

Chin J Plant Ecol ›› 2023, Vol. 47 ›› Issue (1): 88-100.DOI: 10.17521/cjpe.2022.0131
Special Issue: 菌根真菌
• Research Articles • Previous Articles Next Articles
ZHANG Hui1,2,3, ZENG Wen-Jing1,2,*( ), GONG Xin-Tao4, MA Ze-Qing1,2,*(
), GONG Xin-Tao4, MA Ze-Qing1,2,*( )
)
Received:2022-04-11
Accepted:2022-05-19
Online:2023-01-20
Published:2022-07-15
Contact:
*ZENG Wen-Jing(zengwj@igsnrr.ac.cn);MA Ze-Qing(mazq@igsnrr.ac.cn)
Supported by:ZHANG Hui, ZENG Wen-Jing, GONG Xin-Tao, MA Ze-Qing. Relationships between root hairs and mycorrhizal fungi across typical subtropical tree species[J]. Chin J Plant Ecol, 2023, 47(1): 88-100.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2022.0131
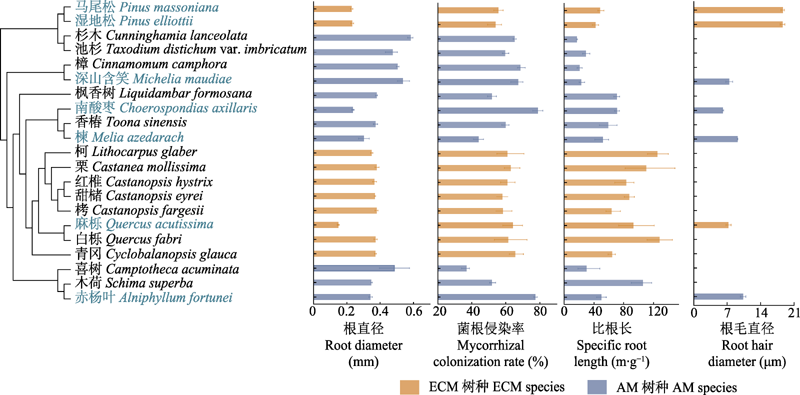
Fig. 1 Phylogeny and variation in traits for 21 typical subtropical tree species (mean ± SE). AM, arbuscular mycorrhiza; ECM, ectomycorrhiza. Names of tree species with root hairs are marked in blue. Root hair diameter was only shown among the seven species with root hairs.
| 物种 Species | 菌根类型 Mycorrhizal type | 根毛出现率 Root hair occurrence rate (%) | 根毛密度 Root hair density (No.·mm-1) | 根毛长度 Root hair length (μm) | 根毛直径 Root hair diameter (μm) |
|---|---|---|---|---|---|
| 深山含笑 Michelia maudiae | AM | 26.97 ± 4.16A | 12.90 ± 1.05A | 20.62 ± 1.43A | 8.32 ± 0.44B |
| 赤杨叶 Alniphyllum fortunei | AM | 51.25 ± 0.85B | 44.30 ± 1.56C | 63.84 ± 4.52C | 10.32 ± 0.36C |
| 南酸枣 Choerospondias axillaris | AM | 58.26 ± 1.85BC | 22.51 ± 1.07B | 36.61 ± 1.59B | 6.09 ± 0.13A |
| 楝 Melia azedarach | AM | 70.35 ± 1.61D | 13.41 ± 1.19A | 43.78 ± 3.18B | 9.71 ± 0.24C |
| 麻栎 Quercus acutissima | ECM | 32.93 ± 3.28A | 15.23 ± 1.00A | 34.42 ± 6.57B | 7.51 ± 0.27B |
| 湿地松 Pinus elliottii | ECM | 61.24 ± 4.09C | 14.09 ± 0.41A | 93.18 ± 2.40D | 19.85 ± 0.34D |
| 马尾松 Pinus massoniana | ECM | 86.27 ± 3.36E | 21.60 ± 1.25B | 161.36 ± 5.68E | 19.99 ± 0.24D |
Table 1 Traits of root hairs in seven typical subtropical tree species (mean ± SE)
| 物种 Species | 菌根类型 Mycorrhizal type | 根毛出现率 Root hair occurrence rate (%) | 根毛密度 Root hair density (No.·mm-1) | 根毛长度 Root hair length (μm) | 根毛直径 Root hair diameter (μm) |
|---|---|---|---|---|---|
| 深山含笑 Michelia maudiae | AM | 26.97 ± 4.16A | 12.90 ± 1.05A | 20.62 ± 1.43A | 8.32 ± 0.44B |
| 赤杨叶 Alniphyllum fortunei | AM | 51.25 ± 0.85B | 44.30 ± 1.56C | 63.84 ± 4.52C | 10.32 ± 0.36C |
| 南酸枣 Choerospondias axillaris | AM | 58.26 ± 1.85BC | 22.51 ± 1.07B | 36.61 ± 1.59B | 6.09 ± 0.13A |
| 楝 Melia azedarach | AM | 70.35 ± 1.61D | 13.41 ± 1.19A | 43.78 ± 3.18B | 9.71 ± 0.24C |
| 麻栎 Quercus acutissima | ECM | 32.93 ± 3.28A | 15.23 ± 1.00A | 34.42 ± 6.57B | 7.51 ± 0.27B |
| 湿地松 Pinus elliottii | ECM | 61.24 ± 4.09C | 14.09 ± 0.41A | 93.18 ± 2.40D | 19.85 ± 0.34D |
| 马尾松 Pinus massoniana | ECM | 86.27 ± 3.36E | 21.60 ± 1.25B | 161.36 ± 5.68E | 19.99 ± 0.24D |
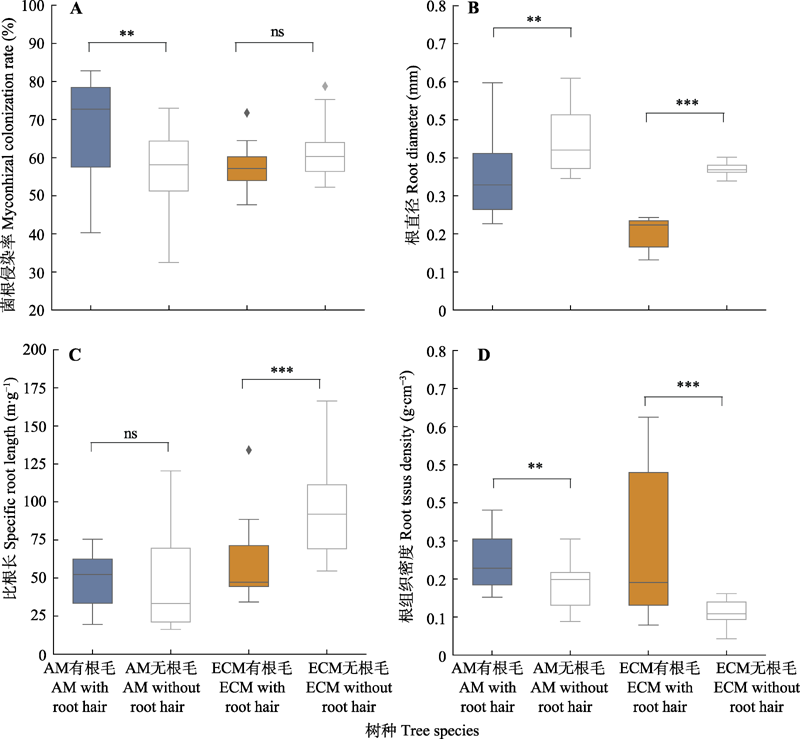
Fig. 3 Comparisons of mycorrhizal colonization rate and absorptive fine root properties (mean ± SE) between typical subtropical tree species with and without root hairs. AM, arbuscular mycorrhiza; ECM, ectomycorrhiza. ns, p > 0.05; **, p < 0.01; ***, p < 0.001.
| 属性 Trait | 最小值 Min | 最大值 Max | 平均数 Mean | 标准误 SE | 变异系数 CV (%) | Blomberg’s K |
|---|---|---|---|---|---|---|
| 根毛出现率 Root hair occurrence rate (%) | 26.97 | 86.30 | 58.26 | 7.79 | 37.25 | 0.29 |
| 根毛密度 Root hair density (No.·mm-1) | 12.90 | 44.30 | 20.58 | 4.22 | 54.28 | 0.40 |
| 根毛长度 Root hair length (μm) | 20.62 | 161.36 | 64.83 | 18.41 | 75.15 | 0.28 |
| 根毛直径 Root hair diameter (μm) | 6.09 | 19.99 | 11.68 | 2.19 | 49.70 | 2.67* |
| 根直径 Root diameter (mm) | 0.15 | 0.54 | 0.29 | 0.05 | 43.10 | 0.66** |
| 比根长 Specific root length (m·g-1) | 23.02 | 83.00 | 52.76 | 7.35 | 55.06 | 0.47 |
| 根组织密度 Root tissue density (g·cm-3) | 0.11 | 0.53 | 0.26 | 0.05 | 53.26 | 0.09 |
| 菌根侵染率 Mycorrhizal colonization rate (%) | 43.99 | 78.88 | 63.10 | 4.82 | 20.22 | 0.47 |
Table 2 Summary of root hair traits, root traits and phylogenetic signals (Blomberg’s K) for seven typical subtropical tree species
| 属性 Trait | 最小值 Min | 最大值 Max | 平均数 Mean | 标准误 SE | 变异系数 CV (%) | Blomberg’s K |
|---|---|---|---|---|---|---|
| 根毛出现率 Root hair occurrence rate (%) | 26.97 | 86.30 | 58.26 | 7.79 | 37.25 | 0.29 |
| 根毛密度 Root hair density (No.·mm-1) | 12.90 | 44.30 | 20.58 | 4.22 | 54.28 | 0.40 |
| 根毛长度 Root hair length (μm) | 20.62 | 161.36 | 64.83 | 18.41 | 75.15 | 0.28 |
| 根毛直径 Root hair diameter (μm) | 6.09 | 19.99 | 11.68 | 2.19 | 49.70 | 2.67* |
| 根直径 Root diameter (mm) | 0.15 | 0.54 | 0.29 | 0.05 | 43.10 | 0.66** |
| 比根长 Specific root length (m·g-1) | 23.02 | 83.00 | 52.76 | 7.35 | 55.06 | 0.47 |
| 根组织密度 Root tissue density (g·cm-3) | 0.11 | 0.53 | 0.26 | 0.05 | 53.26 | 0.09 |
| 菌根侵染率 Mycorrhizal colonization rate (%) | 43.99 | 78.88 | 63.10 | 4.82 | 20.22 | 0.47 |
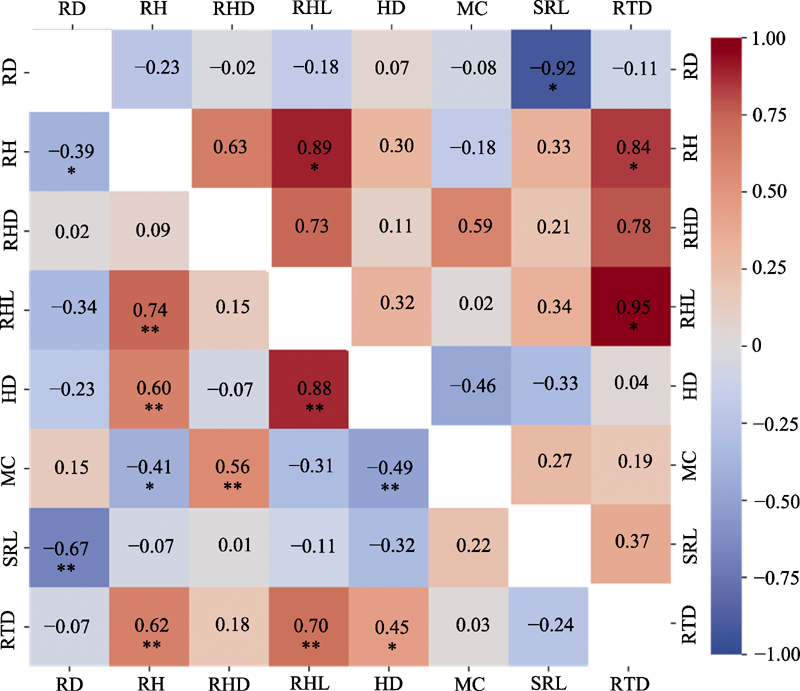
Fig. 4 Pearson correlation coefficients between root hair traits and root traits of seven typical subtropical tree species. HD, root hair diameter; MC, mycorrhizal colonization rate; RD, root diameter; RH, root hair occurrence rate; RHD, root hair density; RHL, root hair length; RTD, root tissue density; SRL, specific root length. Pearson correlation coefficients (below the diagonal) and phylogenetically independent contrasts (above the diagonal) among seven functional traits for seven root hair tree species (n = 28; *, p < 0.05; **, p < 0.01).
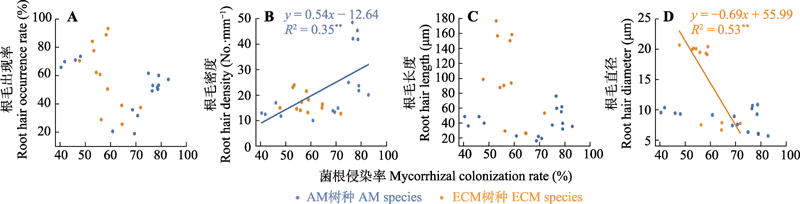
Fig. 5 Correlation between root hair occurrence rate (A), root hair density (B), root hair length (C), root hair diameter (D) and mycorrhizal colonization rate of typical subtropical tree species. AM, arbuscular mycorrhiza (4 species × 4 replicates = 16 points); ECM, ectomycorrhiza (3 species × 4 replicates = 12 points).
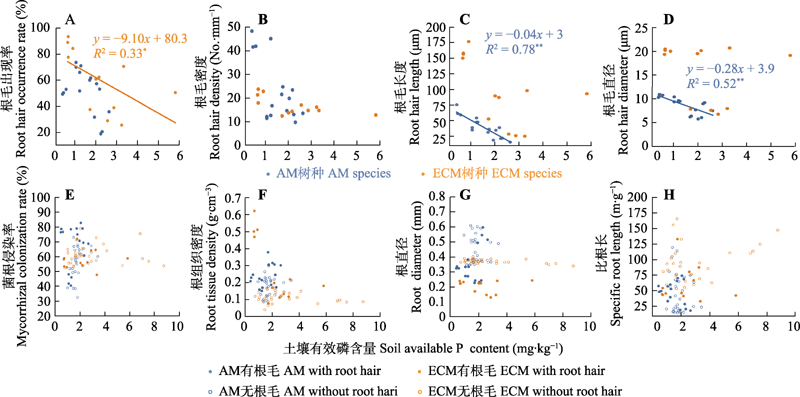
Fig. 6 Correlations of soil available phosphorus (P) content with root hair traits (A-D), mycorrhizal colonization rate (E) and root traits (F-H) of typical subtropical tree species. AM, arbuscular mycorrhiza; ECM, ectomycorrhiz. Relationships between root hair traits and soil available P content were based on data from seven tree species with root hairs (4 AM species × 4 replicates + 3 ECM species × 4 replicates = 28 points). Relationships between mycorrhizal colonization rate, root attributes and soil available P content were based on data of 21 typical subtropical tree species (7 tree species with root hairs × 4 replicates + 14 species without root hairs × 4 replicates = 84 points).
| [1] |
Antunes PM, Lehmann A, Hart MM, Baumecker M, Rillig MC (2012). Long-term effects of soil nutrient deficiency on arbuscular mycorrhizal communities. Functional Ecology, 26, 532-540.
DOI URL |
| [2] | Baylis G (1975). The magnolioid mycorrhiza and mycotrophy in root systems derived from it//Sanders FE, Mosse B, Tinker PB. Endomycorrhizas. Academic Press, New York. 373-389. |
| [3] | Bates TR, Lynch JP (1996). Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell & Environment, 19, 529-538. |
| [4] |
Bichara S, Mazzafera P, de Andrade SAL, (2021). Root morphological changes in response to low phosphorus concentration in eucalypt species. Trees, 35, 1933-1943.
DOI |
| [5] |
Blomberg SP, Garland T, Ives AR (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57, 717-745.
DOI PMID |
| [6] |
Boilard G, Bradley RL, Paterson E, Sim A, Brown LK, George TS, Bainard L, Carubba A (2019). Interaction between root hairs and soil phosphorus on rhizosphere priming of soil organic matter. Soil Biology & Biochemistry, 135, 264-266.
DOI URL |
| [7] |
Brown LK, George TS, Barrett GE, Hubbard SF, White PJ (2013). Interactions between root hair length and arbuscular mycorrhizal colonisation in phosphorus deficient barley (Hordeum vulgare). Plant and Soil, 372, 195-205.
DOI URL |
| [8] |
Brown LK, George TS, Thompson JA, Wright G, Lyon J, Dupuy L, Hubbard SF, White PJ (2012). What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Annals of Botany, 110, 319-328.
DOI PMID |
| [9] | Brundrett M (1991). Mycorrhizas in natural ecosystems. Advances in Ecological Research, 21, 171-313. |
| [10] |
Chen HY, Quan WX, Liu HY, Ding GJ (2022). Effects of Suillus luteus and S. bovinus on the physiological response and nutrient absorption of Pinus massoniana seedlings under phosphorus deficiency. Plant and Soil, 471, 577-590.
DOI |
| [11] |
Chen WL, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016). Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proceedings of the National Academy of Sciences of the United States of America, 113, 8741-8746.
DOI PMID |
| [12] |
da Silva RC, Rondina ABL, Zangaro W, Oliveira HC (2021). Inorganic nitrogen sources alter the root morphology of neotropical tree seedlings from different successional groups. Trees, 35, 875-887.
DOI |
| [13] |
Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tam T, Vijayakumar P, Dolan L (2011). Root hairs: development, growth and evolution at the plant-soil interface. Plant and Soil, 346, 1-14.
DOI URL |
| [14] | Dolan L (2017). Root hair development in grasses and cereals (Poaceae). Current Opinion in Genetics & Development, 45, 76-81. |
| [15] |
Erktan A, Roumet C, Munoz F (2022). Dissecting fine root diameter distribution at the community level captures root morphological diversity. Oikos, 2022, e08907. DOI: 10.1111/oik.08907.
DOI |
| [16] |
Felsenstein J (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1-15.
DOI URL |
| [17] | Fitter AH (2004). Magnolioid roots-hairs, architecture and mycorrhizal dependency. New Phytologist, 64, 15-16. |
| [18] |
Gahoonia TS, Nielsen NE (2004). Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant and Soil, 262, 55-62.
DOI URL |
| [19] | Grierson C, Schiefelbein J (2002). Root hairs//The American Society of Plant Biologists. The Arabidopsis Book. The American Society of Plant Biologists, Rockville, USA. 1-22. |
| [20] |
Guo DL, Li H, Mitchell RJ, Han WX, Hendricks JJ, Fahey TJ, Hendrick RL (2008a). Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytologist, 177, 443-456.
DOI URL |
| [21] |
Guo DL, Xia MX, Wei X, Chang WJ, Liu Y, Wang ZQ (2008b). Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist, 180, 673-683.
DOI URL |
| [22] |
Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS (2013). Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany, 64, 3711-3721.
DOI PMID |
| [23] |
Hajek P, Hertel D, Leuschner C (2013). Intraspecific variation in root and leaf traits and leaf-root trait linkages in eight aspen demes (Populus tremula and P. tremuloides). Frontiers in Plant Science, 4, 415. DOI: 10.3389/fpls.2013.00415.
DOI |
| [24] |
Hou EQ, Luo YQ, Kuang YW, Chen CR, Lu XK, Jiang LF, Luo XZ, Wen DZ (2020). Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nature Communications, 11, 637. DOI: 10.1038/s41467-020-14492-w.
DOI |
| [25] | Jakobsen I, Chen BD, Munkvold L, Lundsgaard T, Zhu YG (2005). Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant, Cell & Environment, 28, 928-938. |
| [26] |
Jaunin F, Hofer RM (1986). Root hair formation and elongation of primary maize roots. Physiologia Plantarum, 68, 653-656.
DOI URL |
| [27] | Kim CM, Dolan L (2016). Correction: root hair defective six-like class I genes promote root hair development in the grass Brachypodium distachyon. PLoS Genetics, 12, 1-18. |
| [28] |
Li T, Lin G, Zhang X, Chen YL, Zhang SB, Chen BD (2014). Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza, 24, 595-602.
DOI PMID |
| [29] |
Liu BT, Li HB, Zhu B, Koide RT, Eissenstat DM, Guo DL (2015). Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytologist, 208, 125-136.
DOI PMID |
| [30] |
Lynch JP (2019). Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytologist, 223, 548-564.
DOI PMID |
| [31] |
Lynch JP, Strock CF, Schneider HM, Sidhu JS, Ajmera I, Galindo-Castañeda T, Klein SP, Hanlon MT (2021). Root anatomy and soil resource capture. Plant and Soil, 466, 21-63.
DOI |
| [32] |
Maherali H (2014). Is there an association between root architecture and mycorrhizal growth response? New Phytologist, 204, 192-200.
DOI PMID |
| [33] |
Ma XM, Li XL, Ludewig U (2021). Arbuscular mycorrhizal colonization outcompetes root hairs in maize under low phosphorus availability. Annals of Botany, 127, 155-166.
DOI PMID |
| [34] | Ma ZQ, Bielenberg DG, Brown KM, Lynch JP (2001). Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell & Environment, 24, 459-467. |
| [35] |
Ma ZQ, Guo DL, Xu XL, Lu MZ, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO (2018). Evolutionary history resolves global organization of root functional traits. Nature, 555, 94-97.
DOI URL |
| [36] |
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo DL, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppälammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, et al. (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytologist, 207, 505-518.
DOI PMID |
| [37] |
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990). A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist, 115, 495-501.
DOI PMID |
| [38] |
Mercado-Blanco J, Prieto P (2012). Bacterial endophytes and root hairs. Plant and Soil, 361, 301-306.
DOI URL |
| [39] |
Miguel MA, Postma JA, Lynch JP (2015). Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiology, 167, 1430-1439.
DOI PMID |
| [40] | Mousa WK, Shearer C, Limay-Rios V, Ettinger CL, Eisen JA, Raizada MN (2016). Root-hair endophyte stacking in finger millet creates a physicochemical barrier to trap the fungal pathogen Fusarium graminearum. Nature Microbiology, 1, 161-167. |
| [41] |
Nestler J, Keyes SD, Wissuwa M (2016). Root hair formation in rice (Oryza sativa L.) differs between root types and is altered in artificial growth conditions. Journal of Experimental Botany, 67, 3699-3708.
DOI URL |
| [42] | Novero M, Genre A, Szczyglowski K, Bonfante P (2008). Root hair colonization by mycorrhizal fungi//Emons AMC, Ketelaar T. Root Hairs. Springer, Berlin. 315-338. |
| [43] |
Orfanoudakis M, Wheeler CT, Hooker JE (2010). Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. . Mycorrhiza, 20, 117-126.
DOI PMID |
| [44] |
Peretto R, Bonfante P, Bettini V, Favaron F, Alghisi P (1995). Polygalacturonase activity and location in arbuscular mycorrhizal roots of Allium porrum L. Mycorrhiza, 5, 157-163.
DOI URL |
| [45] |
Peterson RL, Farquhar ML (1996). Root hairs: specialized tubular cells extending root surfaces. The Botanical Review, 62, 1-40.
DOI URL |
| [46] |
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002). Fine root architecture of nine North American trees. Ecological Monographs, 72, 293-309.
DOI URL |
| [47] |
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil, 321, 305-339.
DOI URL |
| [48] |
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011). Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil, 349, 121-156.
DOI URL |
| [49] |
Rigas S, Ditengou FA, Ljung K, Daras G, Tietz O, Palme K, Hatzopoulos P (2013). Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytologist, 197, 1130-1141.
DOI URL |
| [50] |
Robertson-Albertyn S, Alegria Terrazas R, Balbirnie K, Blank M, Janiak A, Szarejko I, Chmielewska B, Karcz J, Morris J, Hedley PE, George TS, Bulgarelli D (2017). Root hair mutations displace the barley rhizosphere microbiota. Frontiers in Plant Science, 8, 1094. DOI: 10.3389/fpls.2017.01094.
DOI |
| [51] |
Rondina ABL, Tonon BC, Lescano LEAM, Hungria M, Nogueira MA, Zangaro W (2019). Plants of distinct successional stages have different strategies for nutrient acquisition in an Atlantic rain forest ecosystem. International Journal of Plant Sciences, 180, 186-199.
DOI URL |
| [52] |
Ruiz S, Koebernick N, Duncan S, Fletcher DM, Scotson C, Boghi A, Marin M, Bengough AG, George TS, Brown LK, Hallett PD, Roose T (2020). Significance of root hairs at the field scale—Modelling root water and phosphorus uptake under different field conditions. Plant and Soil, 447, 281-304.
DOI PMID |
| [53] |
Salazar-Henao JE, Vélez-Bermúdez IC, Schmidt W (2016). The regulation and plasticity of root hair patterning and morphogenesis. Development, 143, 1848-1858.
DOI PMID |
| [54] |
Schweiger PF, Robson AD, Barrow NJ (1995). Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytologist, 131, 247-254.
DOI URL |
| [55] | Smith SE, Read DJ (2008). Mycorrhizal Symbiosis. 3rd ed. Academic Press, London. |
| [56] |
Smith SE, Smith FA (2012). Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia, 104, 1-13.
DOI PMID |
| [57] |
Tobner CM, Paquette A, Messier C (2013). Interspecific coordination and intraspecific plasticity of fine root traits in North American temperate tree species. Frontiers in Plant Science, 4, 242. DOI: 10.3389/fpls.2013.00242.
DOI |
| [58] |
Tominaga-Wada R, Wada T (2014). Regulation of root hair cell differentiation by R3 MYB transcription factors in tomato and Arabidopsis. Frontiers in Plant Science, 5, 91. DOI: 10.3389/fpls.2014.00091.
DOI |
| [59] |
Turner BL, Brenes-Arguedas T, Condit R (2018). Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature, 555, 367-370.
DOI URL |
| [60] |
Wang YS, Thorup-Kristensen K, Jensen LS, Magid J (2016). Vigorous root growth is a better indicator of early nutrient uptake than root hair traits in Spring wheat grown under low fertility. Frontiers in Plant Science, 7, 865. DOI: 10.3339/fpls.2016.00865.
DOI |
| [61] |
Wang YD, Wang ZL, Wang HM, Guo CC, Bao WK (2012). Rainfall pulse primarily drives litterfall respiration and its contribution to soil respiration in a young exotic pine plantation in subtropical China. Canadian Journal of Forest Research, 42, 657-666.
DOI URL |
| [62] | Wolf AM, Beegle DB (2011). Recommended soil tests for macronutrients//Sims JT, Wolf AM. Recommended Soil Testing Procedures for the Northeastern United States. 3rd ed. Agricultural Experiment Station, University of Delaware, Newark, USA. 39-47. |
| [63] |
Wu QS, Liu CY, Zhang DJ, Zou YN, He XH, Wu QH (2016). Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza, 26, 237-247.
DOI URL |
| [64] | Yu JD, Li Y, Yin DY, Zhou CF, Ma XQ (2017). Response and physiological mechanism of Chinese fir to low phosphorus stress. Forest Research, 30, 566-575. |
| [ 于姣妲, 李莹, 殷丹阳, 周垂帆, 马祥庆 (2017). 杉木对低磷胁迫的响应和生理适应机制. 林业科学研究, 30, 566-575.] | |
| [65] |
Zangaro W, de Almeida Alves R, de Souza PB, Rostirola LV, Lescano LEAM, Rondina ABL, Nogueira MA (2014). Succession and environmental variation influence soil exploration potential by fine roots and mycorrhizal fungi in an Atlantic ecosystem in southern Brazil. Journal of Tropical Ecology, 30, 237-248.
DOI URL |
| [66] |
Zhang CY, Simpson RJ, Kim CM, Warthmann N, Delhaize E, Dolan L, Byrne ME, Wu Y, Ryan PR (2018). Do longer root hairs improve phosphorus uptake? Testing the hypothesis with transgenic Brachypodium distachyon lines overexpressing endogenous RSL genes. New Phytologist, 217, 1654-1666.
DOI URL |
| [67] |
Zhu JM, Zhang CC, Lynch JP (2010). The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology, 37, 313-323.
DOI URL |
| [1] | LIANG Shi-Chu, ZHANG Shu-Min, YU Fei-Hai, DONG Ming. SMALL-SCALE SPATIAL CROSS-CORRELATION BETWEEN RAMET POPULATION VARIABLES OF POTENTILLA REPTANS VAR. SERICOPHYLLA AND SOIL AVAILABLE PHOSPHORUS [J]. Chin J Plant Ecol, 2007, 31(4): 613-618. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn