

Chin J Plan Ecolo ›› 2017, Vol. 41 ›› Issue (8): 914-924.DOI: 10.17521/cjpe.2016.0337
• Reviews • Previous Articles
Ji-Mei HAN1, Wang-Feng ZHANG1, Dong-Liang XIONG2, Jaume FLEXAS2, Ya-Li ZHANG1,*( )
)
Online:2017-08-10
Published:2017-09-29
Contact:
Ya-Li ZHANG
About author:KANG Jing-yao(1991-), E-mail:
Ji-Mei HAN, Wang-Feng ZHANG, Dong-Liang XIONG, Jaume FLEXAS, Ya-Li ZHANG. Mesophyll conductance and its limiting factors in plant leaves[J]. Chin J Plan Ecolo, 2017, 41(8): 914-924.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2016.0337
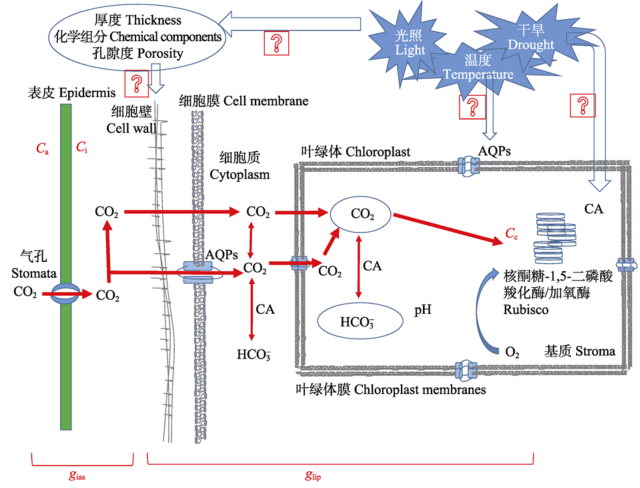
Fig. 1 CO2 transport model. AQPs, aquaporins; Ca, the atmospheric CO2 concentration; Ci, intercellular CO2 concentration; CA, carbonic anhydrase; gias, the gas phase conductance; glip, the liquid phase conductance.
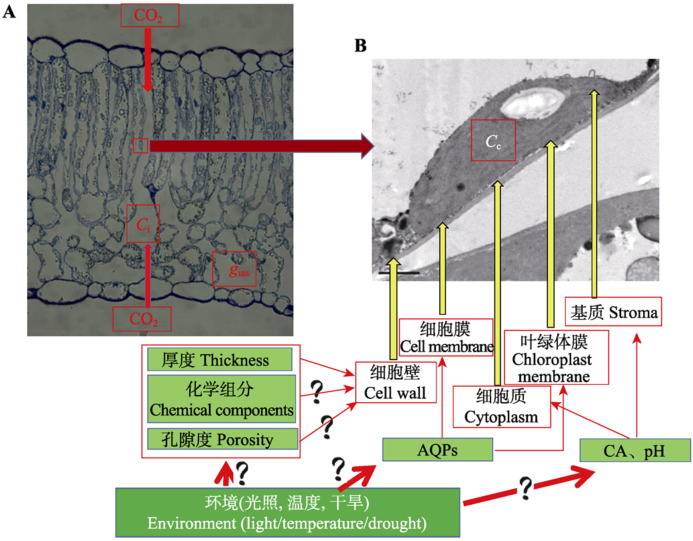
Fig. 2 The diffusion path of CO2 reflected by gm. A, The leaf anatomical structure in cotton by optical microscope, which represents the CO2 gas phase diffusion from the atmosphere into the leaf intercellular air layer; B, The leaf ultra-micro structure in cotton by electron microscope, which represents the CO2 liquid phase diffusion from intercellular into the chloroplast carboxylation site. AQPs, aquaporins; Ci, intercellular CO2 concentration; Cc, CO2 concentration at chloroplast carboxylation site; CA, carbonic anhydrase; gias, the gas phase conductance.
| CO2扩散方式 CO2 diffusion way | CO2运输形态 CO2 transportation form | 阻力来源 Resistance source | 动力来源 Power source | 对外界环境的响应时间 Response time to the external environment | |
|---|---|---|---|---|---|
| 细胞壁 Cell wall | 物理和生化方式 Physics and biochemical mode | CO2 | 厚度、孔隙度、果胶等组分 Thickness, porosity, pectin etc. | CO2浓度差 Difference of CO2 concentration | 最长 Longest |
| 细胞膜 Cell membrane | 物理和生化方式 Physics and biochemical mode | CO2 | 水孔蛋白、膜两侧pH差值 AQPs, the difference of pH on both sides of the membrane | CO2浓度差、跨膜蛋白主动运输 Difference of CO2 concentration, active transport of transmembrane protein | 较短 Shorter |
| 细胞液 Cytoplasm | 生化和物理方 Biochemical and physical mode | CO2, HCO3- | CA、pH、细胞液组分 CA, pH, cytosol component | pH、CA的催化 pH, catalysis of CA | 较短 Shorter |
| 叶绿体膜 Chloroplast membranes | 生化和物理方 Biochemical and physical mode | CO2 | 水孔蛋白、膜两侧CO2浓度差 AQPs, the difference of CO2 concentration on both sides of the membrane | 跨膜蛋白主动运输 Active transport of transmembrane protein | 较短 Shorter |
| 叶绿体基质 Stroma | 生化和物理方式 Biochemical and physical mode) | CO2, HCO3- | CA, pH | pH、CA的催化 pH, catalysis of CA | 最短 Shortest |
Table 1 Diffusion way, transportation form, resistance source, power source when CO2 passes through the ultrastructure components of mesophyll cells and the different response time to the external environment
| CO2扩散方式 CO2 diffusion way | CO2运输形态 CO2 transportation form | 阻力来源 Resistance source | 动力来源 Power source | 对外界环境的响应时间 Response time to the external environment | |
|---|---|---|---|---|---|
| 细胞壁 Cell wall | 物理和生化方式 Physics and biochemical mode | CO2 | 厚度、孔隙度、果胶等组分 Thickness, porosity, pectin etc. | CO2浓度差 Difference of CO2 concentration | 最长 Longest |
| 细胞膜 Cell membrane | 物理和生化方式 Physics and biochemical mode | CO2 | 水孔蛋白、膜两侧pH差值 AQPs, the difference of pH on both sides of the membrane | CO2浓度差、跨膜蛋白主动运输 Difference of CO2 concentration, active transport of transmembrane protein | 较短 Shorter |
| 细胞液 Cytoplasm | 生化和物理方 Biochemical and physical mode | CO2, HCO3- | CA、pH、细胞液组分 CA, pH, cytosol component | pH、CA的催化 pH, catalysis of CA | 较短 Shorter |
| 叶绿体膜 Chloroplast membranes | 生化和物理方 Biochemical and physical mode | CO2 | 水孔蛋白、膜两侧CO2浓度差 AQPs, the difference of CO2 concentration on both sides of the membrane | 跨膜蛋白主动运输 Active transport of transmembrane protein | 较短 Shorter |
| 叶绿体基质 Stroma | 生化和物理方式 Biochemical and physical mode) | CO2, HCO3- | CA, pH | pH、CA的催化 pH, catalysis of CA | 最短 Shortest |
| [1] |
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002). Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesisin vivo. Plant Physiology, 130, 1992-1998.
DOI URL PMID |
| [2] |
Boex-Fontvieille E, Jossier M, Davanture M, Zivy M, Hodges M, Tcherkez G (2014). Differential protein phosphorylation regulates chloroplast movement in response to strong light and darkness inArabidopsis thaliana. Plant Molecular Biology Reporter, 32, 987-1001.
DOI URL |
| [3] |
Bongi G, Loreto F (1989). Gas-exchange properties of salt stressed olive (Olea europea L.) leaves.Plant Physiology, 90, 1408-1416.
DOI URL PMID |
| [4] |
Boron W, Endeward V, Gros G, Musa-Aziz R, Pohl P (2011). Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels.Chemphyschem, 12, 1017-1019.
DOI URL PMID |
| [5] |
Burnell JN, Suzuki I, Sugiyama T (1990). Light induction and the effect of nitrogen status upon the activity of carbonic anhydrase in maize leaves.Plant Physiology, 94, 384-387.
DOI URL |
| [6] |
Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000). Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress.Journal of Experimental Botany, 51, 61-70.
DOI URL PMID |
| [7] |
Cochard H, Nardini A, Coll L (2004). Hydraulic architecture of leafblades: Where is the main resistance?Plant, Cell & Environment, 27, 1257-1267.
DOI URL |
| [8] |
Diaz-Espejo A, Nicolás E, Fernández JE (2007). Seasonal evolution of diffusional limitations and photosynthetic capacity in olive under drought.Plant, Cell & Environment, 30, 922-933.
DOI URL PMID |
| [9] | Ethier GJ, Livingston NJ (2004). On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model.Plant, Cell & Environment, 27, 137-153. |
| [10] |
Evans JR, Kaldenhoff R, Genty B, Terashima I (2009). Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany, 60, 2235-2248.
DOI URL PMID |
| [11] |
Evans JR, Shatrkey TD, Berry JA, Farquhar GD (1986). Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants.Australian Journal of Plant Physiology, 13, 281-292.
DOI URL |
| [12] |
Evans JR, von Caemmere S (1996). Carbon dioxide diffusion inside leaves.Plant Physiology, 110, 339-346.
DOI URL PMID |
| [13] |
Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994). The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco.Australian Journal of Plant Physiology, 21, 475-495.
DOI URL |
| [14] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.Planta, 149, 78-90.
DOI URL |
| [15] |
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets ü, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012). Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Science, 193-194, 70-84.
DOI URL PMID |
| [16] |
Flexas J, Bota J, Cifre J, Escalona JM, Galmés J, Gulías J, Lefi EK, Martinez-Canellas SF, Moreno MT, Ribas-Carbo M (2004). Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Annal of Applied Biology, 144, 273-283.
DOI URL |
| [17] |
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H (2002). Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Functional Plant Biology, 29, 461-471.
DOI URL |
| [18] |
Flexas J, Diaz-Espejo A (2015). Interspecific differences in temperature response of mesophyll conductance: Food for thought on its origin and regulation.Plant, Cell & Environment, 38, 625-628.
DOI URL |
| [19] |
Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano HO, Ribas-Carbó M (2007). Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves.Plant, Cell & Environment, 30, 1284-1298.
DOI URL PMID |
| [20] |
Flexas J, Ribas-Carbó M, Bota J, Galmés J, Henkle M, Martínez-Ca?ellas S, Medrano H (2006a). Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration.New Phytologist, 172, 73-82.
DOI URL PMID |
| [21] | Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008). Mesophyll conductance to CO2: Current knowledge and future prospects.Plant, Cell & Environment, 31, 602-621. |
| [22] | Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R (2006b). Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo. The Plant Journal, 48, 427-439. |
| [23] |
Flexas J, Scoffoni C, Gago J, Sack L (2013). Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination.Journal of Experimental Botany, 64, 3965-3981.
DOI URL PMID |
| [24] |
Galmés J, Medrano H, Flexas J (2006). Acclimation of Rubisco specificity factor to drought in tobacco: Discrepancies between in vitro and in vivo estimations.Journal of Experimental Botany, 57, 3659-3667.
DOI URL PMID |
| [25] |
Galmés J, Medrano H, Flexas J (2007). Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist, 175, 81-93.
DOI URL |
| [26] |
Gillon JS, Yakir D (2000). Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves.Plant Physiology, 123, 201-213.
DOI URL |
| [27] |
Gu L, Sun Y (2013). Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods.Plant, Cell & Environment, 37, 1231-1249.
DOI URL PMID |
| [28] |
Han JM, Meng HF, Wang SY, Jiang CD, Liu F, Zhang WF, Zhang YL (2016). Variability of mesophyll conductance and its relationship with water use efficiency in cotton leaves under drought pretreatment.Journal of Plant Physiology, 194, 61-71.
DOI URL PMID |
| [29] | Hanba YT, Kogami H, Terashima I (2002). The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand.Plant, Cell & Environment, 25, 1021-1030. |
| [30] |
Hanba YT, Miyazawa SI, Terashima I (1999). The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm temperate forests.Functional Ecology, 13, 632-639.
DOI URL |
| [31] |
Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004). Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants.Plant Cell Physiology, 45, 521-529.
URL PMID |
| [32] |
Harley PC, Loreto F, Marco GD, Sharkey TD (1992). Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2.Plant Physiology, 98, 1429-1436.
DOI URL PMID |
| [33] |
Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR (2009). Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls.Journal of Experimental Botany, 60, 2303-2314.
DOI URL PMID |
| [34] |
Heckwolf M, Pater D, Hanson DT, Kaldenhoff R (2011). The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator.Plant Journal, 67, 795-804.
DOI URL PMID |
| [35] |
Hub JS, de Groot BL (2006). Does CO2 permeate through aquaporin-1?Biophysical Journal, 91, 842-848.
DOI URL PMID |
| [36] |
Hub JS, de Groot BL (2008). Mechanism of selectivity in aquaporins and aquaglyceroporins.Proceedings of the National Academy of Sciences of the United States of America, 105, 1198-1203.
DOI URL PMID |
| [37] |
Jia WS, Davies WJ (2007). Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals.Plant Physiology, 143, 68-77.
DOI URL PMID |
| [38] |
Kelly G, Sade N, Attia Z, Secchi F, Zwieniecki M, Holbrook NM, Levi A, Alchanatis V, Moshelion M, Granot D (2014). Relationship between hexokinase and the aquaporin PIP1 in the regulation of photosynthesis and plant growth.PLOS ONE, 9, e87888. doi:10.1371/journal.pone. 0087888.
DOI URL PMID |
| [39] |
Laisk A, Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Kull O (2005). Adjustment of leaf photosynthesis to shade in a natural canopy: Rate parameters.Plant, Cell & Environment, 28, 375-388.
DOI URL |
| [40] |
Li Y, Gao YX, Xu XM, Shen QR, Guo SW (2009). Light- saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration.Journal of Experimental Botany, 60, 2351-2360.
DOI URL PMID |
| [41] |
Loreto F, Harley PC, Di Marco G, Sharkey TD (1992). Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology, 98, 1437-1443.
DOI URL PMID |
| [42] |
Loreto F, Tsonev T, Centritto M (2009). The impact of blue light on leaf mesophyll conductance.Journal of Experimental Botany, 60, 2283-2290.
DOI URL PMID |
| [43] |
Makino A, Sakashita H, Hidema J, Mae T, Ojima K, Osmond B (1992). Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance.Plant Physiology, 100, 1737-1743.
DOI URL PMID |
| [44] |
Missner A, Kugler P, Antonenko YN, Pohl P (2008). Passive transport across bilayer lipid membranes: Overton continues to rule.Proceedings of the National Academy of Sciences of the United States of America, 1778, 2154-2156.
DOI URL |
| [45] | Miyazawa SI, Yoshimura S, Shinzaki Y, Maeshima M, Miyake C (2008). Relationship between mesophyll CO2 gas diffusion conductance and leaf plasma-membrane-type aquaporin contents in tobacco plants grown under drought conditions.Photosynthesis, 91, 805-808. |
| [46] |
Moualeu-Ngangue DP, Chen T-W, Stutzel H (2016). A new method to estimate photosynthetic parameters through net assimilation reteintercellular space CO2 concentration (A-Ci) curve and chlorophyll fluorescence measurements.New Phytologist, 213, 1543-1554.
DOI URL PMID |
| [47] |
Niinemets ü, Reichstein M (2003a). Controls on the emission of plant volatiles through stomata: A sensitivity analysis.Journal of Geophysical Research, 108, 4211. doi: 4210.1029/2002JD002626.
DOI URL |
| [48] |
Niinemets ü, Reichstein M (2003b). Controls on the emission of plant volatiles through stomata: Sensitivity or insensitivity of the emission rates to stomatal closure explained.Journal of Geophysical Research, 108, 4208. doi: 4210.1029/2002JD002620.
DOI URL |
| [49] |
Niinemets ü, Diaz-Espejo A, Flexas J, Galmés J, Warren CR (2009). Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field.Journal of Experimental Botany, 60, 2271-2282.
DOI URL |
| [50] |
Otto B, Uehlein N, Sdorra S, Fischer M, Ayaz M, Belastegui- Macadam X, Heckwolf M, Lachnit M, Pede N, Priem N (2010). Aquaporin tetramer composition modifies the function of tobacco aquaporins.Journal of Biological Chemistry, 285, 31253-31260.
DOI URL |
| [51] |
Pakatas A, Stavrakas D, Fisarakis I (2003). Relationship between CO2 assimilation and leafanatomical characteristics of two grapevine cultivars.Agronomie, 23, 293-296.
DOI URL |
| [52] |
Perez-Martin A, Michelazzo C, Torres-Ruiz JM, Flexas J, Fernández JE, Sebastiani L, Diaz-Espejo A (2014). Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins.Journal of Experimental Botany, 65, 3143-3156.
DOI URL |
| [53] |
Piel C, Frak E, Le Roux X, Genty B (2002). Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut.Journal of Experimental Botany, 53, 2423-2430.
DOI URL PMID |
| [54] |
Price DG, von Caemmerer S, Evans JR, Yu JW, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger M (1994). Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation.Planta, 193, 331-340.
DOI URL |
| [55] |
Sack L, Holbrook NM (2006). Leaf hydraulics.Annual Review of Plant Biology, 57, 361-381.
DOI URL |
| [56] |
Sack L, Streeter CM, Holbrook NM (2004). Hydraulic analysis of water flow through leaves of sugar maple and red oak.Plant Physiology, 134, 1824-1833.
DOI URL PMID |
| [57] |
Sade N, Gallé A, Flexas J, Lerner S, Peleg G, Yaaran A, Moshelion M (2014). Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions.Planta, 239, 357-366.
DOI URL |
| [58] |
Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, Moshelion M (2010). The Role of tobacco aquaporin 1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress.Plant Physiology, 152, 245-254.
DOI URL PMID |
| [59] |
Sage TL, Sage RF (2009). The functional anatomy of rice leaves: Implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice.Plant Cell Physiology, 50, 756-772.
DOI URL PMID |
| [60] |
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves.Plant, Cell & Environment, 30, 1035-1040.
DOI URL PMID |
| [61] | Syvertsen JP, Lloyd J, Meconchie C, Kriedbmann PE, Farquhar GD (1995). On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves.Plant Cell Physiology, 18, 149-157. |
| [62] |
Terashima I, Araya T, Miyazawa S-I, Sone K, Yano S (2005). Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise.Annals of Botany, 95, 507-519.
DOI URL PMID |
| [63] |
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006). Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. Journal of Experimental Botany, 57, 343-354.
DOI URL PMID |
| [64] |
Terashima I, Hanba YT, Tholen D, Niinemets U (2011). Leaf functional anatomy in relation to photosynthesis.Plant Physiology, 155, 108-116.
DOI URL PMID |
| [65] | Terashima I, Hikosaka K (1995). Comparative ecophysiology/ anatomy of leaf and canopy photosynthesis.Plant, Cell & Environment, 18, 1111-1128. |
| [66] |
Terashima I, Ono K (2002). Effects of HgCl2 on CO2 dependence of leaf photosynthesis: Evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane.Plant Cell Physiology, 43, 70-78.
URL PMID |
| [67] |
Théroux-Rancourt G, Gilbert ME (2017). The light response of mesophyll conductance is controlled by structure across leaf profiles. Plant, Cell & Environment, 40, 726-740.
DOI URL PMID |
| [68] |
Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I (2008). The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell & Environment, 31, 1688-1700.
DOI URL PMID |
| [69] |
Tholen D, Ethier G, Genty B, Pepin S, Zhu XG (2012). Variable mesophyll conductance revisited: Theoretical background and experimental implications.Plant, Cell & Environment, 35, 2087-2103.
DOI URL PMID |
| [70] |
Tholen D, Zhu XG (2011). The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiology, 156, 90-105.
DOI URL |
| [71] |
Tomás M, Flexas J, Copolovici L, Galmes J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets ü (2013). Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models.Journal of Experimental Botany, 64, 2269-2281.
DOI URL |
| [72] |
Tomás M, Medrano H, Brugnoli E, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J (2014). Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency.Australian Journal of Grape and Wine Research, 20, 272-280.
DOI URL |
| [73] |
Tosens T, Niinemets ü, Vislap V, Eichelmann H, Castro Diez P (2012a). Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: How structure constrains function.Plant, Cell & Environment, 35, 839-856.
DOI URL PMID |
| [74] |
Tosens T, Niinemets ü, Westoby M, Wright IJ (2012b). Anatomicalbasis of variation in mesophyll resistance in eastern Australian sclerophylls: News of a long and winding path.Journal of Experimental Botany, 63, 5105-5119.
DOI URL PMID |
| [75] |
Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R (2008). Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell, 20, 648-657.
DOI URL |
| [76] |
von Caemmerer S, Evans JR (1991). Determination of the average partial pressure of CO2 in chloroplast from leaves of several C3 plants.Australian Journal of Plant Physiology, 18, 287-305.
DOI URL |
| [77] |
Warren CR (2004). The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply.Journal of Experimental Botany, 55, 2313-2321.
DOI URL PMID |
| [78] |
Warren CR (2008). Stand aside stomata, another actor deserves centre stage: The forgotten role of the internal conductance to CO2 transfer.Journal of Experimental Botany, 59, 1475-1487.
DOI URL PMID |
| [79] |
Warren CR, Low M, Matyssek R, Tausz M (2007). Internal conductance to CO2 transfer of adult Fagus sylvatica: Variation between sun and shade leaves and due to free-air ozone fumigation.Environmental and Experimental Botany, 59, 130-138.
DOI URL |
| [80] |
Williams TG, Flanagan LB, Coleman JR (1996). Photosynthetic gas exchange and discrimination against 13CO2, and C18O16O in tobacco plants modified by an antisense construct to have low chloroplastic carbonic anhydrase.Plant Physiology, 112, 319-326.
DOI URL PMID |
| [81] |
Xiong D, Flexas J, Yu T, Peng S, Huang J (2016). Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 inOryza. New Phytologist, 213, 572-583.
DOI URL PMID |
| [82] | Xiong D, Liu X, Liu L, Douthe C, Li Y, Peng S, Huang J (2015b). Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice.Plant, Cell & Environment, 38, 2541-2550. |
| [83] |
Xiong D, Yu T, Zhang T, Li Y, Peng S, Huang J (2015a). Leaf hydraulic conductance is coordinated with leaf morpho- anatomical traits and nitrogen status in the genusOryza. Journal of Experimental Botany, 66, 741-748.
DOI URL PMID |
| [84] |
Yamori W, Nagai T, Makino A (2011). The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species.Plant, Cell & Environment, 34, 764-777.
DOI URL PMID |
| [85] |
Yamori W, Noguchi K, Hanba YT, Terashima I (2006). Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures.Plant Cell Physiology, 47, 1069-1080.
DOI URL PMID |
| [1] | SACHURA , ZHANG Xia, ZHU Lin, KANG Saruul. Leaf anatomical changes of Cleistogenes songorica under long-term grazing with different intensities in a desert steppe [J]. Chin J Plant Ecol, 2024, 48(3): 331-340. |
| [2] | LI Wei-Bin, ZHANG Hong-Xia, ZHANG Yu-Shu, CHEN Ni-Na. Influence of diurnal asymmetric warming on carbon sink capacity in a broadleaf Korean pine forest in Changbai Mountains, China [J]. Chin J Plant Ecol, 2023, 47(9): 1225-1233. |
| [3] | JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses [J]. Chin J Plant Ecol, 2023, 47(7): 988-997. |
| [4] | LIU Hai-Yan, ZANG Sha-Sha, ZHANG Chun-Xia, ZUO Jin-Cheng, RUAN Zuo-Xi, WU Hong-Yan. Photochemical reaction of photosystem II in diatoms under phosphorus starvation and its response to high light intensity [J]. Chin J Plant Ecol, 2023, 47(12): 1718-1727. |
| [5] | YUAN Yuan, MU Yan-Mei, DENG Yu-Jie, LI Xin-Hao, JIANG Xiao-Yan, GAO Sheng-Jie, ZHA Tian- Shan, JIA Xin. Effects of land cover and phenology changes on the gross primary productivity in an Artemisia ordosica shrubland [J]. Chin J Plant Ecol, 2022, 46(2): 162-175. |
| [6] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [7] | JIN Chuan, LI Xin-Hao, JIANG Yan, XU Ming-Ze, TIAN Yun, LIU Peng, JIA Xin, ZHA Tian- Shan. Relative changes and regulation of photosynthetic energy partitioning components in Artemisia ordosica during growing season [J]. Chin J Plant Ecol, 2021, 45(8): 870-879. |
| [8] | WU Hong-Min, SHUANG Sheng-Pu, ZHANG Jin-Yan, CUN Zhu, MENG Zhen-Gui, LI Long-Gen, SHA Ben-Cai, CHEN Jun-Wen. Photodamage to photosystem in a typically shade-tolerant species Panax notoginseng exposed to a sudden increase in light intensity [J]. Chin J Plant Ecol, 2021, 45(4): 404-419. |
| [9] | YE Zi-Piao, YU Feng, AN Ting, WANG Fu-Biao, KANG Hua-Jing. Investigation on CO2-response model of stomatal conductance for plants [J]. Chin J Plant Ecol, 2021, 45(4): 420-428. |
| [10] | WU Jian-Bo, WANG Xiao-Dan. Analyzing leaf anatomical structure of dominant species Stipa purpurea adapting to alpine and drought environment at Qingzang Plateau [J]. Chin J Plant Ecol, 2021, 45(3): 265-273. |
| [11] | LI Jing, WANG Xin, WANG Zhen-Hua, WANG Bin, WANG Cheng-Zhang, DENG Mei-Feng, LIU Ling-Li. Effects of ozone and aerosol pollution on photosynthesis of poplar leaves [J]. Chin J Plant Ecol, 2020, 44(8): 854-863. |
| [12] | JI Ruo-Xuan, YU Xiao, CHANG Yuan, SHEN Chao, BAI Xue-Qia, XIA Xin-Li, YIN Wei-Lun, LIU Chao. Geographical provenance variation of leaf anatomical structure of Caryopteris mongholica and its significance in response to environmental changes [J]. Chin J Plant Ecol, 2020, 44(3): 277-286. |
| [13] | LI Xu, WU Ting, CHENG Yan, TAN Na-Dan, JIANG Fen, LIU Shi-Zhong, CHU Guo-Wei, MENG Ze, LIU Ju-Xiu. Ecophysiological adaptability of four tree species in the southern subtropical evergreen broad-leaved forest to warming [J]. Chin J Plant Ecol, 2020, 44(12): 1203-1214. |
| [14] | LIU Xiao-Ming, YANG Xiao-Fang, WANG Xuan, ZHANG Shou-Ren. Effects of simulated nitrogen deposition on growth and photosynthetic characteristics of Quercus wutaishanica and Acer pictum subsp. mono in a warm-temperate deciduous broad- leaved forest [J]. Chin J Plant Ecol, 2019, 43(3): 197-207. |
| [15] | LI Xin-Hao, YAN Hui-Juan, WEI Teng-Zhou, ZHOU Wen-Jun, JIA Xin, ZHA Tian-Shan. Relative changes of resource use efficiencies and their responses to environmental factors in Artemisia ordosica during growing season [J]. Chin J Plant Ecol, 2019, 43(10): 889-898. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn