

植物生态学报 ›› 2017, Vol. 41 ›› Issue (4): 489-496.DOI: 10.17521/cjpe.2016.0091
• • 上一篇
杨升1,2,*, 张华新2, 陈秋夏1, 杨秀艳2
收稿日期:2016-03-09
接受日期:2016-09-21
出版日期:2017-04-10
发布日期:2017-05-19
通讯作者:
杨升
基金资助:Sheng YANG1,2,*, Hua-Xin ZHANG2, Qiu-Xia CHEN1, Xiu-Yan YANG2
Received:2016-03-09
Accepted:2016-09-21
Online:2017-04-10
Published:2017-05-19
Contact:
Sheng YANG
摘要:
为了进一步从离子动态运输方面了解沙枣(Elaeagnus angustifolia)耐盐机制和揭示沙枣种源间的K+/Na+平衡调控差异, 该研究利用非损伤微测技术(non-invasive micro-test technology, NMT)测定银川种源(盐敏感型)和阿拉尔种源(耐盐型)沙枣幼苗根系在3种不同NaCl处理方式下的离子流: 1)在150 mmol·L-1 NaCl胁迫24 h后的Na+和K+离子流; 2) NaCl瞬时处理后的K+和H+的动态离子流; 3)先NaCl胁迫24 h, 再用Na+/H+逆向转运体抑制剂阿米洛利(Amiloride)和K+通道抑制剂氯化四乙胺(TEA)处理后的Na+和K+离子流。结果表明: NaCl胁迫24 h后, 沙枣根系Na+和K+外排净流量显著增加, 并且银川种源沙枣幼苗根系Na+净流量显著低于阿拉尔种源, 净流量分别为720和912 pmol·cm-2·s-1, 而K+外流净流量显著高于阿拉尔种源。瞬时NaCl处理后, 沙枣根系K+的外流迅速增加, 并且银川种源的K+外排净流量始终高于阿拉尔种源, 而H+由内流转为外排, 阿拉尔种源的H+净外流量大于银川种源。NaCl和NaCl + Amiloride处理下, 阿拉尔种源沙枣幼苗Na+外流的净流量均大于银川种源, 但K+外流的净流量均小于银川种源, 而在对照和NaCl + TEA处理下, Na+和K+的净流量在两个种源间无明显差异。研究证明NaCl胁迫造成根系Na+积累和K+外流, 沙枣幼苗为减少Na+积累, 通过根系Na+/H+逆向转运体将Na+从体内排出, 并且耐盐型种源沙枣幼苗根系在NaCl胁迫时能更好地维持体内的K+/Na+平衡, 其原因主要在于具有较强的Na+外排能力和较弱的K+流失。该研究可以为进一步发掘优良耐盐沙枣种质资源提供理论参考依据。
杨升, 张华新, 陈秋夏, 杨秀艳. 沙枣幼苗根尖离子流对NaCl胁迫的响应. 植物生态学报, 2017, 41(4): 489-496. DOI: 10.17521/cjpe.2016.0091
Sheng YANG, Hua-Xin ZHANG, Qiu-Xia CHEN, Xiu-Yan YANG. Responses of apical ion fluxes to NaCl stress in Elaeagnus angustifolia seedlings. Chinese Journal of Plant Ecology, 2017, 41(4): 489-496. DOI: 10.17521/cjpe.2016.0091
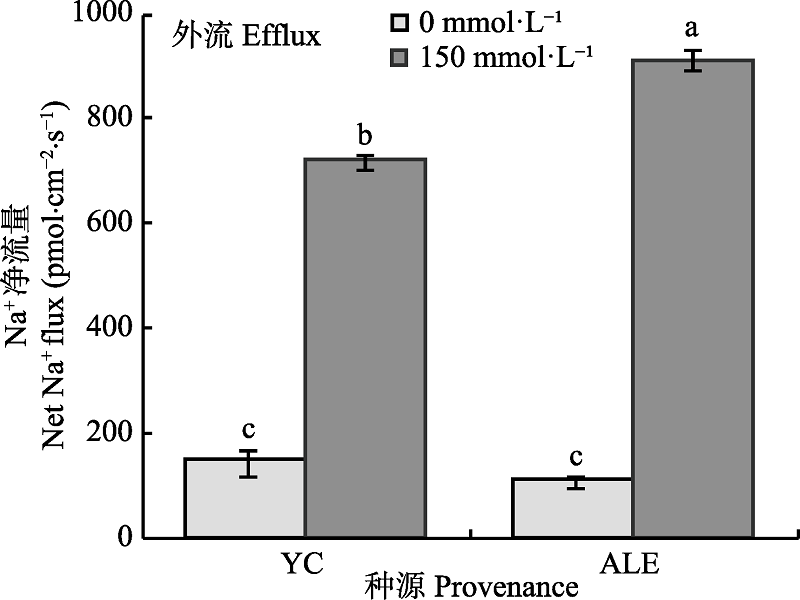
图1 NaCl胁迫对两个种源沙枣幼苗根系稳态Na+流的影响(平均值±标准误差, n = 4)。ALE, 阿拉尔; YC, 银川。不同小写字母表示不同处理和种源间差异显著(p < 0.05)。
Fig. 1 Effects of NaCl stress on steady-state Na+ flux at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences between different treatments and provenances (p < 0.05).
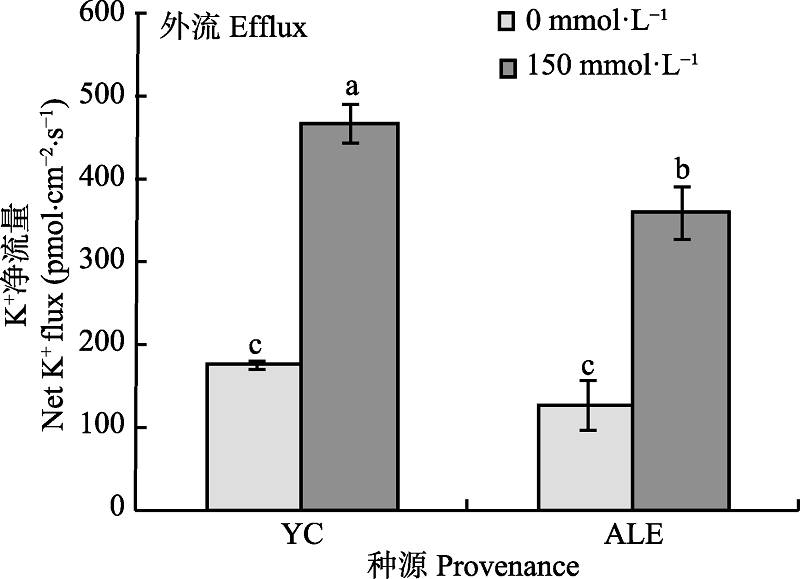
图2 NaCl胁迫对两个种源沙枣幼苗根系稳态K+流的影响(平均值±标准误差, n = 4)。ALE, 阿拉尔; YC, 银川。不同小写字母表示不同处理和种源间差异显著(p < 0.05)。
Fig. 2 Effects of NaCl stress on steady-state K+ flux at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences between different treatments and provenances (p < 0.05).
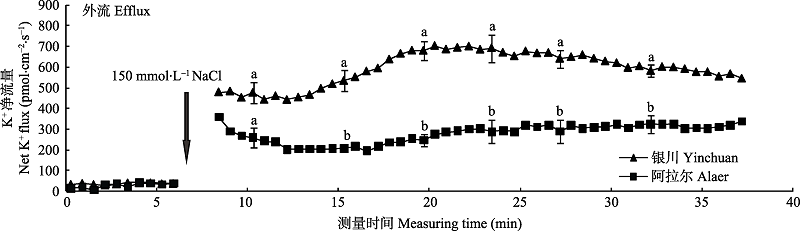
图3 NaCl胁迫诱导两个种源沙枣幼苗根系瞬时K+流的动态差异(平均值±标准误差, n = 4)。不同小写字母表示同一时间点种源间差异显著(p < 0.05)。
Fig. 3 Differences of NaCl stress on transient K+ kinetics at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). Different lowercase letters indicate significant differences between provenances at the same time (p < 0.05).
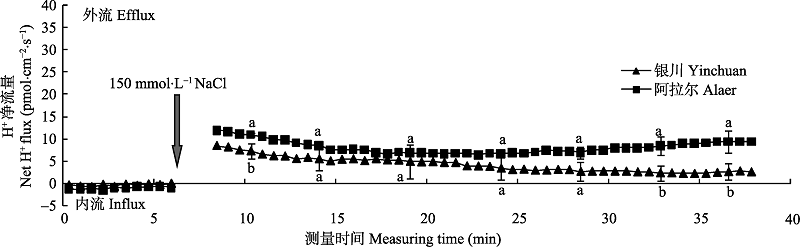
图4 NaCl胁迫诱导两个种源沙枣幼苗根系瞬时H+流的动态差异(平均值±标准误差, n = 4)。不同小写字母表示同一时间点种源间差异显著(p < 0.05)。
Fig. 4 Differences of NaCl stress on transient H+ kinetics at apical regions of two provenances of Elaeagnus angustifolia (mean ± SE, n = 4). Different lowercase letters indicate significant differences between provenances at the same time (p < 0.05).
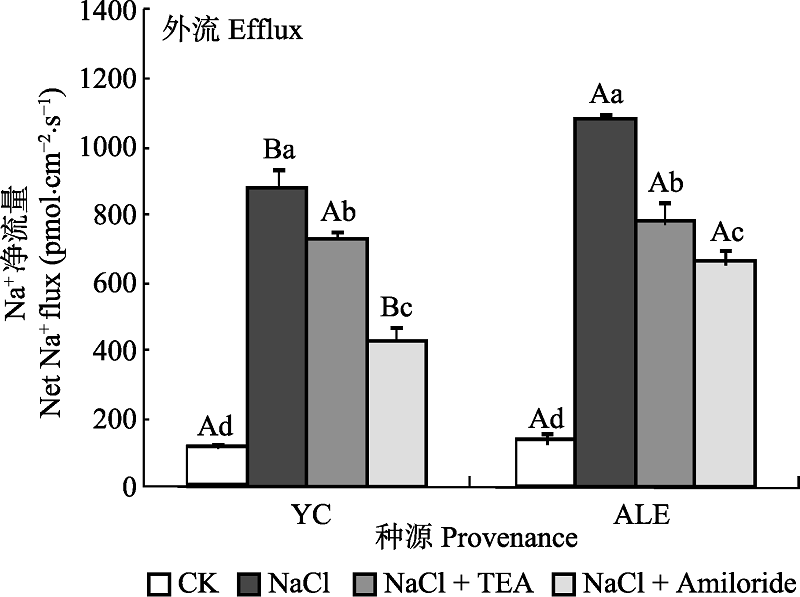
图5 两种抑制剂对NaCl胁迫后两个种源沙枣根系Na+流的影响(平均值±标准误差, n = 4)。ALE, 阿拉尔; YC, 银川。不同小写字母表示同一种源不同处理间差异显著(p < 0.05); 不同大写字母表示相同处理不同种源间差异显著(p < 0.05)。
Fig. 5 Effects of two kinds of inhibitors on net Na+ flux at apical regions of two provenances of Elaeagnus angustifolia treated by NaCl stress (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences among different treatments in the same provenance (p < 0.05), While different capital letters indicate significant difference between provenances at the same treatment (p < 0.05).
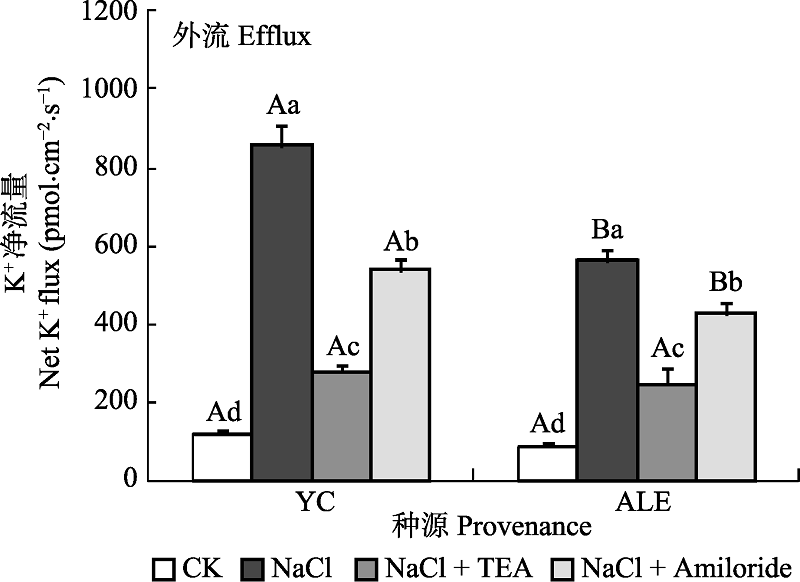
图6 两种抑制剂对NaCl胁迫后两个种源沙枣根系K+流的影响(平均值±标准误差, n = 4)。ALE, 阿拉尔; YC, 银川。不同小写字母表示同一种源不同处理间差异显著(p < 0.05); 不同大写字母表示相同处理不同种源间差异显著(p < 0.05)。
Fig. 6 Effects of two kinds of inhibitors on net K+ flux at apical regions of two provenances of Elaeagnus angustifolia treated by NaCl stress (mean ± SE, n = 4). ALE, Alaer; YC, Yinchuan. Different lowercase letters indicate significant differences among different treatments in the same provenance (p < 0.05), While different capital letters indicate significant difference between provenances at the same treatment (p < 0.05).
| [1] | Britto DT, Kronzucker HJ (2008). Cellular mechanisms of potassium transport in plants.Physiologia Plantarum, 133, 637-650. |
| [2] | Chao DY, Dilkes B, Luo H, Douqlas A, Yakubova E, Lahner B, Salt DE (2013). Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science, 341, 658-659. |
| [3] | Chen GP, Wang HZ, Shi NN, Chen SY (2006). Na+/H+ antiporter and its relationship with plant salt tolerance.China Biotechnology, 26(5), 101-106. (in Chinese with English abstract)[陈观平, 王慧中, 施农农, 陈受宜 (2006). Na+/H+逆向转运蛋白与植物耐盐性的关系研究进展. 中国生物工程杂志, 26(5), 101-106.] |
| [4] | Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005). Screening plants for salt tolerance by measuring K+ flux: A case study for barely.Plant, Cell & Environment, 28, 1230-1246. |
| [5] | Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabala S (2007a). Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley.Plant Physiology, 145, 1714-1725. |
| [6] | Chen Z, Shabala S, Mendham N, Newman I, Zhang GP, Zhou MX (2008). Combining ability of salinity tolerance on the basis of NaCl-induced K flux from roots of barley.Crop Science, 48, 1382-1388. |
| [7] | Chen Z, Zhou M, Newman IA, Mendham NJ, Zhang GP, Shabala S (2007b). Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance.Functional Plant Biology, 34, 150-162. |
| [8] | Coskun D, Britto DT, Jean YK, Kabir I, Tolay I, Torun AA, Kronzucker HJ (2013). K+ efflux and retention in response to NaCl stress do not predict salt tolerance in contrasting genotypes of rice (Oryza sativa L.). PLOS ONE, 8(2), e57767. doi: 10.1371/journal.pone.0057767. |
| [9] | Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008). A root’s ability to retain K+ correlates with salt tolerance in wheat.Journal of Experimental Botany, 59, 2697-2706. |
| [10] | Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S (2011). Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: In planta quantification methods.Plant, Cell & Environment, 34, 947-961. |
| [11] | Cuin TA, Zhou M, Parsons D, Shabala S (2012). Genetic behaviour of physiological traits conferring cytosolic K+/Na+ homeostasis in wheat.Plant Biology, 14, 438-446. |
| [12] | Demidchik V, Maathuis FJM (2007). Physiological roles of nonselective cation channels in plants: From salt stress to signaling and development.New Phytologist, 175, 384-404. |
| [13] | Feki K, Quintero FJ, Khoudi H, Leidi EO, Masmoudi K, Pardo JM, Brini F (2014). A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Reportsl Reports, 33, 277-288. |
| [14] | Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K (2012). Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Molecular Biology, 79, 137-155. |
| [15] | Guan WK, Xu N (2012). Research situation and resources utilization of Elaeagnus angustifolia. Anhui Agricultural Science Bulletin, 18(19), 119-121. (in Chinese with English abstract)[管文轲, 徐娜 (2012). 沙枣资源利用研究与开发现状述评. 安徽农学通报, 18(19), 119-121.] |
| [16] | Guo LJ, Wang YT (2008). Conservation research and prospects of Elaeagnus germplasm resources and utilization values. Chinese Wild Plant Resources, 27(5), 32-34. (in Chinese with English abstract)[郭丽君, 王玉涛 (2008). 沙枣种质资源特性及利用价值. 中国野生植物资源, 27(5), 32-34.] |
| [17] | Kong X, Luo Z, Dong H, Eneji AE, Li W (2012). Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton.Journal of Experimental Botany, 63, 2105-2116. |
| [18] | Liu BY (2007). Study on Ecophysiological Response and Ion Distribution of Elaeagnus angustifolia to Salt Stress. Master degree dissertation, Tianjin Normal University, Tianjin. 27-53. (in Chinese with English abstract)[刘宝玉 (2007). 盐胁迫下沙枣生理生态响应与离子分配研究. 硕士学位论文, 天津师范大学, 天津. 27-53.] |
| [19] | Liu ZX (2013). Physiological Mechanism of Heterogeneous Responses of Elaeagnus angustifolia to NaCl and Na2SO4 Stress. PhD dissertation, Chinese Academy of Forestry, Beijing. 100-104. (in Chinese with English abstract)[刘正祥 (2013). 沙枣对氯化钠和硫酸钠胁迫异质性响应的生理机制. 博士学位论文, 中国林业科学研究院, 北京. 100-104.] |
| [20] | Liu ZX, Zhang HX, Yang XY, Liu T, Di WB (2014). Growth, and cationic absorption, transportation and allocation of Elaeagnus angustifolia seedlings under NaCl stress. Acta Ecologica Sinica, 34, 326-336. (in Chinese with English abstract)[刘正祥, 张华新, 杨秀艳, 刘涛, 狄文彬 (2014). NaCl胁迫下沙枣幼苗生长和阳离子吸收、运输与分配特性. 生态学报, 34, 326-336.] |
| [21] | Ma Q, Bao AK, Wu GQ, Wang SM (2011). Plasma membrane Na+/H+ antiporter is involved in plant salt tolerance.Chinese Bulletin of Botany, 46, 206-215. (in Chinese with English abstract)[马清, 包爱科, 伍国强, 王锁民 (2011). 质膜Na+/H+逆向转运蛋白与植物耐盐性. 植物学报, 46, 206-215.] |
| [22] | Maathuis FJM (2006). The role of monovalent cation transporters in plant responses to salinity.Journal of Experimental Botany, 57, 1137-1147. |
| [23] | Maathuis FJM, Amtmann A (1999). K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios.Annals of Botany, 84, 123-133. |
| [24] | Olias R, Eljakaoui Z, Li JU, Morales PD, Marin-manzano MC, Pardo JM, Belver A (2009). The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs.Plant, Cell & Environment, 32, 904-916. |
| [25] | Rausch T, Kirsch M, Löw R, Lehr A, Viereck R, An ZG (1996). Salt stress responses of higher plants: The role of proton pumps and Na+/H+-antiporters.Journal of Plant Physiology, 148, 425-433. |
| [26] | Shabala S, Cuin TA (2008). Potassium transport and plant salt tolerance.Physiologia Plantarum, 133, 651-669. |
| [27] | Shi HZ, Lee B, Wu SJ, Zhu JK (2003). Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnology, 21, 81-85. |
| [28] | Shi HZ, Ishitani M, Kim C, Zhu JK (2000). The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences of the United States of America, 97, 6896-6901. |
| [29] | Sun J (2011). Signaling Network in the Perception of Salt Stress and Ionic Homeostasis Regulation in Populus euphratica. PhD dissertation, Beijing Forestry University, Beijing. 26-28. (in Chinese with English abstract)[孙健 (2011). 胡杨响应盐胁迫与离子平衡调控信号网络研究. 博士学位论文, 北京林业大学, 北京. 26-28.] |
| [30] | Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009). NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species.Plant Physiology, 149, 1141-1153. |
| [31] | Wegner LH, Stefano G, Shabala L, Rossi M, Mancuso M, Shabala S (2011). Sequential depolarization of root cortical and stelar cells induced by an acute salt shock—Implications for Na+ and K+ transport into xylem vessels. Plant, Cell & Environment, 34, 859-869. |
| [32] | Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong Z (2009). Overexpression of SOS (salt overly sensitive) genes increases salt tolerance in transgenic Arabidopsis. Molecular Plant, 2, 22-31. |
| [33] | Yang S, Liu T, Zhang HX, Li HY, Zhang L (2014). Growth and physiological characteristics of Elaeagnus angustifolia L. under salt stress. Journal of Fujian College of Forestry, 34(1), 64-70. (in Chinese with English abstract)[杨升, 刘涛, 张华新, 李焕勇, 张丽 (2014). 盐胁迫下沙枣幼苗的生长表现和生理特性. 福建林学院学报, 34(1), 64-70.] |
| [34] | Yang S, Zhang HX, Liu T, Wu HW, Yang XY, Ni JW, Chen QX (2016). Study on ion metabolism characteristics of Elaeagnus angustifolia L. seedlings under NaCl stress. Forest Research, 29(1), 140-146. (in Chinese with English abstract)[杨升, 张华新, 刘涛, 武海雯, 杨秀艳, 倪建伟, 陈秋夏 (2016). NaCl胁迫下沙枣幼苗的离子代谢特性. 林业科学研究, 29(1), 140-146.] |
| [35] | Yang S, Zhang HX, Yang XY, Chen QX, Wu HW (2015). Differential growth performance ofElaeagnus angustifolia L. provenances under NaCl stress. Scientia Silvae Sinicae, 51(9), 51-58. (in Chinese with English abstract)[杨升, 张华新, 杨秀艳, 陈秋夏, 武海雯 (2015). NaCl胁迫下不同种源沙枣的生长表现差异. 林业科学, 51(9), 51-58.] |
| [36] | Yue Y, Zhang M, Zhang J, Duan L, Li Z (2012). SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio.Journal of Plant Physiology, 169, 255-261. |
| [37] | Zhang BZ, Cao ZY, Zhao KF (1992). A study on some physiological properties ofElaeagnus angustifolia under salt stress condition. Scientia Silvae Sinicae, 28(2), 187-189. (in Chinese with English abstract)[张宝泽, 曹子谊, 赵可夫 (1992). 盐分胁迫下沙枣某些生理特性的研究. 林业科学, 28(2), 187-189.] |
| [1] | 安凡, 李宝银, 钟全林, 程栋梁, 徐朝斌, 邹宇星, 张雪, 邓兴宇, 林秋燕. 不同种源刨花楠苗木生长与主要功能性状对氮添加的响应[J]. 植物生态学报, 2023, 47(12): 1693-1707. |
| [2] | 张小燕, WEE Kim Shan Alison, KAJITA Tadashi, 曹坤芳. 种源地对两种红树叶片结构和功能的影响: 对温度的适应性遗传[J]. 植物生态学报, 2021, 45(11): 1241-1250. |
| [3] | 韩大勇, 张维, 努尔买买提•依力亚斯, 杨允菲. 植物种群更新的补充限制[J]. 植物生态学报, 2021, 45(1): 1-12. |
| [4] | 纪若璇, 于笑, 常远, 沈超, 白雪卡, 夏新莉, 尹伟伦, 刘超. 蒙古莸叶片解剖结构的地理种源变异及其对环境变化响应的意义[J]. 植物生态学报, 2020, 44(3): 277-286. |
| [5] | 吕晋慧,任磊,李艳锋,王玄,赵夏陆,张春来. 不同基因型茶菊对盐胁迫的响应[J]. 植物生态学报, 2013, 37(7): 656-664. |
| [6] | 王殿, 袁芳, 王宝山, 陈敏. 能源植物杂交狼尾草对NaCl胁迫的响应及其耐盐阈值[J]. 植物生态学报, 2012, 36(6): 572-577. |
| [7] | 王玉猛, 任立飞, 田秋英, 刘洪升, 李凌浩, 张文浩. 根茎在羊草响应短期NaCl胁迫过程中的作用[J]. 植物生态学报, 2006, 30(6): 954-959. |
| [8] | 袁琳, 克热木·伊力, 张利权. NaCl胁迫对阿月浑子实生苗活性氧代谢与细胞膜稳定性的影响[J]. 植物生态学报, 2005, 29(6): 985-991. |
| [9] | 张文辉, 段宝利, 周建云, 刘祥君. 不同种源栓皮栎幼苗叶片水分关系和保护酶活性对干旱胁迫的响应[J]. 植物生态学报, 2004, 28(4): 483-490. |
| [10] | 洪伟, 吴承祯. 杉木种源胸径生长地理变异规律的研究[J]. 植物生态学报, 1998, 22(2): 186-192. |
| [11] | 任文伟, 罗岫泉, 郑师章. 不同种源羊草的SOD、POD的活性及丙二醛含量的比较[J]. 植物生态学报, 1997, 21(1): 77-82. |
| [12] | 唐季林, 徐化成. 油松不同种源在北京地区水势和蒸腾速率的比较研究[J]. 植物生态学报, 1992, 16(2): 97-107. |
| [13] | 俞新妥, 卢建煌, 王锦上. 不同种源马尾松水分生理生态的比较研究[J]. 植物生态学报, 1991, 15(4): 355-365. |
| [14] | 李晓洁, 徐化成. 华山松种子及其发芽习性的地理变异研究[J]. 植物生态学报, 1991, 15(1): 27-35. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()