, Wang QC, Cui DH (2006). Impact of soil cadmium contamination on chlorophyll fluorescence characters and biomass accumulation of four broad-leaved tree species seedlings.Chinese Journal of Applied Ecology, 17, 769-772. (in Chinese with English abstract)[曹玲, 王庆成, 崔东海 (2006). 土壤镉污染对四种阔叶树苗木叶绿素荧光特性和生长的影响. 应用生态学报, 17, 769-772.] |
| [6] | Chen LH, Han Y, Jiang H, Korpelainen H, Li CY (2011). Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. Journal of Experimental Botany, 62, 5037-5050. |
| [7] | Chen LH, Hu XW, Yang WQ, Xu ZF, Zhang DJ, Gao S (2015). The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicology and Environmental Safety, 113, 460-468. |
| [8] | Chen LH, Zhang DJ, Yang WQ, Liu Y, Zhang L, Gao S (2016). Sex-specific responses of Populus deltoides to Glomus intraradices colonization and Cd pollution. Chemosphere, 155, 196-206. |
| [9] | Chen LH, Zhang L, Tu LH, Xu ZF, Zhang J, Gao S (2014). Sex-related differences in physiological and ultrastructural responses of Populus cathayana to Ni toxicity. Acta Physiologiae Plantarum, 36, 1937-1946. |
| [10] | Cocozza C, Trupiano D, Lustrato G, Alfano G, Vitullo D, Falasca A, Lomaglio T, de Felice V, Lima G, Ranalli G, Scippa S, Tognetti R (2015). Challenging synergistic activity of poplar-bacteria association for the Cd phytostabilization.Environmental Science and Pollution Research, 22, 19546-19561. |
| [11] | DalCorso G, Farinati S, Maistri S, Furini A (2008). How plants cope with cadmium: Staking all on metabolism and gene expression.Journal of Integrative Plant Biology, 50, 1268-1280. |
| [12] | Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013). Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton.Ecotoxicology and Environmental Safety, 96, 242-249. |
| [13] | Farquhar GD, Von SV, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.Planta, 149, 78-90. |
| [14] | Gómez-Cadenas A, Vives V, Zandalinas SI, Manzi M, Sánchez-Pérez AM, Pérez-Clemente RM, Arbona V (2015). Abscisic acid: A versatile phytohormone in plant signaling and beyond.Current Protein and Peptide Science, 16, 413-434. |
| [15] | Hayward AR, Coates KE, Galer AL, Hutchinson TC, Emery RN (2013). Chelator profiling in Deschampsia cespitosa(L.) Beauv. reveals a Ni reaction, which is distinct from the ABA and cytokinin associated response to Cd. Plant Physiology and Biochemistry, 64, 84-91. |
| [16] | Huang J, Ling WT, Sun YD, Liu J (2012). Impacts of arbuscular mycorrhizal fungi inoculation on the uptake of cadmium and zinc by Alfalfa in contaminated soil. Journal of Agro-Environment Science, 31, 99-105. (in Chinese with English abstract)[黄晶, 凌婉婷, 孙艳娣, 刘娟 (2012). 丛枝菌根真菌对紫花苜蓿吸收土壤中镉和锌的影响. 农业环境科学学报, 31, 99-105.] |
| [17] | Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I (2007). Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytologist, 175, 655-674. |
| [18] | Li DJ, Mo JM, Fang YT, Jiang YQ (2004). Ecophysiological responses of woody plants to elevated nitrogen deposition.Journal of Tropical and Subtropical Botany, 12, 482-488. (in Chinese with English abstract)[李德军, 莫江明, 方运霆, 江远清 (2004). 木本植物对高氮沉降的生理生态响应. 热带亚热带植物学报, 12, 482-488.] |
| [19] | Li YL, Jin ZX, Li JM, Guo SM, Guan M (2015). Effects of soil microbe inoculation on the growth and photosynthetic physiology of Elsholtzia splendens under copper stress. Acta Ecologica Sinica, 35, 3926-3937. (in Chinese with English abstract)[李月灵, 金则新, 李钧敏, 郭素民, 管铭 (2015). 接种土壤微生物对铜胁迫下海州香薷生长及光合生理的影响. 生态学报, 35, 3926-3937.] |
| [20] | Loth-Pereda V, Orsini E, Courty PE, Lota F, Kohler A, Diss L, Blaudez D, Chalot M, Nehls U, Bucher M, Martin F (2011). Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa. Plant Physiology, 156, 2141-2154. |
| [21] | Ma HY (2007). Changes of Endogenous Hormones in Grapevine During Its Development. Master degree dissertation, Northwest Agriculture and Forestry University, Yangling, Shaanxi. (in Chinese with English abstract)[马海燕 (2007). 葡萄生长过程中内源激素含量变化的研究. 硕士学位论文, 西北农林科技大学, 陕西杨凌.] |
| [22] | Pallara G, Todeschini V, Lingua G, Camussi A, Racchi ML (2013). Transcript analysis of stress defence genes in a white poplar clone inoculated with the arbuscular mycorrhizal fungus Glomus mosseae and grown on a polluted soil. Plant Physiology and Biochemistry, 63, 131-139. |
| [23] | Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013). The dual effect of abscisic acid on stomata.New Phytologist, 197, 65-72. |
| [24] | Pereira MP, de Almeida Rodrigues LC, Corrêa FF, de Castro EM, Ribeiro VE, Pereira FJ (2016). Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees, 30, 807-814. |
| [25] | Prusty R, Grisafi P, Fink GR (2004). The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America, 101, 4153-4157. |
| [26] | Ran Q, Zhong ZC (2015). Effect of AMF on the photosynthetic characteristics and growth of maize seedlings under the stress of drought, high calcium and their combination in karst area.Acta Ecologica Sinica, 35, 460-467. (in Chinese with English abstract)[冉琼, 钟章成 (2015). 模拟岩溶旱钙土壤基质中AM真菌对玉米幼苗光合生长的影响. 生态学报, 35, 460-467.] |
| [27] | Rozpądek P, Wężowicz K, Nosek M, Ważny R, Tokarz K, Lembicz M, Miszalski Z, Turnau K (2015). The fungal endophyteEpichloë typhina improves photosynthesis efficiency of its host orchard grass(Dactylis glomerata). Planta, 242, 1025-1035. |
| [28] | Sanità di Toppi L, Gabbrielli R (1999). Response to cadmium in higher plants.Environmental and Experimental Botany, 41, 105-130. |
| [29] | Smirnoff N, Cumbes QJ (1989). Hydroxyl radical scavenging activity of compatible solutes.Phytochemistry, 28, 1057-1060. |
| [30] | Stroiński A, Giżewska K, Zielezińska M (2013). Abscisic acid is required in transduction of cadmium signal to potato roots.Biologia Plantarum, 57, 121-127. |
| [31] | Wang SZ, Jin ZX, Li YL, Gu YF (2015). Effects of arbuscular mycorrhizal fungi inoculation on the photosynthetic pigment contents, anti-oxidation capacity and membrane lipid peroxidation of Elsholtzia splendens leaves under copper stress. Acta Ecologica Sinica, 35, 7699-7708. (in Chinese with English abstract)[王穗子, 金则新, 李月灵, 谷银芳 (2015). 铜胁迫条件下AMF对海州香薷光合色素含量、抗氧化能力和膜脂过氧化的影响. 生态学报, 35, 7699-7708.] |
| [32] | Xu X, Peng GQ, Wu CC, Korpelainen H, Li CY (2008). Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiology, 28, 1751-1759. |
| [33] | Zhao HX, Li Y, Duan BL, Korpelainen H, Li CY (2009). Sex-related adaptive responses of Populus cathayana to photoperiod transitions. Plant, Cell & Environment, 32, 1401-1411. |
| [1] | SHI Meng-Jiao, LI Bin, YI Li-Ta, LIU Mei-Hua. Sexual divergence of Populus deltoides seedlings growth and ecophysiological response to drought and rewatering [J]. Chin J Plant Ecol, 2023, 47(8): 1159-1170. |
| [2] | LIN Xia-Zhen, LIU Lin, DONG Ting-Ting, FANG Qi-Bo, GUO Qing-Xue. Effects of non-structural carbohydrate and nitrogen allocation on the ability of Populus deltoides and P. cathayana to resist soil salinity stress [J]. Chin J Plant Ecol, 2021, 45(9): 961-971. |
| [3] | Jian-Guo CAI, Meng-Qi WEI, Yi ZHANG, Yun-Long WEI. Effects of shading on photosynthetic characteristics and chlorophyll fluorescence parameters in leaves of Hydrangea macrophylla [J]. Chin J Plan Ecolo, 2017, 41(5): 570-576. |
| [4] | HU Chu-Qi,LIU Jin-Ke,WANG Tian-Hong,WANG Wen-Lin,LU Shan,ZHOU Chang-Fang. Influence of three types of salt stress on photosynthesis in Spartina alterniflora and Phragmites australis [J]. Chin J Plan Ecolo, 2015, 39(1): 92-103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn