

Chin J Plant Ecol ›› 2021, Vol. 45 ›› Issue (9): 961-971.DOI: 10.17521/cjpe.2021.0240
• Research Articles • Previous Articles Next Articles
LIN Xia-Zhen1, LIU Lin2, DONG Ting-Ting2, FANG Qi-Bo2, GUO Qing-Xue2,*( )
)
Received:2021-06-28
Accepted:2021-08-11
Online:2021-09-20
Published:2021-11-18
Contact:
GUO Qing-Xue
LIN Xia-Zhen, LIU Lin, DONG Ting-Ting, FANG Qi-Bo, GUO Qing-Xue. Effects of non-structural carbohydrate and nitrogen allocation on the ability of Populus deltoides and P. cathayana to resist soil salinity stress[J]. Chin J Plant Ecol, 2021, 45(9): 961-971.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2021.0240
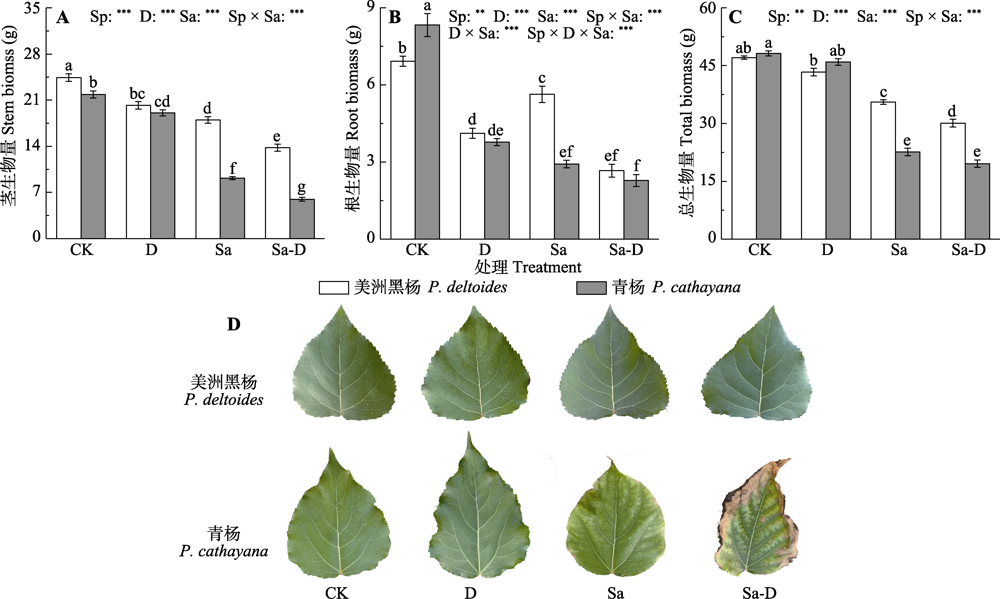
Fig. 1 Stem biomass (A), root biomass (B), total biomass (C) and leaf performance (D) of Populus deltoides and P. cathayana under different treatments (mean ± SE). CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). **, 0.001 < p ≤0.01; ***, p ≤ 0.001.
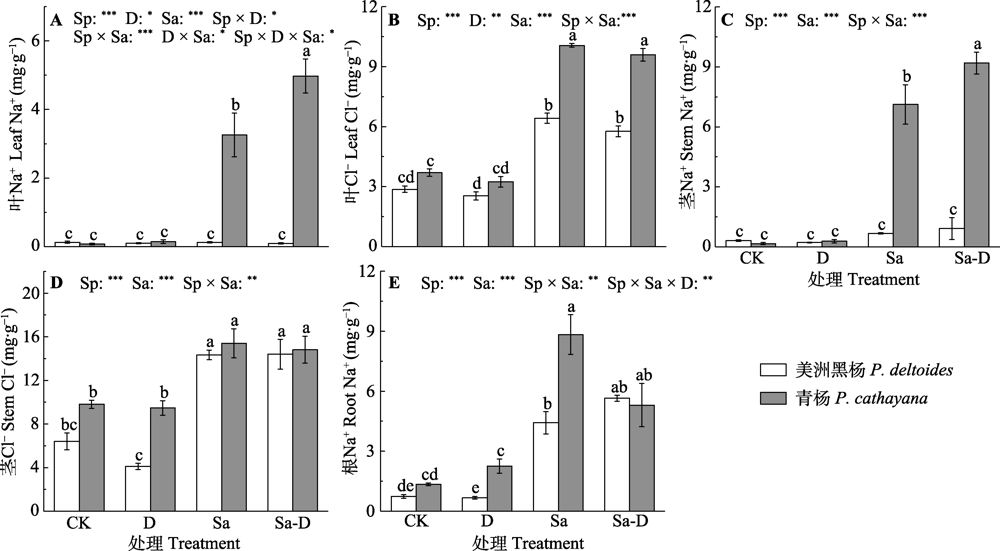
Fig. 2 Na+ and Cl- concentrations of leaf, stem and root in both Populus deltoides and P. cathayana under different treatments (mean ± SE). Root Cl- concentration was not tested because the root biomass of P. cathayana in the salt stress and defoliation treatment was not sufficient. CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
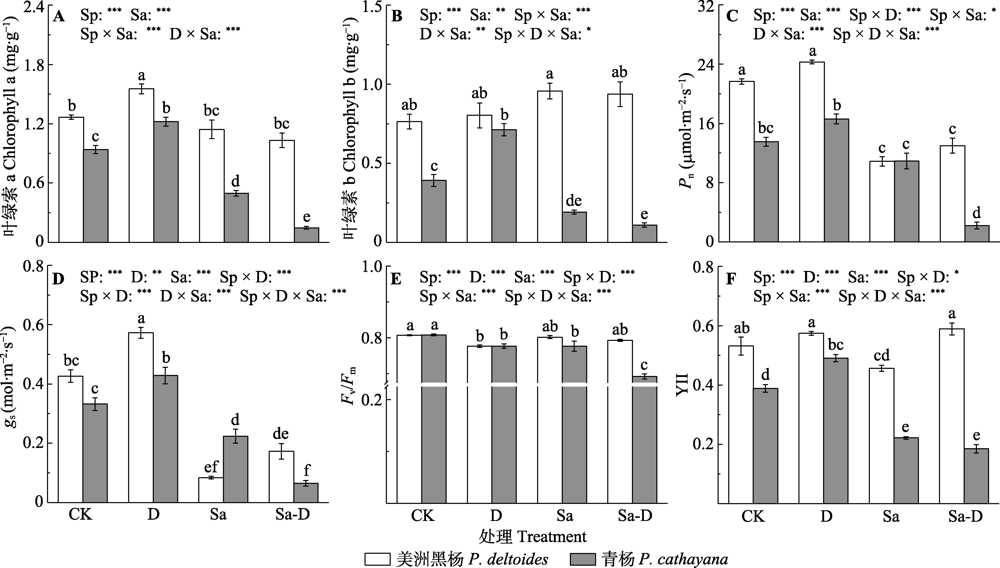
Fig. 3 The leaf photosynthetic and chlorophyll fluorescence traits of Populus deltoides and P. cathayana under different treatments (mean ± SE). Fv/Fm, maximum yield of primary photochemistry; gs, stomatal conductance; Pn, net photosynthetic rate; YII, effective quantum yield of PSII. CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
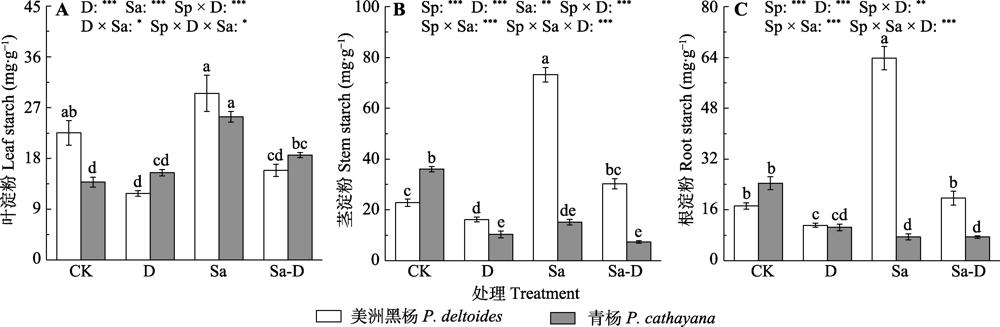
Fig. 4 Leaf, stem and root starch concentration of Populus deltoides and P. cathayana under different treatments (mean ± SE). CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
| 处理 Treatment | 可溶性糖 Soluble sugars (mg·g-1) | 蔗糖 Sucrose (mg·g-1) | ||||
|---|---|---|---|---|---|---|
| 叶 Leaf | 茎 Stem | 根 Root | 叶 Leaf | 茎 Stem | 根 Root | |
| 美洲黑杨 P. deltoides | ||||||
| 对照 Control (CK) | 134.1 ± 4.4a | 71.2 ± 2.2a | 51.6 ± 1.3b | 75.6 ± 3.9a | 27.6 ± 1.9ab | 27.6 ± 1.4b |
| 去叶 Defoliation (D) | 143.1 ± 4.6a | 58.4 ± 1.9bc | 33.0 ± 1.3e | 73.8 ± 4.3a | 26.5 ± 2.6abc | 19.3 ± 1.4c |
| 盐胁迫 Salt stress (Sa) | 144.9 ± 6.0a | 67.2 ± 3.0ab | 60.3 ± 1.8a | 79.4 ± 5.8a | 35.0 ± 4.4a | 37.7 ± 3.2a |
| 盐胁迫与去叶 Salt stress and Defoliation (Sa-D) | 149.0 ± 2.3a | 54.1 ± 1.6c | 45.1 ± 1.1bc | 75.4 ± 3.9a | 25.3 ± 3.2abc | 22.0 ± 1.4bc |
| 青杨 P. cathayana | ||||||
| CK | 144.6 ± 3.4a | 59.7 ± 1.8bc | 39.4 ± 1.5cd | 61.0 ± 2.4ab | 27.0 ± 3.3abc | 19.4 ± 0.6c |
| D | 141.6 ± 1.1a | 44.4 ± 1.9d | 38.7 ± 2.0cd | 47.2 ± 2.8b | 17.8 ± 2.0bc | 21.2 ± 1.8bc |
| Sa | 113.6 ± 5.5b | 34.6 ± 1.4e | 29.9 ± 3.4e | 49.0 ± 8.1b | 15.2 ± 1.6bc | 14.5 ± 1.2cd |
| Sa-D | 113.0 ± 4.2b | 31.0 ± 1.1e | 18.5 ± 1.2f | 44.5 ± 3.4b | 14.6 ± 1.2c | 11.0 ± 1.4d |
| p | ||||||
| 物种 Species (Sp) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| D | 0.432 | <0.001 | <0.001 | 0.076 | 0.012 | <0.001 |
| Sa | 0.001 | <0.001 | 0.094 | 0.483 | 0.264 | 0.636 |
| Sp × D | 0.168 | 0.216 | <0.001 | 0.352 | 0.894 | <0.001 |
| Sp × Sa | <0.001 | <0.001 | <0.001 | 0.138 | 0.010 | <0.001 |
| D × Sa | 0.833 | 0.044 | 0.174 | 0.598 | 1.000 | 0.012 |
| Sp × D × Sa | 0.549 | 0.035 | 0.010 | 0.388 | 0.034 | 0.676 |
Table 1 Soluble sugars and sucrose concentration of leaf, stem and root in both Populus deltoides and P. cathayana under different treatments (mean ± SE)
| 处理 Treatment | 可溶性糖 Soluble sugars (mg·g-1) | 蔗糖 Sucrose (mg·g-1) | ||||
|---|---|---|---|---|---|---|
| 叶 Leaf | 茎 Stem | 根 Root | 叶 Leaf | 茎 Stem | 根 Root | |
| 美洲黑杨 P. deltoides | ||||||
| 对照 Control (CK) | 134.1 ± 4.4a | 71.2 ± 2.2a | 51.6 ± 1.3b | 75.6 ± 3.9a | 27.6 ± 1.9ab | 27.6 ± 1.4b |
| 去叶 Defoliation (D) | 143.1 ± 4.6a | 58.4 ± 1.9bc | 33.0 ± 1.3e | 73.8 ± 4.3a | 26.5 ± 2.6abc | 19.3 ± 1.4c |
| 盐胁迫 Salt stress (Sa) | 144.9 ± 6.0a | 67.2 ± 3.0ab | 60.3 ± 1.8a | 79.4 ± 5.8a | 35.0 ± 4.4a | 37.7 ± 3.2a |
| 盐胁迫与去叶 Salt stress and Defoliation (Sa-D) | 149.0 ± 2.3a | 54.1 ± 1.6c | 45.1 ± 1.1bc | 75.4 ± 3.9a | 25.3 ± 3.2abc | 22.0 ± 1.4bc |
| 青杨 P. cathayana | ||||||
| CK | 144.6 ± 3.4a | 59.7 ± 1.8bc | 39.4 ± 1.5cd | 61.0 ± 2.4ab | 27.0 ± 3.3abc | 19.4 ± 0.6c |
| D | 141.6 ± 1.1a | 44.4 ± 1.9d | 38.7 ± 2.0cd | 47.2 ± 2.8b | 17.8 ± 2.0bc | 21.2 ± 1.8bc |
| Sa | 113.6 ± 5.5b | 34.6 ± 1.4e | 29.9 ± 3.4e | 49.0 ± 8.1b | 15.2 ± 1.6bc | 14.5 ± 1.2cd |
| Sa-D | 113.0 ± 4.2b | 31.0 ± 1.1e | 18.5 ± 1.2f | 44.5 ± 3.4b | 14.6 ± 1.2c | 11.0 ± 1.4d |
| p | ||||||
| 物种 Species (Sp) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| D | 0.432 | <0.001 | <0.001 | 0.076 | 0.012 | <0.001 |
| Sa | 0.001 | <0.001 | 0.094 | 0.483 | 0.264 | 0.636 |
| Sp × D | 0.168 | 0.216 | <0.001 | 0.352 | 0.894 | <0.001 |
| Sp × Sa | <0.001 | <0.001 | <0.001 | 0.138 | 0.010 | <0.001 |
| D × Sa | 0.833 | 0.044 | 0.174 | 0.598 | 1.000 | 0.012 |
| Sp × D × Sa | 0.549 | 0.035 | 0.010 | 0.388 | 0.034 | 0.676 |
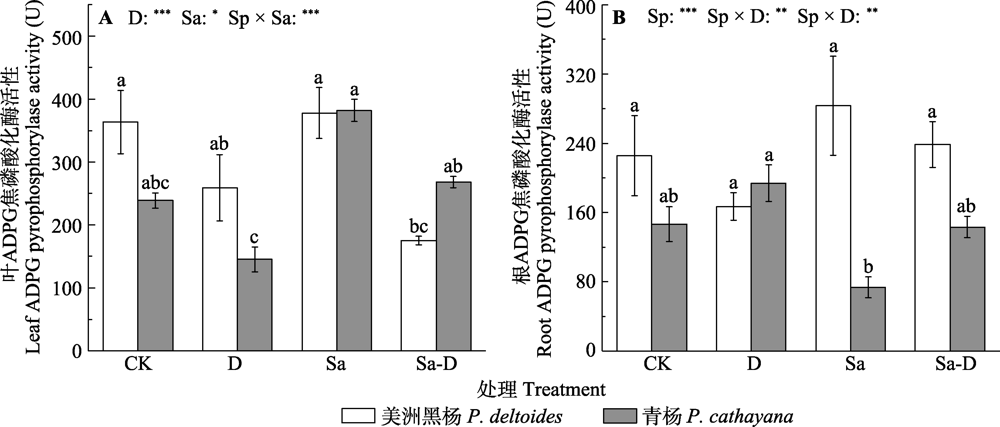
Fig. 5 Adenosine diphosphate glucose (ADPG) pyrophosphorylase activity in the leaf and root of Populus deltoides and P. cathayana under different treatments (mean ± SE). CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
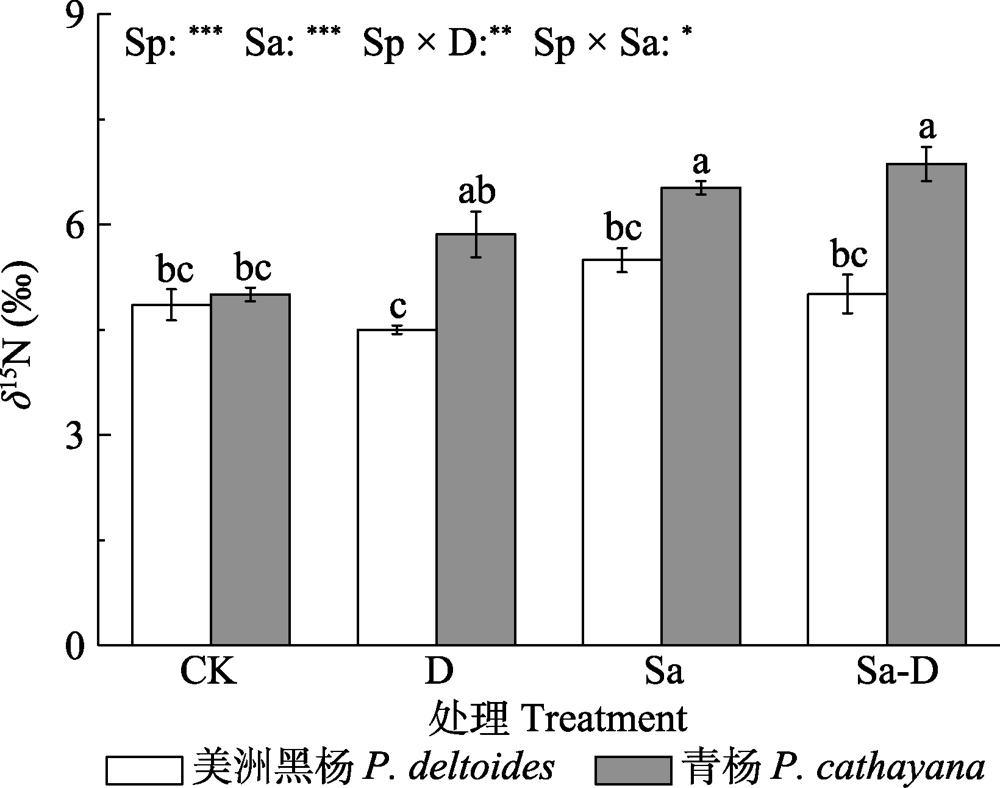
Fig. 6 Nitrogen stable isotope ratio (δ15N) in leaves of Populus deltoides and P. cathayana under different treatments (mean ± SE). CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
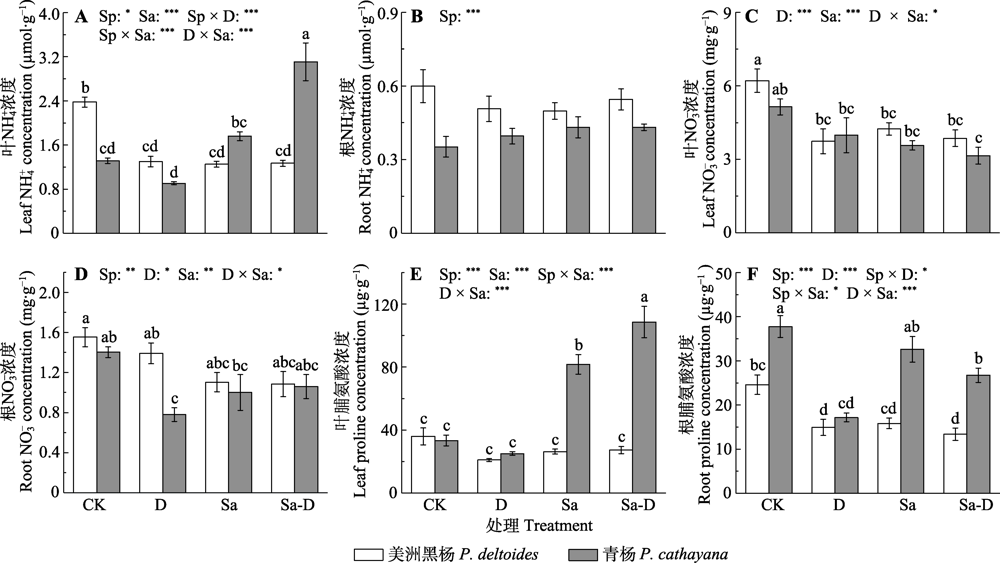
Fig. 7 NH4+, NO3- and proline concentrations in the leaf and root of Populus deltoides and P. cathayana under different treatments (mean ± SE). CK, control; D, defoliation; Sa, salt stress; Sa-D, salt stress and defoliation; Sp, species. Different lowercase letters indicate significant differences among treatments (p < 0.05). *, 0.01 < p ≤ 0.05; **, 0.001 < p ≤ 0.01; ***, p ≤ 0.001.
| 处理 Treatment | 谷氨酸脱氢酶 GDH (U) | 谷氨酰胺合成酶 GS (U) | ||
|---|---|---|---|---|
| 叶 Leaf | 根 Root | 叶 Leaf | 根 Root | |
| 美洲黑杨 P. deltoides | ||||
| 对照 Control (CK) | 202.1 ± 25.3cd | 63.7 ± 7.7b | 7.5 ± 1.7a | 5.3 ± 0.5 |
| 去叶 Defoliation (D) | 111.6 ± 26.2de | 82.6 ± 4.9b | 9.5 ± 1.6a | 4.6 ± 0.4 |
| 盐胁迫 Salt stress (Sa) | 411.9 ± 40.6b | 129.3 ± 9.3a | 11.3 ± 1.8a | 4.6 ± 0.4 |
| 盐胁迫与去叶 Salt stress and Defoliation (Sa-D) | 685.1 ± 22.6a | 49.2 ± 9.4b | 13.7 ± 1.5a | 3.8 ± 0.2 |
| 青杨 P. cathayana | ||||
| CK | 114.6 ± 15.2de | 73.5 ± 7.1b | 7.2 ± 0.9a | 3.5 ± 0.3 |
| D | 58.3 ± 12.6e | 85.7 ± 9.1b | 7.9 ± 0.9a | 3.7 ± 0.9 |
| Sa | 248.8 ± 36.7c | 78.4 ± 11.4b | 4.1 ± 0.5a | 3.5 ± 0.2 |
| Sa-D | 248.1 ± 28.1c | 78.2 ± 11.2b | 5.8 ± 0.9a | 3.8 ± 0.2 |
| p | ||||
| 物种 Spices (Sp) | <0.001 | 0.726 | <0.001 | 0.004 |
| D | 0.114 | 0.063 | 0.078 | 0.491 |
| Sa | <0.001 | 0.253 | 0.454 | 0.242 |
| Sp × D | 0.004 | 0.007 | 0.582 | 0.136 |
| Sp × Sa | <0.001 | 0.181 | 0.001 | 0.216 |
| D × Sa | <0.001 | <0.001 | 0.736 | 0.920 |
| Sp × D × Sa | <0.001 | 0.002 | 0.880 | 0.888 |
Table 2 Glutamate dehydrogenase (GDH) and glutamine synthase (GS) activity of leaf and root in both Populus deltoides and P. cathayana under different treatments (mean ± SE)
| 处理 Treatment | 谷氨酸脱氢酶 GDH (U) | 谷氨酰胺合成酶 GS (U) | ||
|---|---|---|---|---|
| 叶 Leaf | 根 Root | 叶 Leaf | 根 Root | |
| 美洲黑杨 P. deltoides | ||||
| 对照 Control (CK) | 202.1 ± 25.3cd | 63.7 ± 7.7b | 7.5 ± 1.7a | 5.3 ± 0.5 |
| 去叶 Defoliation (D) | 111.6 ± 26.2de | 82.6 ± 4.9b | 9.5 ± 1.6a | 4.6 ± 0.4 |
| 盐胁迫 Salt stress (Sa) | 411.9 ± 40.6b | 129.3 ± 9.3a | 11.3 ± 1.8a | 4.6 ± 0.4 |
| 盐胁迫与去叶 Salt stress and Defoliation (Sa-D) | 685.1 ± 22.6a | 49.2 ± 9.4b | 13.7 ± 1.5a | 3.8 ± 0.2 |
| 青杨 P. cathayana | ||||
| CK | 114.6 ± 15.2de | 73.5 ± 7.1b | 7.2 ± 0.9a | 3.5 ± 0.3 |
| D | 58.3 ± 12.6e | 85.7 ± 9.1b | 7.9 ± 0.9a | 3.7 ± 0.9 |
| Sa | 248.8 ± 36.7c | 78.4 ± 11.4b | 4.1 ± 0.5a | 3.5 ± 0.2 |
| Sa-D | 248.1 ± 28.1c | 78.2 ± 11.2b | 5.8 ± 0.9a | 3.8 ± 0.2 |
| p | ||||
| 物种 Spices (Sp) | <0.001 | 0.726 | <0.001 | 0.004 |
| D | 0.114 | 0.063 | 0.078 | 0.491 |
| Sa | <0.001 | 0.253 | 0.454 | 0.242 |
| Sp × D | 0.004 | 0.007 | 0.582 | 0.136 |
| Sp × Sa | <0.001 | 0.181 | 0.001 | 0.216 |
| D × Sa | <0.001 | <0.001 | 0.736 | 0.920 |
| Sp × D × Sa | <0.001 | 0.002 | 0.880 | 0.888 |
| [1] |
Ahmad P, Hashem A, Abd-Allah EF, Alqarawi AA, John R, Egamberdieva D, Gucel S (2015). Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Frontiers in Plant Science, 6, 868. DOI: 10.3389/fpls.2015.00868.
DOI |
| [2] | Bai AX, Lu XY (2020). Effects of calcium and calcium effectors on antioxidant system and osmotic adjustment substances content of sour jujube (Ziziphus jujuba var. spinosa) seedlings under NaCl stress. Plant Physiology Journal, 56, 1910-1920. |
| [ 白爱兴, 鲁晓燕 (2020). 钙和钙效应剂对NaCl胁迫下酸枣幼苗抗氧化系统及渗透调节物质含量的影响. 植物生理学报, 56, 1910-1920.] | |
| [3] |
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang LX (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Frontiers in Plant Science, 10, 1068. DOI: 10.3389/fpls.2019.01068.
DOI URL |
| [4] |
Behdad A, Mohsenzadeh S, Azizi M (2021). Growth, leaf gas exchange and physiological parameters of two Glycyrrhiza glabra L. populations subjected to salt stress condition. Rhizosphere, 17, 100319. DOI: 10.1016/j.rhisph.2021.100319.
DOI URL |
| [5] |
Cao X, Jia JB, Li H, Li MC, Liang ZS, Liu TX, Liu WG, Peng CH, Luo ZB (2012). Photosynthesis, water use efficiency and stable carbon isotope composition are associated with anatomical properties of leaf and xylem in six poplar species. Plant Biology, 14, 612-620.
DOI PMID |
| [6] | Chen J, Dong TF, Duan BL, Korpelainen H, Niinemets Ü, Li CY (2015). Sexual competition and N supply interactively affect the dimorphism and competiveness of opposite sexes in Populus cathayana. Plant, Cell & Environment, 38, 1285-1298. |
| [7] | Chen SL, Li JK, Bi WF, Wang SS (2001). Genotypic variation in accumulation of salt ions betaine and sugars in poplar under conditions of salt stress. Chinese Bulletin of Botany, 18, 587-596. |
| [ 陈少良, 李金克, 毕望富, 王沙生 (2001). 盐胁迫条件下杨树盐分与甜菜碱及糖类物质变化. 植物学通报, 18, 587-596.] | |
| [8] | Evans JR, Seemann JR (1989). The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences and control. Plant Biology, 183-205. |
| [9] |
Faseela P, Sinisha AK, Brestič M, Puthur JT (2019). Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica, 58, 293-3005.
DOI URL |
| [10] |
Ferrero DML, Piattoni CV, Asencion Diez MD, Rojas BE, Hartman MD, Ballicora MA, Iglesias AA (2020). Phosphorylation of ADP-glucose pyrophosphorylase during wheat seeds development. Frontiers in Plant Science, 11, 1058. DOI: 10.3389/fpls.2020.01058.
DOI PMID |
| [11] |
Guo QQ, Turnbull MH, Song JC, Roche J, Novak O, Späth J, Jameson PE, Love J (2017). Depletion of carbohydrate reserves limits nitrate uptake during early regrowth in Lolium perenne L. Journal of Experimental Botany, 68, 1569-1583.
DOI URL |
| [12] |
Guo QX, Wu XY, Korpelainen H, Li CY (2020). Stronger intra- specific competition aggravates negative effects of drought on the growth of Cunninghamia lanceolata. Environmental and Experimental Botany, 175, 104042. DOI: 10.1016/j.envexpbot.2020.104042.
DOI URL |
| [13] | Han LX, Ouyang DJ, Zhang GX (2020). Growth Na+ and K+ distribution and osmotic regulation of Chionanthus retusus seedlings under NaCl stress. Acta Botanica Boreali- Occidentalia Sinica, 40, 502-509. |
| [ 韩丽霞, 欧阳敦君, 张鸽香 (2020). NaCl胁迫对流苏幼苗生长、钠钾离子分布及渗透调节物质的影响. 西北植物学报, 40, 502-509.] | |
| [14] |
Hartmann H, Trumbore S (2016). Understanding the roles of nonstructural carbohydrates in forest trees-From what we can measure to what we want to know. New Phytologist, 211, 386-403.
DOI PMID |
| [15] |
Hüve K, Bichele I, Ivanova H, Keerberg O, Parnik T, Rasulov B, Tobias M, Niinemets Ü (2012). Temperature responses of dark respiration in relation to leaf sugar concentration. Physiologia Plantarum, 144, 320-334.
DOI URL |
| [16] | Lin SJ, Sun M (2017). Analysis of physiological response and salt tolerance mechanism of Crossostephium chinense and four species of Chrysanthemum under salt stress. Acta Botanica Boreali-Occidentalia Sinica, 37, 1137-1144. |
| [ 林双冀, 孙明 (2017). 盐胁迫下芙蓉菊与4种菊属植物生理响应特征及其耐盐机理分析. 西北植物学报, 37, 1137-1144.] | |
| [17] |
Liu M, Korpelainen H, Li CY (2021a). Sexual differences and sex ratios of dioecious plants under stressful environments. Journal of Plant Ecology, 14, 920-933.
DOI URL |
| [18] |
Liu M, Liu XC, Du XH, Korpelainen H, Niinemets Ü, Li CY (2021b). Anatomical variation of mesophyll conductance due to salt stress in Populus cathayana females and males growing under different inorganic nitrogen sources. Tree Physiology, 41, 1462-1478.
DOI URL |
| [19] |
Luo J, Zhou J, Li H, Shi WG, Polle A, Lu MZ, Sun XM, Luo ZB (2015). Global poplar root and leaf transcriptomes reveal links between growth and stress responses under nitrogen starvation and excess. Tree Physiology, 35, 1283-1302.
DOI URL |
| [20] |
Macneill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ (2017). Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. Journal of Experimental Botany, 68, 4433-4453.
DOI URL |
| [21] |
Meuriot F, Morvan-Bertrand A, Noiraud-Romy N, Decau ML, Escobar-Gutiérrez AJ, Gastal F, Prud'homme MP (2018). Short-term effects of defoliation intensity on sugar remobilization and N fluxes in ryegrass. Journal of Experimental Botany, 69, 3975-3986.
DOI URL |
| [22] |
Munns R, Tester M (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651-681.
DOI PMID |
| [23] |
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M, Kangasjärvi J, Jiang XN, Polle A (2005). Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiology, 139, 1762-1772.
PMID |
| [24] |
Sathee L, Jha SK, Rajput OS, Singh D, Kumar S, Kumar A (2021). Expression dynamics of genes encoding nitrate and ammonium assimilation enzymes in rice genotypes exposed to reproductive stage salinity stress. Plant Physiology and Biochemistry, 165, 161-172.
DOI URL |
| [25] | Secchi F, Zwieniecki MA (2011). Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling. Plant, Cell & Environment, 34, 514-524. |
| [26] |
Signori-Müller C, Oliveira RS, Barros FdV, Tavares JV, Gilpin M, Diniz FC, Zevallos MJM, Yupayccana CAS, Acosta M, Bacca J, Cruz Chino RS, Aramayo Cuellar GM, Cumapa ERM, Martinez F, Pérez Mullisaca FM, et al. (2021). Non-structural carbohydrates mediate seasonal water stress across Amazon forests. Nature Communication, 12, 2310. DOI: 10.1038/s41467-021-22378-8.
DOI URL |
| [27] | Takashima T, Hikosaka K, Hirose T (2004). Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant, Cell & Environment, 27, 1047-1054. |
| [28] |
Wang ZQ, Yuan YZ, Ou JQ, Lin QH, Zhang CF (2007). Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. Journal of Plant Physiology, 164, 695-701.
DOI URL |
| [29] | Wen XM, Xian T, Liu JY, Xu X, Dong TF (2021). Effects of defoliation on growth and non-structural carbohydrates in female and male Populus cathayana. Chinese Journal of Ecology, 40, 41-48. |
| [ 文小梅, 鲜婷, 刘俊雁, 胥晓, 董廷发 (2021). 去叶对青杨雌雄植株生长和非结构性碳水化合物的影响. 生态学杂志, 40, 41-48.] | |
| [30] |
Wu XY, Liu JT, Meng QQ, Fang SY, Kang JY, Guo QX (2021). Differences in carbon and nitrogen metabolism between male and female Populus cathayana in response to deficient nitrogen. Tree Physiology, 41, 119-133.
DOI URL |
| [31] |
Xu XH, Diao HJ, Qin CY, Hao J, Shen Y, Dong KH, Wang CH (2021). Response of soil net nitrogen mineralization to different levels of nitrogen addition in a saline-alkaline grassland of northern China. Chinese Journal of Plant Ecology, 45, 85-95.
DOI URL |
| [ 徐小惠, 刁华杰, 覃楚仪, 郝杰, 申颜, 董宽虎, 王常慧 (2021). 华北盐渍化草地土壤净氮矿化速率对不同水平氮添加的响应. 植物生态学报, 45, 85-95.] | |
| [32] | Zhao R, Chen SL (2020). The salt-stress signaling network involved in the regulation of ionic and ROS homeostasis in poplar. Scientia Sinica (Vitae), 50, 167-175. |
| 赵瑞, 陈少良 (2020). 杨树耐盐性调控的离子平衡与活性氧平衡信号网络. 中国科学: 生命科学, 50, 167-175.] | |
| [33] |
Zhou HH, Li WH (2015). Responses and adaptation of xylem hydraulic conductivity to salt stress in Populus euphratica. Chinese Journal of Plant Ecology, 39, 81-91.
DOI URL |
|
[ 周洪华, 李卫红 (2015). 胡杨木质部水分传导对盐胁迫的响应与适应. 植物生态学报, 39, 81-91.]
DOI |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn