

Chin J Plant Ecol ›› 2023, Vol. 47 ›› Issue (3): 331-347.DOI: 10.17521/cjpe.2021.0452
Special Issue: 光合作用
• Research Articles • Previous Articles Next Articles
ZHANG Jin-Yan, CUN Zhu, SHUANG Sheng-Pu, HONG Jie, MENG Zhen-Gui, CHEN Jun-Wen( )
)
Received:2021-12-06
Accepted:2022-05-20
Online:2023-03-20
Published:2023-02-28
Contact:
CHEN Jun-Wen
Supported by:ZHANG Jin-Yan, CUN Zhu, SHUANG Sheng-Pu, HONG Jie, MENG Zhen-Gui, CHEN Jun-Wen. Steady-state and dynamic photosynthetic characteristics of shade-tolerant species Panax notoginseng in response to nitrogen levels[J]. Chin J Plant Ecol, 2023, 47(3): 331-347.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2021.0452
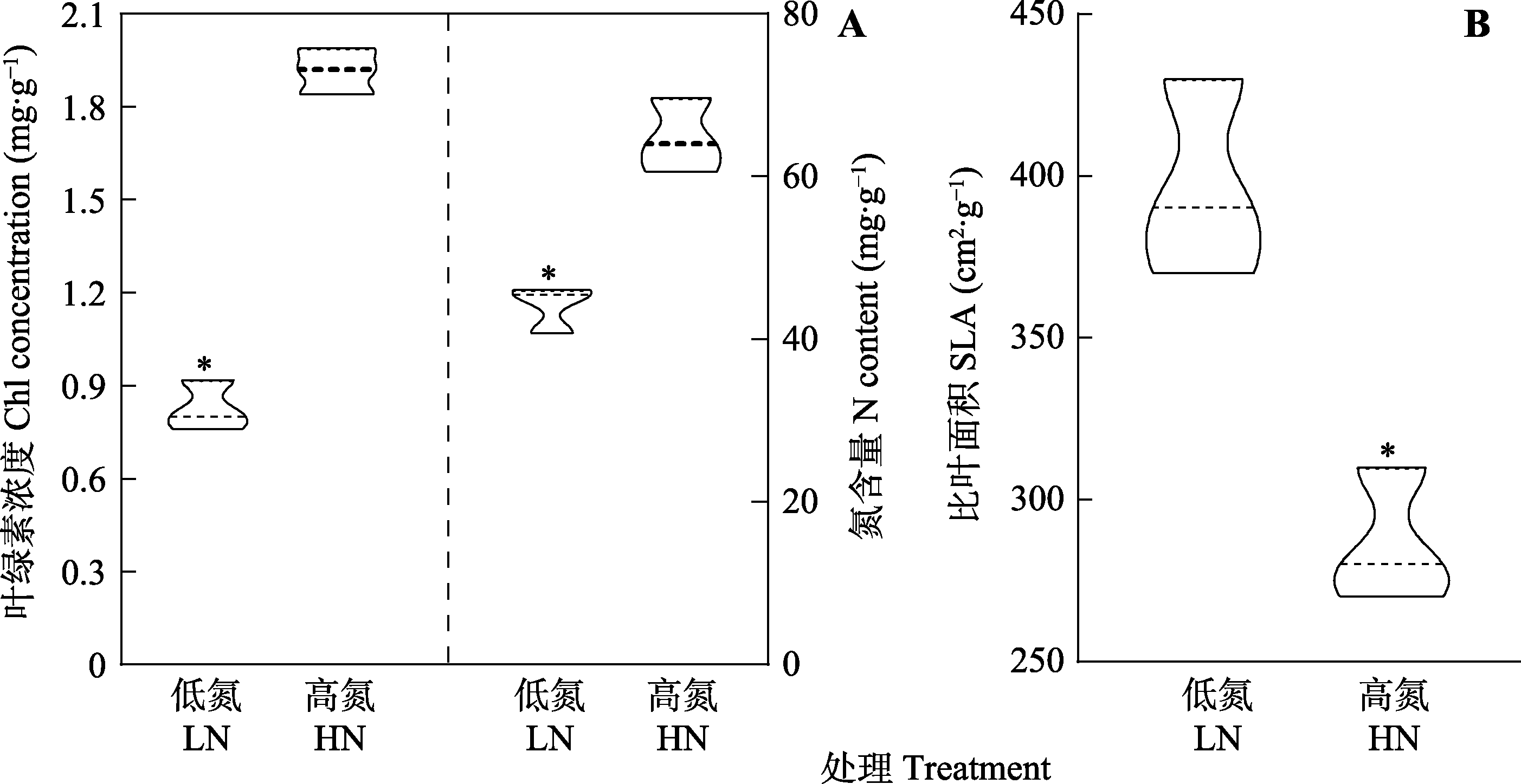
Fig. 1 Leaf nitrogen (N) content, chlorophyll (Chl) concentration and specific leaf area (SLA) of Panax notoginseng at different N levels. HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two N treatments (p < 0.05).
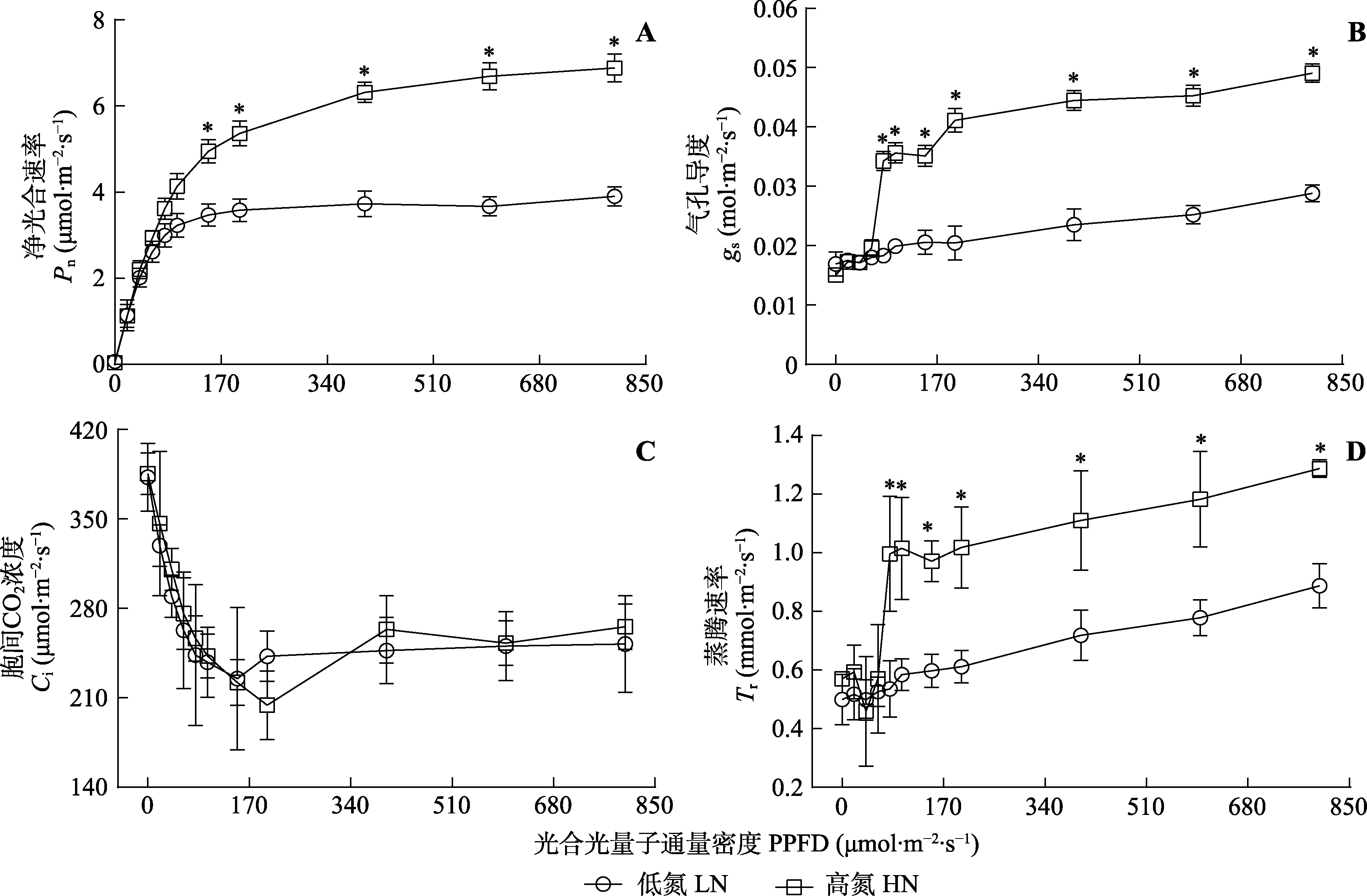
Fig. 2 Net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) of Panax notoginseng in response to photosynthetic photon flux density (PPFD) at two different nitrogen levels (mean ± SD). HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two nitrogen treatments (p < 0.05).
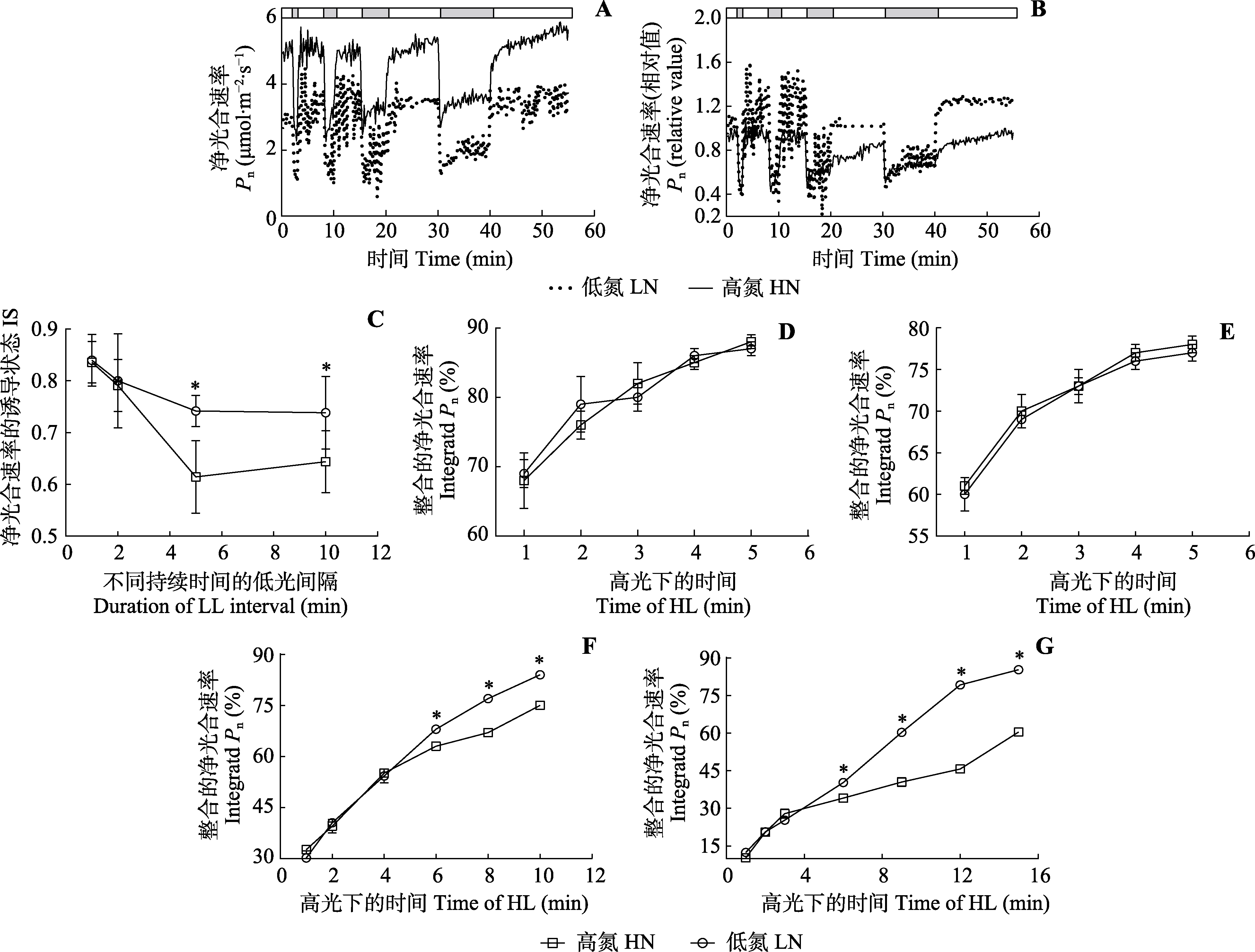
Fig. 3 Gas exchange parameters of leaves of Panax notoginseng under irregular dynamic light condition (mean ± SD). A, B, Net photosynthetic rates (Pn) under low nitrogen (LN) and high nitrogen (HN) conditions. The bars at the top of the figures show the net photosynthetic rates under high light (HL, 800 μmol·m-2·s-1, white) and low light (LL, 50 μmol·m-2·s-1, grey) conditions. The leaves were exposed to high light for 20-40 min until the net photosynthetic rates stabilized. After that, the leaves were exposed to changing light conditions. The grey bars from left to right represent low light at 60, 120, 300 and 600 s. The white bars from left to right represent high light at 300, 300, 600 and 900 s. C, Induction state of net photosynthetic rates (IS) after low light intervals of different durations. D-G, Integrated net photosynthetic rates during high light period after 60, 120, 300 and 600 s low light intervals. * indicates significant differences between the two nitrogen treatments (p < 0.05).
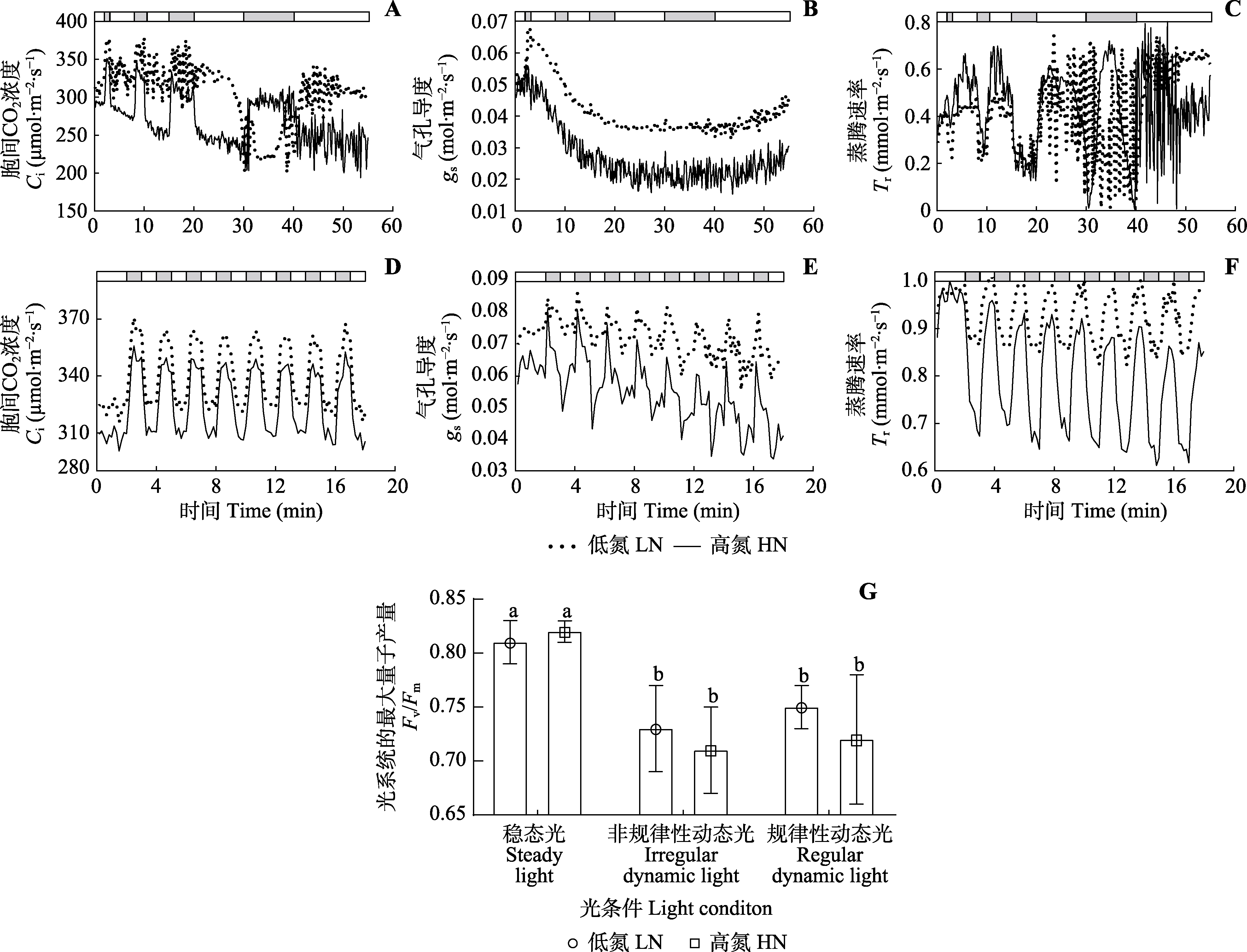
Fig. 4 Intercellular CO2 concentration (Ci), stomatal conductance (gs) and transpiration rate (Tr) under two dynamic light conditions, and photosystem II photoinhibition induced by dynamic light of Panax notoginseng (mean ± SD). The bars at the top of A?F show the high light (800 μmol·m?2·s?1, white) and low light (50 μmol·m?2·s?1, grey) periods. Fv/Fm, maximum photochemistry efficiency of photosystem II; HN, high nitrogen; LN, low nitrogen. Different lowercase letters in G indicate significant differences (p < 0.05).
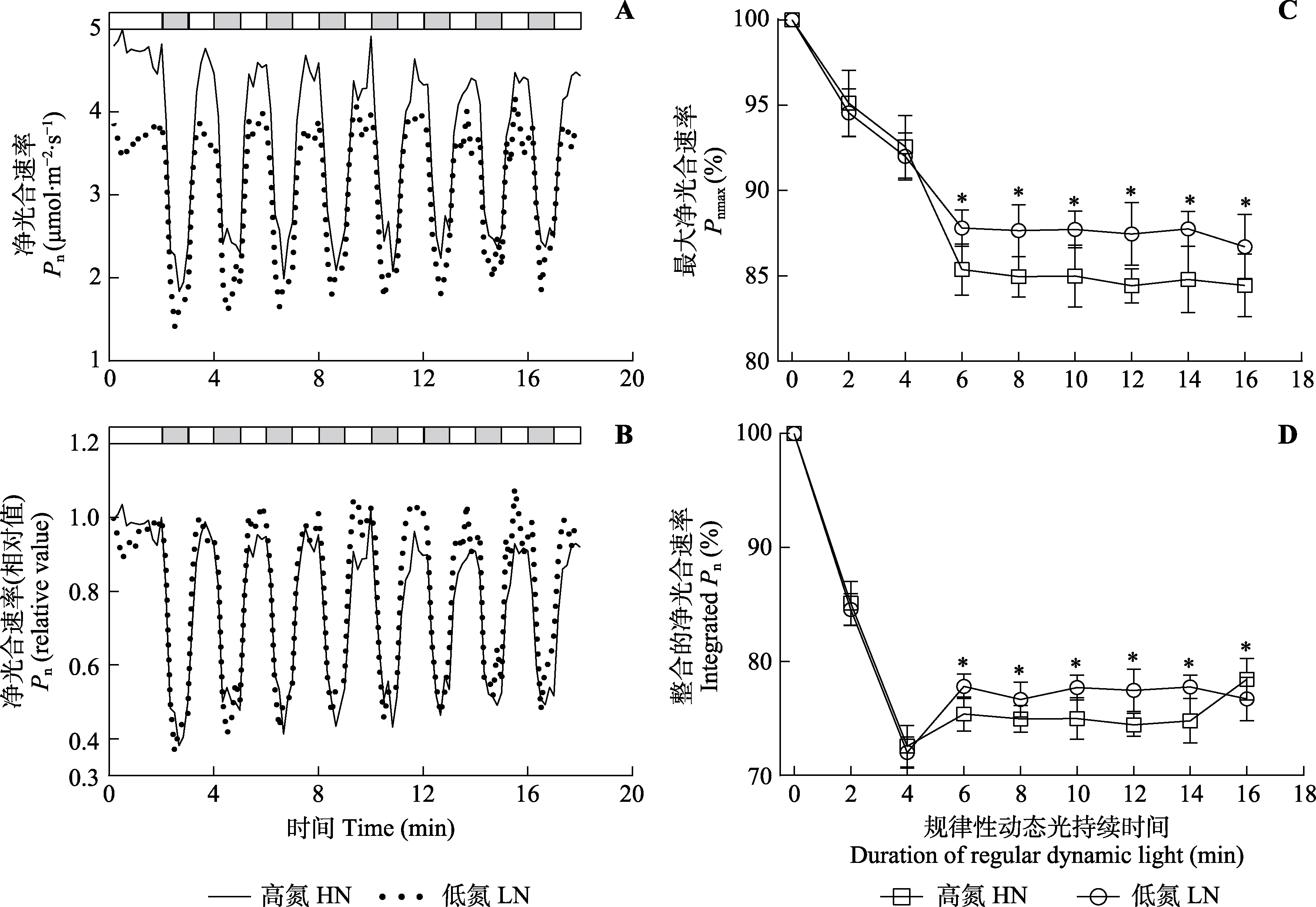
Fig. 5 Gas exchange parameters of Panax notoginseng under regular dynamic light condition (mean ± SD). In A and B, after photosynthetic rate was stabilized, the light intensity alternated between high light (HL, 800 μmol·m-2·s-1, white) and low light (LL, 50 μmol·m-2·s-1, grey) every 60 s under fluctuating light. In C, net photosynthetic rate (Pn) under steady-state high light was 100%, net photosynthetic rate under fluctuating light was the percentage of net photosynthetic rate under steady-state high light. Pnmax, maximum net photosynthetic rate. HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two nitrogen treatments (p < 0.05).
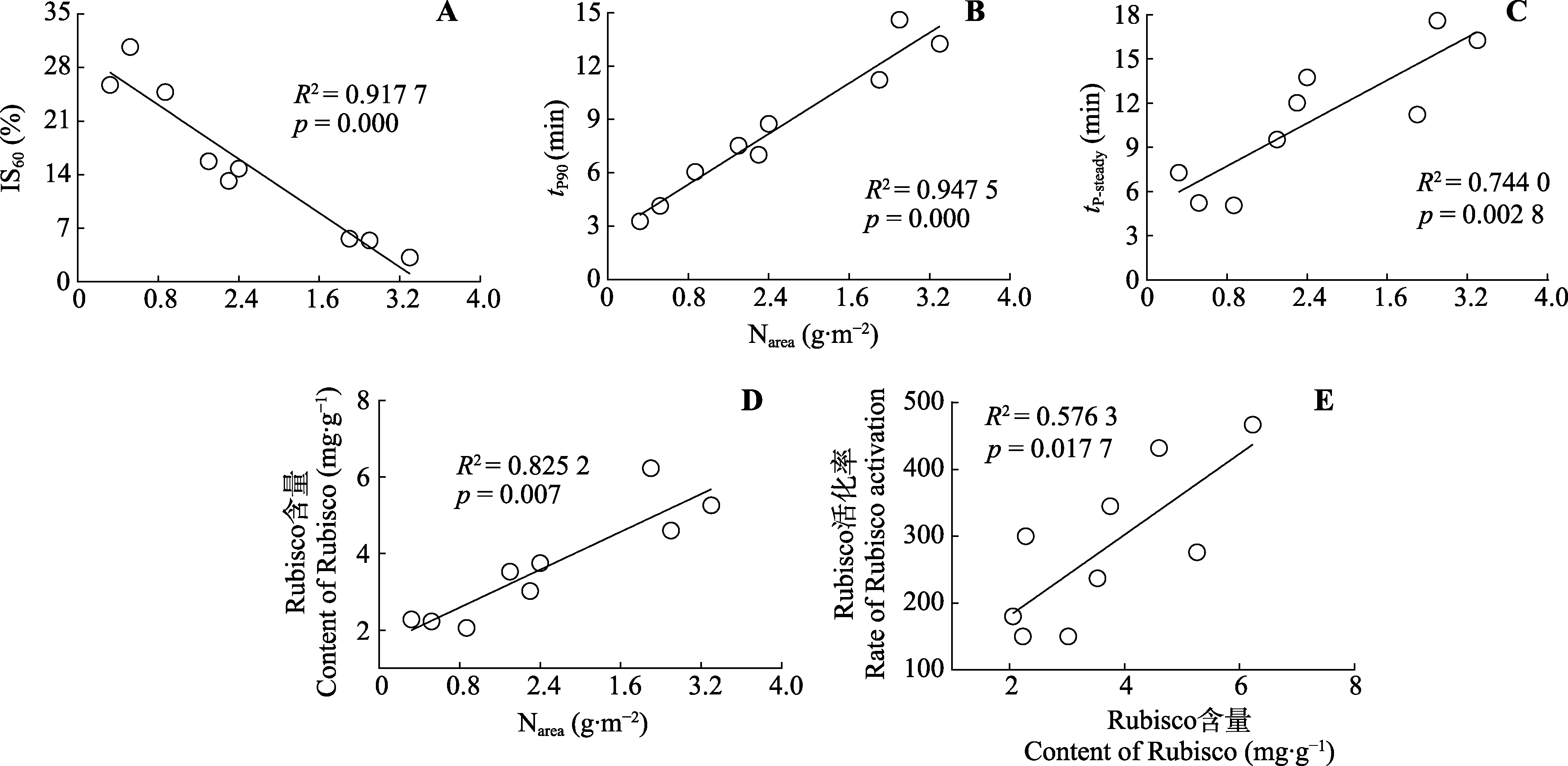
Fig. 6 Correlation between gas exchange parameters of Panax notoginseng. IS60, induction state at 60 s of light; Narea, nitrogen content per unit leaf area; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; tP90, time required to reach 90% of photosynthetic steady state; tP-steady, time required to reach 100% of photosynthetic steady state.
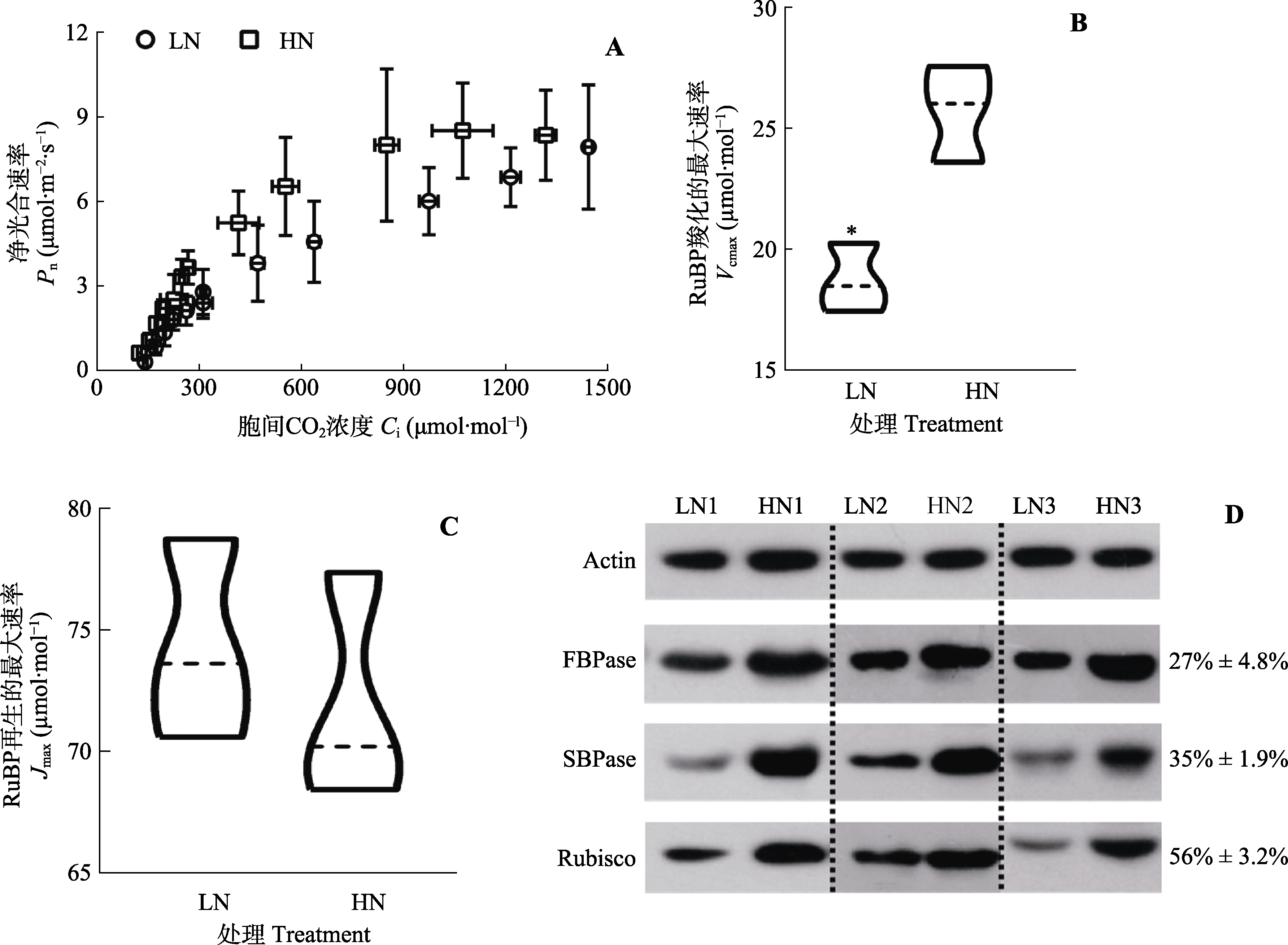
Fig. 7 Ribulose 1,5-bisphosphate carboxylation (RuBP) and regeneration capacity of Panax notoginseng leaves exposed to low-nitrogen (LN) and high-nitrogen (HN). D, Immunoblot analysis of 1,5-bisphosphate carboxylase (Rubisco), scenedesmus heptulose-1,7-bisphosphatase (SBPase) and fructose-1,6-bisphosphatase (FBPase), with the numbers on the right indicates the percentage of protein content of low-nitrogen leaves as a percentage of protein content of high-nitrogen leaves. Ci, intercellular CO2 concentration; Jmax, maximum rate of RuBP-regeneration; Pn, net photosynthetic rate; Vcmax, maximum carboxylation efficiency. * indicates significant differences between the two nitrogen treatments (p < 0.05).
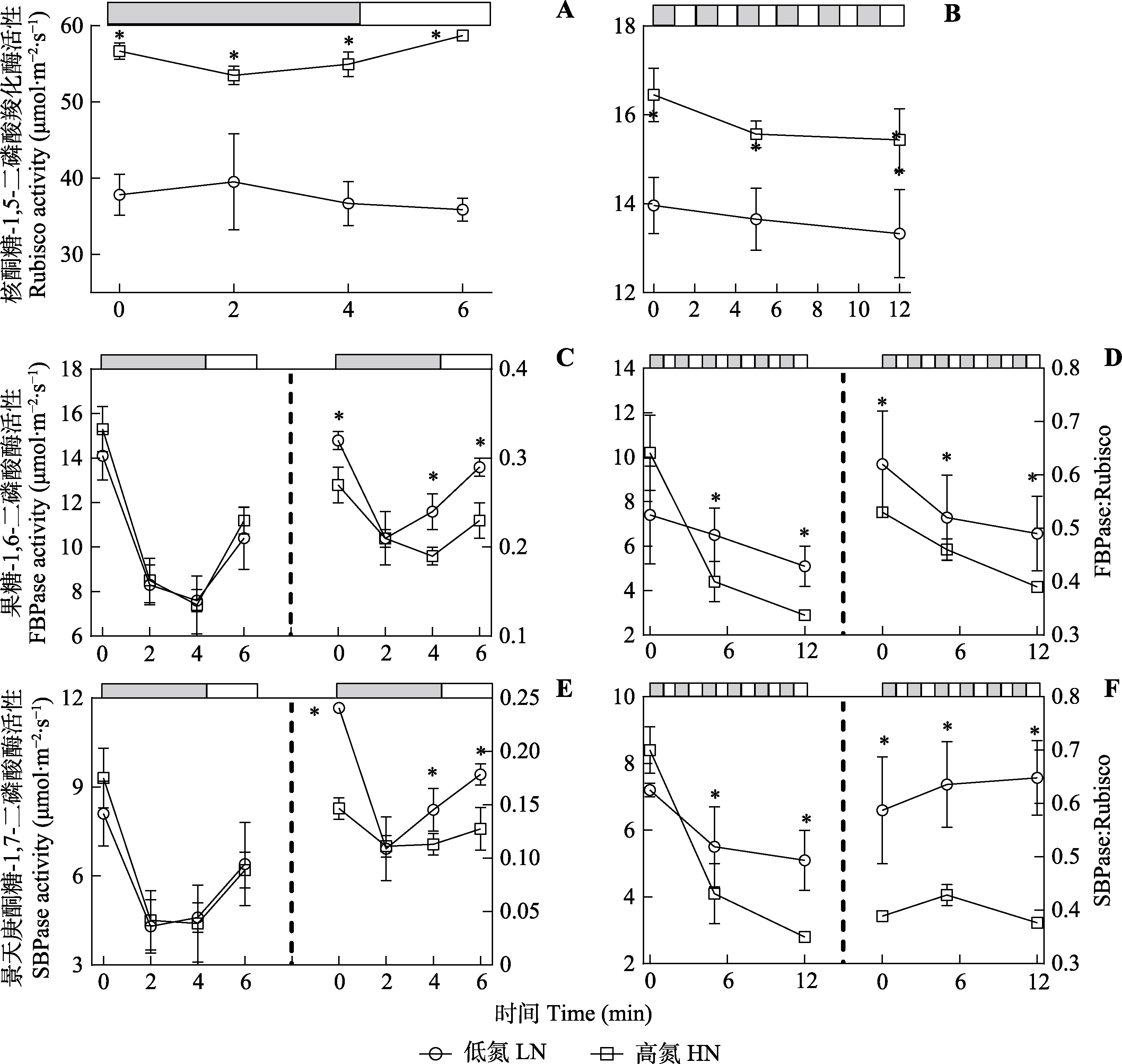
Fig. 8 Enzyme activities of Panax notoginseng under steady-state and dynamic light conditions (mean ± SD). The bars at the top of each figure show the high light (800 μmol·m-2·s-1, white) and low light (50 μmol·m-2·s-1, grey) periods. Under irregular dynamic light condition (A, C, E), leaves were exposed under high light (800 μmol·m-2·s-1) condition for 20-40 min and then the leaves were exposed to low light (50 μmol·m-2·s-1) for 240 s and then the light was converted to high light for 120 s. Under regular dynamic light condition (B, D, F), leaves were treated with high light for 20-40 min, followed by alternating high light (800 μmol·m-2·s-1) and low light (50 μmol·m-2·s-1) every 120 s for 12 min. FBPase, fructose-1,6-bisphosphatase; Rubisco, 1,5-bisphosphate carboxylase; SBPase, scenedesmusheptulose-1,7-bisphosphatase. HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two nitrogen treatments (p < 0.05).
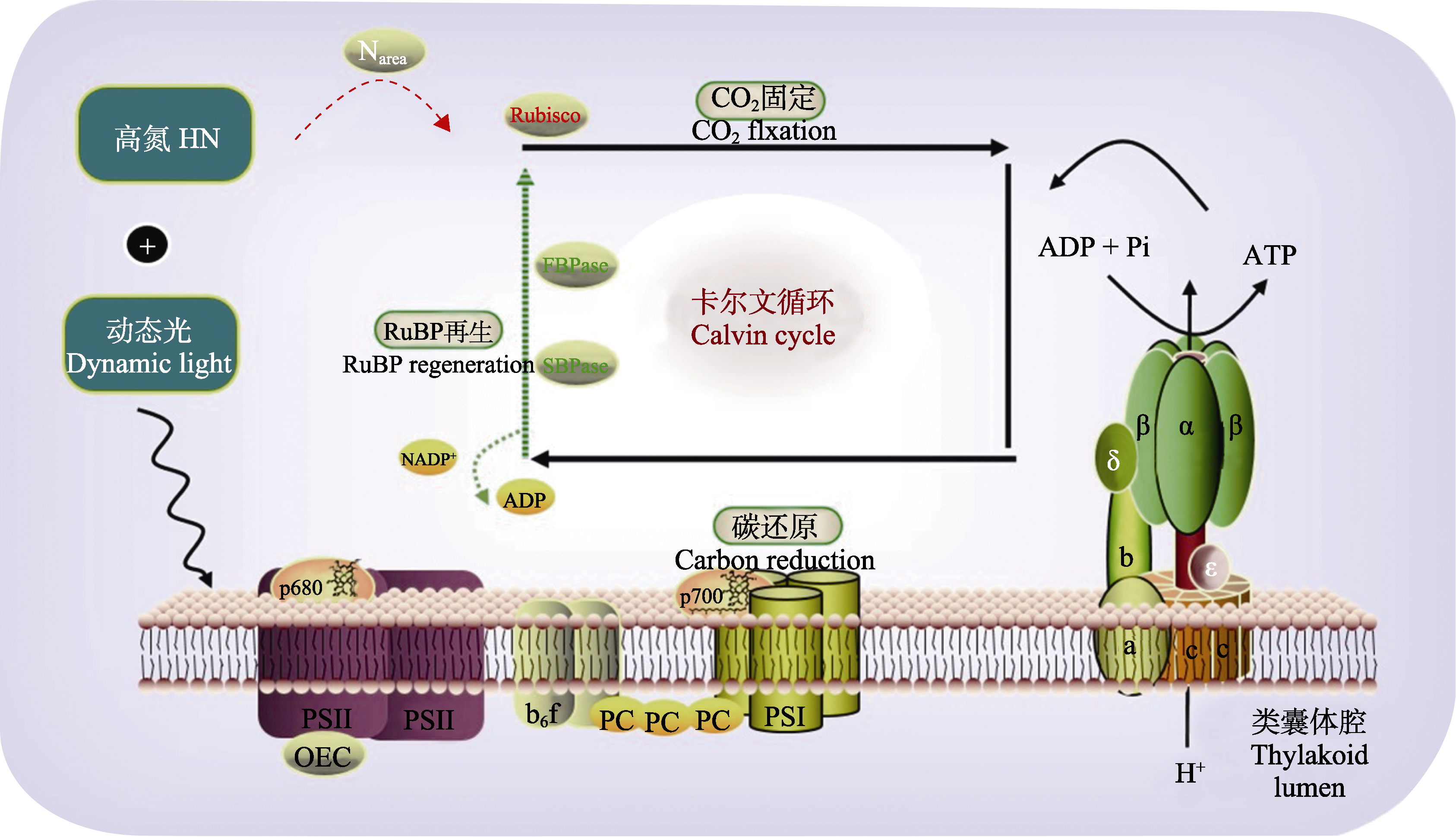
Fig. 9 High nitrogen (HN) exacerbates the decline of photosynthetic induction rate in a typically shade-tolerant species Panax notoginseng. The protein content of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in high-nitrogen leaves was higher than that of fructose-1,6-bisphosphatase (FBPase) and scenedesmus heptulose-1,7-bisphosphatase (SBPase); during the high light phase of dynamic light, HN leaves required activation of a higher proportion of FBPase and SBPase and a longer time to restore photosynthetic rate. The photosynthetic induction in the dynamic light of HN condition after low light interval was mainly limited by SBPase and FBPase reactivation rather than by Rubisco activity. The model shows the process of photosynthetic electron transfer, the red dashed line indicates that the process is fully activated and the green dashed line indicates that the process is inhibited, the red font indicates an increase in the amount (concentration) of the substance and the green font indicates a decrease in the amount (concentration) of the substance. ADP, adenosine diphosphate; ATP, adenosine triphosphate; OEC, oxygen-evolving complex; PC, plastocyanin; Pi, phosphate group; PSI, photosystem I; PSII, photosystem II. a, b, c, β, δ, α and ε, different subunits of ATP synthase; ADP, adenosine diphosphate; ATP, adenosine triphosphate; b6f, cytochrome b6-f complex; FBPase, fructose-1,6-bisphosphatase; Narea, N content per unit of leaf area; NADP+, nicotinamide adenine dinucleotide phosphate; OEC, oxygen-evolving complex; p680, chlorophyll II; p700, chlorophyl; PC, plastocyanin; Pi, phosphate group; PSI, photosystem I; PSII, photosystem II; RuBP, ribulose-1,5-disphosphate; Rubisco, ribulose-1,5-bisphosphate carboxylase; SBPase, sedoheptulose-1,7-bisphosphatase.
| [1] | Cai ZQ, Qi X, Cao KF (2004). Response of stomatal characteristics and its plasticity to different light intensities in leaves of seven tropical woody seedlings. Chinese Journal of Applied Ecology, 15, 201-204. |
| [蔡志全, 齐欣, 曹坤芳 (2004). 七种热带雨林树苗叶片气孔特征及其可塑性对不同光照强度的响应. 应用生态学报, 15, 201-204.] | |
| [2] |
Carmo-Silva AE, Salvucci ME (2013). The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiology, 161, 1645-1655.
DOI PMID |
| [3] |
Chen JW, Kuang SB, Long GQ, Yang SC, Meng ZG, Li LG, Chen ZJ, Zhang GH (2016). Photosynthesis, light energy partitioning, and photoprotection in the shade-demanding species Panax notoginseng under high and low level of growth irradiance. Functional Plant Biology, 43, 479-491.
DOI URL |
| [4] |
Chen JW, Zhang Q, Li XS, Cao KF (2011). Steady and dynamic photosynthetic responses of seedlings from contrasting successional groups under low-light growth conditions. Physiologia Plantarum, 141, 84-95.
DOI URL |
| [5] | Cun Z, Zhang JY, Chen JW (2020). Effects of nitrogen addition on growth, photosynthetic characteristics and saponin content in two-year-old Panax notoginseng. Chinese Journal of Ecology, 39, 1101-1111. |
| [寸竹, 张金燕, 陈军文 (2020). 氮添加对二年生三七生长、光合特性及皂苷含量的影响. 生态学杂志, 39, 1101-1111.] | |
| [6] |
Cun Z, Zhang JY, Wu HM, Zhang L, Chen JW (2021). High nitrogen inhibits photosynthetic performance in a shade-tolerant and N-sensitive species Panax notoginseng. Photosynthesis Research, 147, 283-300.
DOI |
| [7] |
de Souza AP, Wang Y, Orr DJ, Carmo-Silva E, Long SP (2020). Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytologist, 225, 2498-2512.
DOI PMID |
| [8] |
Ernstsen J, Woodrow IE, Mott KA (1997). Responses of Rubisco activation and deactivation rates to variations in growth-light conditions. Photosynthesis Research, 52, 117-125.
DOI URL |
| [9] |
Evans JR, Clarke VC (2019). The nitrogen cost of photosynthesis. Journal of Experimental Botany, 70, 7-15.
DOI PMID |
| [10] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
DOI PMID |
| [11] | Fu Z, Xie SQ, Xu WG, Yan S, Chen JW (2016). Characteristics of photosynthesis and light energy partitioning in Amorphophallus xiei grown along a light-intensity gradient. Chinese Journal of Applied Ecology, 27, 1177-1188. |
|
[付忠, 谢世清, 徐文果, 岩所, 陈军文 (2016). 不同光照强度下谢君魔芋的光合作用及能量分配特征. 应用生态学报, 27, 1177-1188.]
DOI |
|
| [12] | Gao F, Wang RS, Xu HS, Wang DM, Yang ZR (2016). Effects of water and fertilizer coupling on photosynthetic characteristics of maize leaves in ear position at filling stage in an apple-maize intercropping system in Losses Plateau of west Shanxi Province, China. Chinese Journal of Applied Ecology, 27, 2477-2490. |
|
[高飞, 王若水, 许华森, 王冬梅, 杨宗儒 (2016). 晋西黄土区水肥调控对苹果-玉米间作系统玉米灌浆期穗位叶光合生理特性的影响. 应用生态学报, 27, 2477-2490.]
DOI |
|
| [13] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [14] | Grieco M, Roustan V, Dermendjiev G, Rantala S, Jain A, Leonardelli M, Neumann K, Berger V, Engelmeier D, Bachmann G, Ebersberger I, Aro EM, Weckwerth W, Teige M (2020). Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant, Cell & Environment, 43, 1484-1500. |
| [15] |
Han JM, Zhang WF, Xiong DL, Flexas J, Zhang YL (2017). Mesophyll conductance and its limiting factors in plant leaves. Chinese Journal of Plant Ecology, 41, 914-924.
DOI URL |
|
[韩吉梅, 张旺锋, 熊栋梁, Flexas J, 张亚黎 (2017). 植物光合作用叶肉导度及主要限制因素研究进展. 植物生态学报, 41, 914-924.]
DOI |
|
| [16] |
Hauser T, Popilka L, Hartl FU, Hayer-Hartl M (2015). Role of auxiliary proteins in Rubisco biogenesis and function. Nature Plants, 1, 15065. DOI: 10.1038/nplants.2015.65.
DOI |
| [17] | He P, Cheng F, Yang M, He FZ (2020). Light environment analysis of artificial and natural canopy of Parashorea chinensis in Guangxi. Journal of Northeast Forestry University, 48(7), 29-33. |
| [何鹏, 程飞, 杨梅, 何方中 (2020). 广西望天树人工林和天然林冠层光环境特征与其生长的关系. 东北林业大学学报, 48(7), 29-33.] | |
| [18] |
Howard TP, Metodiev M, Lloyd JC, Raines CA (2008). Thioredoxin-mediated reversible dissociation of a stromal multiprotein complex in response to changes in light availability. Proceedings of the National Academy of Sciences of the United States of America, 105, 4056-4061.
DOI PMID |
| [19] |
Ikeuchi M, Uebayashi N, Sato F, Endo T (2014). Physiological functions of PsbS-dependent and PsbS-independent NPQ under naturally fluctuating light conditions. Plant and Cell Physiology, 55, 1286-1295.
DOI PMID |
| [20] | Jiang QQ, Liu C, Hu ZH, Yu LF, Yang ZQ, Chen ST (2021). Effects of different levels of elevated CO2 concentration and nitrogen fertilization on chlorophyll fluorescence characteristics of rice. Acta Ecologica Sinica, 41, 4953-4962. |
| [姜倩倩, 刘超, 胡正华, 于凌飞, 杨再强, 陈书涛 (2021). 不同CO2浓度升高和氮肥水平对水稻叶绿素荧光特性的影响. 生态学报, 41, 4953-4962.] | |
| [21] |
Jin C, Li XH, Jiang Y, Xu MZ, Tian Y, Liu P, Jia X, Zha TS (2021). Relative changes and regulation of photosynthetic energy partitioning components in Artemisia ordosica during growing season. Chinese Journal of Plant Ecology, 45, 870-879.
DOI URL |
|
[靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山 (2021). 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制. 植物生态学报, 45, 870-879.]
DOI |
|
| [22] |
Kaiser E, Kromdijk J, Harbinson J, Heuvelink E, Marcelis LFM (2016a). Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance. Annals of Botany, 119, 191-205.
DOI URL |
| [23] |
Kaiser E, Matsubara S, Harbinson J, Heuvelink E, Marcelis LFM (2018). Acclimation of photosynthesis to lightflecks in tomato leaves: interaction with progressive shading in a growing canopy. Physiologia Plantarum, 162, 506-517.
DOI URL |
| [24] |
Kaiser E, Morales A, Harbinson J (2017a). Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiology, 176, 977-989.
DOI URL |
| [25] |
Kaiser E, Morales A, Harbinson J, Heuvelink E, Prinzenberg AE, Marcelis LFM (2016b). Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Scientific Reports, 6, 31252. DOI: 10.1038/srep31252.
DOI |
| [26] |
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM (2014). Dynamic photosynthesis in different environmental conditions. Journal of Experimental Botany, 66, 2415-2426.
DOI URL |
| [27] |
Kaiser E, Zhou D, Heuvelink E, Harbinson J, Morales A, Marcelis LFM (2017b). Elevated CO2 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. Journal of Experimental Botany, 68, 5629-5640.
DOI URL |
| [28] |
Kang H, Zhu X, Yamori W, Tang Y (2020). Concurrent increases in leaf temperature with light accelerate photosynthetic induction in tropical tree seedlings. Frontiers in Plant Science, 11, 1216. DOI: 10.3389/fpls.2020.01216.
DOI |
| [29] |
Kono M, Kawaguchi H, Mizusawa N, Yamori W, Suzuki Y, Terashima I (2019). Far-red light accelerates photosynthesis in the low-light phases of fluctuating light. Plant and Cell Physiology, 61, 192-202.
DOI URL |
| [30] | Kuang SB, Xu XZ, Meng ZG, Zhang GH, Yang SC, Chen ZJ, Wei FG, Chen JW (2015). Effects of light transmittance on plant growth and root ginsenoside content of Panax notoginseng. Chinese Journal of Applied and Environmental Biology, 21, 279-286. |
| [匡双便, 徐祥增, 孟珍贵, 张广辉, 杨生超, 陈中坚, 魏富刚, 陈军文 (2015). 不同透光率对三七生长特征及根皂苷含量的影响. 应用与环境生物学报, 21, 279-286.] | |
| [31] |
Lang Y, Wang M, Zhang GC, Zhao QK (2013). Experimental and simulated light responses of photosynthesis in leaves of three tree species under different soil water conditions. Photosynthetica, 51, 370-378.
DOI URL |
| [32] |
Lawson T, Kramer DM, Raines CA (2012). Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Current Opinion in Biotechnology, 23, 215-220.
DOI PMID |
| [33] | Leakey ADB, Press MC, Scholes JD (2003). Patterns of dynamic irradiance affect the photosynthetic capacity and growth of dipterocarp tree seedlings. Oecologia, 135, 184-193. |
| [34] |
LeBauer DS, Treseder KK (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89, 371-379.
PMID |
| [35] | Li M, Li YC, Niu XG, Ma F, Wei N, Hao XY, Dong LB, Guo LP (2021). Effects of elevated atmospheric CO2 concentration and nitrogen fertilizer on the yield of summer maize and carbon and nitrogen metabolism after flowering. Scientia Agricultura Sinica, 54, 3647-3665. |
|
[李明, 李迎春, 牛晓光, 马芬, 魏娜, 郝兴宇, 董李冰, 郭李萍 (2021). 大气CO2浓度升高与氮肥互作对玉米花后碳氮代谢及产量的影响. 中国农业科学, 54, 3647-3665.]
DOI |
|
| [36] | Li Q, Luo YH, Yu DH, Kong FL, Yang SM, Yuan JC (2015). Effects of low nitrogen stress on photosynthetic characteristics and chlorophyll fluorescence parameters of maize cultivars tolerant to low nitrogen stress at the seedling stage. Journal of Plant Nutrition and Fertilizer, 21, 1132-1141. |
| [李强, 罗延宏, 余东海, 孔凡磊, 杨世民, 袁继超 (2015). 低氮胁迫对耐低氮玉米品种苗期光合及叶绿素荧光特性的影响. 植物营养与肥料学报, 21, 1132-1141.] | |
| [37] | Li Y (2011). Studies on Mechanisms of the Effects of Different Nitrogen Supplies on Photosynthesis and Photosynthetic Nitrogen Use Efficiency of Rice Plants. PhD dissertation, Nanjing Agricultural University, Nanjing. |
| [李勇 (2011). 氮素营养对水稻光合作用与光合氮素利用率的影响机制研究. 博士学位论文, 南京农业大学, 南京.] | |
| [38] |
Li YT, Li Y, Li YN, Liang Y, Sun Q, Li G, Liu P, Zhang ZS, Gao HY (2020). Dynamic light caused less photosynthetic suppression, rather than more, under nitrogen deficit conditions than under sufficient nitrogen supply conditions in soybean. BMC Plant Biology, 20, 339. DOI: 10.1186/s12870-020-02516-y.
DOI |
| [39] |
Li ZZ, Liu DH, Zhao SW, Jiang CD, Shi L (2014). Mechanisms of photoinhibition induced by high light in Hosta grown outdoors. Chinese Journal of Plant Ecology, 38, 720-728.
DOI |
|
[李志真, 刘东焕, 赵世伟, 姜闯道, 石雷 (2014). 环境强光诱导玉簪叶片光抑制的机制. 植物生态学报, 38, 720-728.]
DOI |
|
| [40] | Liu J, Last RL (2017). A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proceedings of the National Academy of Sciences of the United States of America, 114, E8110-E8117. |
| [41] |
Liu J, Zhang J, Estavillo GM, Luo T, Hu L (2021). Leaf N content regulates the speed of photosynthetic induction under fluctuating light among canola genotypes (Brassica napus L.). Physiologia Plantarum, 172, 1844-1852.
DOI URL |
| [42] |
Long SP, Bernacchi CJ (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany, 54, 2393-2401.
DOI PMID |
| [43] |
Marri L, Zaffagnini M, Collin V, Issakidis-Bourguet E, Lemaire SD, Pupillo P, Sparla F, Miginiac-Maslow M, Trost P (2009). Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Molecular Plant, 2, 259-269.
DOI URL |
| [44] |
Matthews JSA, Vialet-Chabrand S, Lawson T (2018). Acclimation to fluctuating light impacts the rapidity of response and diurnal rhythm of stomatal conductance. Plant Physiology, 176, 1939-1951.
DOI PMID |
| [45] |
Mu XH, Chen YL (2021). The physiological response of photosynthesis to nitrogen deficiency. Plant Physiology and Biochemistry, 158, 76-82.
DOI PMID |
| [46] |
Naranjo B, Diaz-Espejo A, Lindahl M, Cejudo FJ (2016). Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. Journal of Experimental Botany, 67, 1951-1964.
DOI PMID |
| [47] |
Niedermaier S, Schneider T, Bahl MO, Matsubara S, Huesgen PF (2020). Photoprotective acclimation of the Arabidopsis thaliana leaf proteome to fluctuating light. Frontiers in Genetics, 11, 154. DOI: 10.3389/fgene.2020.00154.
DOI |
| [48] |
Niyogi KK, Truong TB (2013). Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Current Opinion in Plant Biology, 16, 307-314.
DOI PMID |
| [49] |
Ohkubo S, Tanaka Y, Yamori W, Adachi S (2020). Rice cultivar takanari has higher photosynthetic performance under fluctuating light than koshihikari, especially under limited nitrogen supply and elevated CO2. Frontiers in Plant Science, 11, 1308. DOI: 10.3389/fpls.2020.01308.
DOI |
| [50] |
Pilon C, Snider JL, Sobolev V, Chastain DR, Sorensen RB, Meeks CD, Massa AN, Walk T, Singh B, Earl HJ (2018). Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). Journal of Plant Physiology, 231, 124-134.
DOI URL |
| [51] |
Raines CA (2003). The Calvin cycle revisited. Photosynthesis Research, 75, 1-10.
DOI PMID |
| [52] |
Rascher U, Nedbal L (2006). Dynamics of photosynthesis in fluctuating light. Current Opinion in Plant Biology, 9, 671-678.
DOI PMID |
| [53] |
Scheibe R, Fickenscher K, Ashton AR (1986). Studies on the mechanism of the reductive activation of NADP-malate dehydrogenase by thioredoxin m and low-molecular-weight thiols. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 870, 191-197.
DOI URL |
| [54] |
Seuter A, Busch M, Hain R (2002). Overexpression of the potential herbicide target sedoheptulose-1,7-bisphosphatase from Spinacia oleracea in transgenic tobacco. Molecular Breeding, 9, 53-61.
DOI URL |
| [55] |
Slattery RA, Walker BJ, Weber APM, Ort DR (2017). The impacts of fluctuating light on crop performance. Plant Physiology, 176, 990-1003.
DOI URL |
| [56] | Soleh MA, Tanaka Y, Nomoto Y, Iwahashi Y, Nakashima K, Fukuda Y, Long SP, Shiraiwa T (2016). Factors underlying genotypic differences in the induction of photosynthesis in soybean [Glycine max (L.) Merr]. Plant, Cell & Environment, 39, 685-693. |
| [57] |
Sun H, Shi Q, Zhang SB, Huang W (2022). The response of photosystem I to fluctuating light is influenced by leaf nitrogen content in tomato. Environmental and Experimental Botany, 193, 104665. DOI: 10.1016/j.envexpbot.2021.104665.
DOI |
| [58] |
Sun J, Ye M, Peng S, Li Y (2016). Nitrogen can improve the rapid response of photosynthesis to changing irradiance in rice (Oryza sativa L.) plants. Scientific Reports, 6, 31305. DOI: 10.1038/srep31305.
DOI |
| [59] |
Terashima I, Matsuo M, Suzuki Y, Yamori W, Kono M (2021). Photosystem I in low light-grown leaves of Alocasia odora, a shade-tolerant plant, is resistant to fluctuating light-induced photoinhibition. Photosynthesis Research, 149, 69-82.
DOI PMID |
| [60] |
Warren CR, Dreyer E, Adams MA (2003). Photosynthesis-Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees, 17, 359-366.
DOI |
| [61] | Wei Z (2021). Physiological and Molecular Mechanisms of Rice in Response to Fluctuating Light. PhD dissertation, Shandong Agricultural University, Taian, Shangdong. |
| [卫泽 (2021). 水稻响应波动光的生理及分子机制. 博士学位论文, 山东农业大学, 山东泰安.] | |
| [62] |
Woodrow IE, Mott KA (1989). Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in spinach. Functional Plant Biology, 16, 487. DOI: 10.1071/pp9890487.
DOI |
| [63] |
Wu HM, Shuang SP, Zhang JY, Cun Z, Meng ZG, Li LG, Sha BC, Chen JW (2021). Photodamage to photosystem in a typically shade-tolerant species Panax notoginseng exposed to a sudden increase in light intensity. Chinese Journal of Plant Ecology, 45, 404-419.
DOI URL |
|
[武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文 (2021). 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制. 植物生态学报, 45, 404-419.]
DOI |
|
| [64] | Xiong DL (2016). Coordination of Leaf Morpho-Anatomical Traits, Photosynthesis and Leaf Hydraulic Conductance in Oryza. PhD dissertation, Huazhong Agricultural University, Wuhan. |
| [熊栋梁 (2016). 水稻叶片结构对水力导度与光合作用的影响及其机理. 博士学位论文, 华中农业大学, 武汉.] | |
| [65] |
Xiong DL, Flexas J, Yu TT, Peng SB, Huang JL (2017). Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytologist, 213, 572-583.
DOI URL |
| [66] | Xu XZ, Zhang JY, Zhang GH, Long GQ, Yang SC, Chen ZJ, Wei FG, Chen JW (2018). Effects of light intensity on photosynthetic capacity and light energy allocation in Panax notoginseng. Chinese Journal of Applied Ecology, 29, 193-204. |
|
[徐祥增, 张金燕, 张广辉, 龙光强, 杨生超, 陈中坚, 魏富刚, 陈军文 (2018). 光强对三七光合能力及能量分配的影响. 应用生态学报, 29, 193-204.]
DOI |
|
| [67] | Yamori W, Kusumi K, Iba K, Terashima I (2020). Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant, Cell & Environment, 43, 1230-1240. |
| [68] | Ye SH (2004). Experimental Course of Plant Physiology and Biochemistry. Yunnan Science and Technology Press, Kunming. |
| [叶尚红 (2004). 植物生理生化实验教程. 云南科技出版社, 昆明.] | |
| [69] | Ye ZP, Yu Q (2008). Comparison of new and several classical models of photo-synthesis in response to irradiance. Journal of Plant Ecology (Chinese Version), 32, 1356-1361. |
|
[叶子飘, 于强 (2008). 光合作用光响应模型的比较. 植物生态学报, 32, 1356-1361.]
DOI |
|
| [70] |
Yin XY, Schapendonk AHCM, Struik PC (2018). Exploring the optimum nitrogen partitioning to predict the acclimation of C3 leaf photosynthesis to varying growth conditions. Journal of Experimental Botany, 70, 2435-2447.
DOI URL |
| [71] | Yuan JM, He L, Yang XQ, Xu ZP, Kong WX, Zhao QL, Qu WL, Lei X (2020). Light response curve of photosynthesis of three Phyllanthus emblica provenances in dry-hot valley of Jinsha River. Acta Agriculturae Jiangxi, 32, 55-60. |
| [袁建民, 何璐, 杨晓琼, 许智萍, 孔维喜, 赵琼玲, 瞿文林, 雷虓 (2020). 干热河谷区3个种源余甘子的光响应曲线特性研究. 江西农业学报, 32, 55-60.] | |
| [72] |
Zhang JY, Cun Z, Chen JW (2020a). Photosynthetic performance and photosynthesis-related gene expression coordinated in a shade-tolerant species Panax notoginseng under nitrogen regimes. BMC Plant Biology, 20, 273. DOI: 10.1186/s12870-020-02434-z.
DOI |
| [73] |
Zhang JY, Cun Z, Wu HM, Chen JW (2020b). Integrated analysis on biochemical profiling and transcriptome revealed nitrogen-driven difference in accumulation of saponins in a medicinal plant Panax notoginseng. Plant Physiology and Biochemistry, 154, 564-580.
DOI URL |
| [74] |
Zhang JY, Shuang SP, Zhang L, Xie SQ, Chen JW (2021a). Photosynthetic and photoprotective responses to steady-state and fluctuating light in the shade-demanding crop Amorphophallus xiei grown in intercropping and monoculture systems. Frontiers in Plant Science, 12, 663473. DOI: 10.3389/fpls.2021.663473.
DOI |
| [75] |
Zhang JY, Xie SQ, Yan S, Xu WG, Chen JW (2021b). Light energy partitioning and photoprotection from excess light energy in shade-tolerant plant Amorphophallus xiei under steady-state and fluctuating high light. Acta Physiologiae Plantarum, 43, 1-17.
DOI |
| [76] |
Zhang JY, Xu XZ, Kuang SB, Cun Z, Wu HM, Shuang SP, Chen JW (2021c). Constitutive activation of genes involved in triterpene saponins enhances the accumulation of saponins in three-year-old Panax notoginseng growing under moderate light intensity. Industrial Crops and Products, 171, 113938. DOI: 10.1016/j.indcrop.2021.113938.
DOI |
| [77] |
Zhang JY, Zhang QH, Shuang SP, Cun Z, Wu HM, Chen JW (2021d). The responses of light reaction of photosynthesis to dynamic sunflecks in a typically shade-tolerant species Panax notoginseng. Frontiers in Plant Science, 12, 718981. DOI: 10.3389/fpls.2021.718981.
DOI |
| [78] |
Zhang QQ, Peng SB, Li Y (2019). Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. Journal of Experimental Botany, 70, 5259-5269.
DOI URL |
| [79] | Zhao SJ, Cang J (2016). Experimental Guide for Plant Physiology. Chinese Agriculture Press, Beijing. |
| [赵世杰, 苍晶 (2016). 植物生理学实验指导. 中国农业出版社, 北京.] |
| [1] | WU Hong-Min, SHUANG Sheng-Pu, ZHANG Jin-Yan, CUN Zhu, MENG Zhen-Gui, LI Long-Gen, SHA Ben-Cai, CHEN Jun-Wen. Photodamage to photosystem in a typically shade-tolerant species Panax notoginseng exposed to a sudden increase in light intensity [J]. Chin J Plant Ecol, 2021, 45(4): 404-419. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn