

Chin J Plant Ecol ›› 2023, Vol. 47 ›› Issue (7): 988-997.DOI: 10.17521/cjpe.2022.0153
Special Issue: 光合作用
• Research Articles • Previous Articles Next Articles
JIANG Hai-Gang1, ZENG Yun-Hong1, TANG Hua-Xin1, LIU Wei1, LI Jie-Lin1, HE Guo-Hua1, QIN Hai-Yan1, WANG Li-Chao1, Victor RESCO de DIOS1,2,*( ), YAO Yin-An1,*(
), YAO Yin-An1,*( )
)
Received:2022-04-18
Accepted:2022-09-28
Online:2023-07-20
Published:2023-07-21
Contact:
*(Yao YA, Supported by:JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses[J]. Chin J Plant Ecol, 2023, 47(7): 988-997.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2022.0153
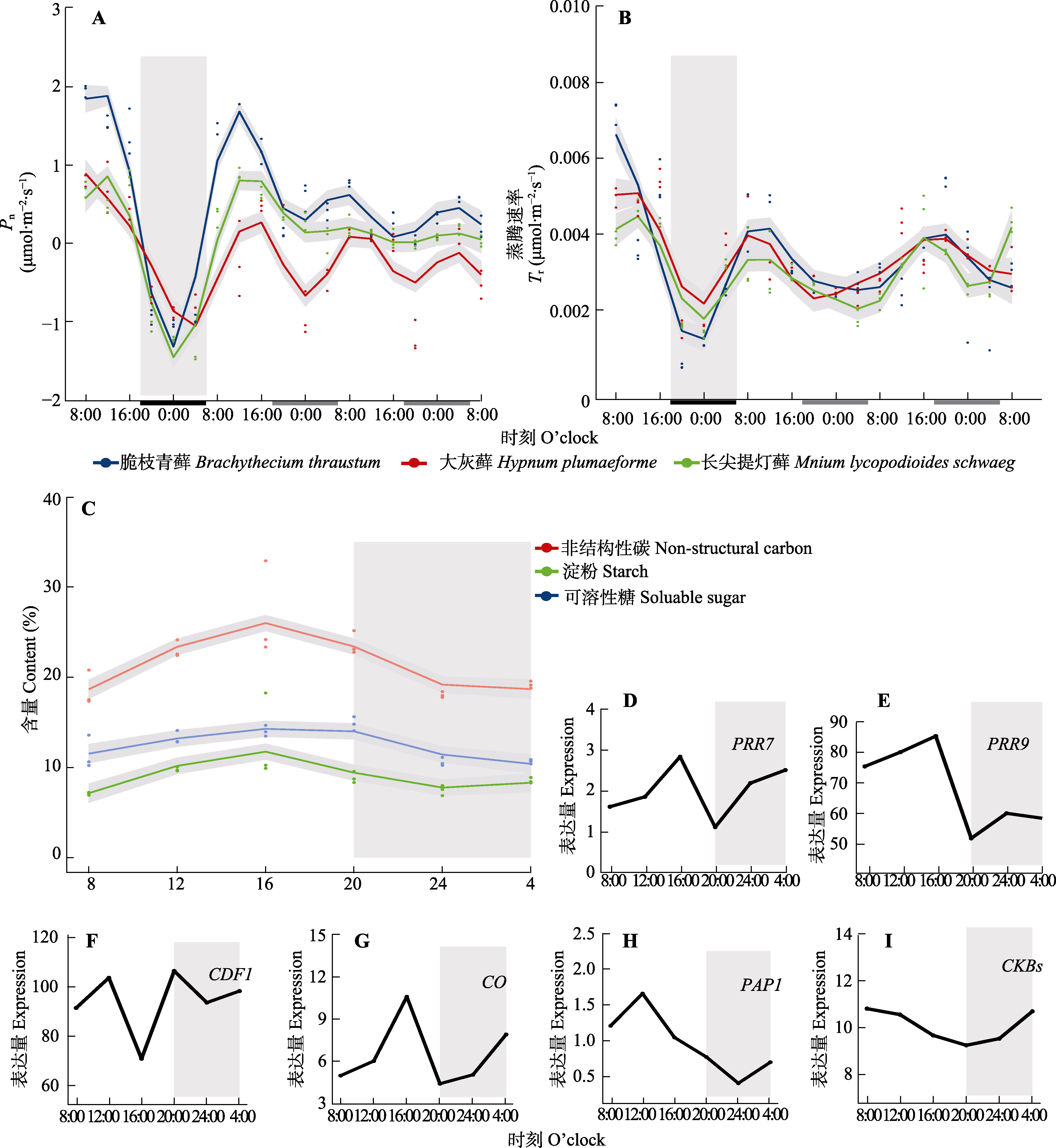
Fig. 1 Circadian rhythms of core circadian clock genes and rhythm regulation-related genes fitted to photosynthetic assimilation rate (Pn), transpiration rate (Tr), and non-structural carbon (NSC) in Brachythecium thraustum、Hypnum plumaeforme and Mnium lycopodioides using generalized additive model (GAM). The gray part in A and B corresponds to the last night of the 15 d normal photoperiod. The black and white parts on the horizontal coordinates represent the night and daytime of the plant under natural conditions, respectively, while the gray part represents the darkness of the plant under natural conditions but given light. The gray part in C-I represents the night under full light conditions on the second day (LL2d) under external conditions. D-I represent expression of clock-regulated genes in the moss Ceratodon purpureus. CDF1, CYCLING DOF FACTOR1; CKB, CASEIN KINASE II BETA CHAIN; CO, Constans; PAP1, PRODUCTION OF ANTHOCYANIN PIGMENT 1; PRR7, PSEUDO RESPONSE REGULATOR 7; PRR9, PSEUDO RESPONSE REGULATOR.
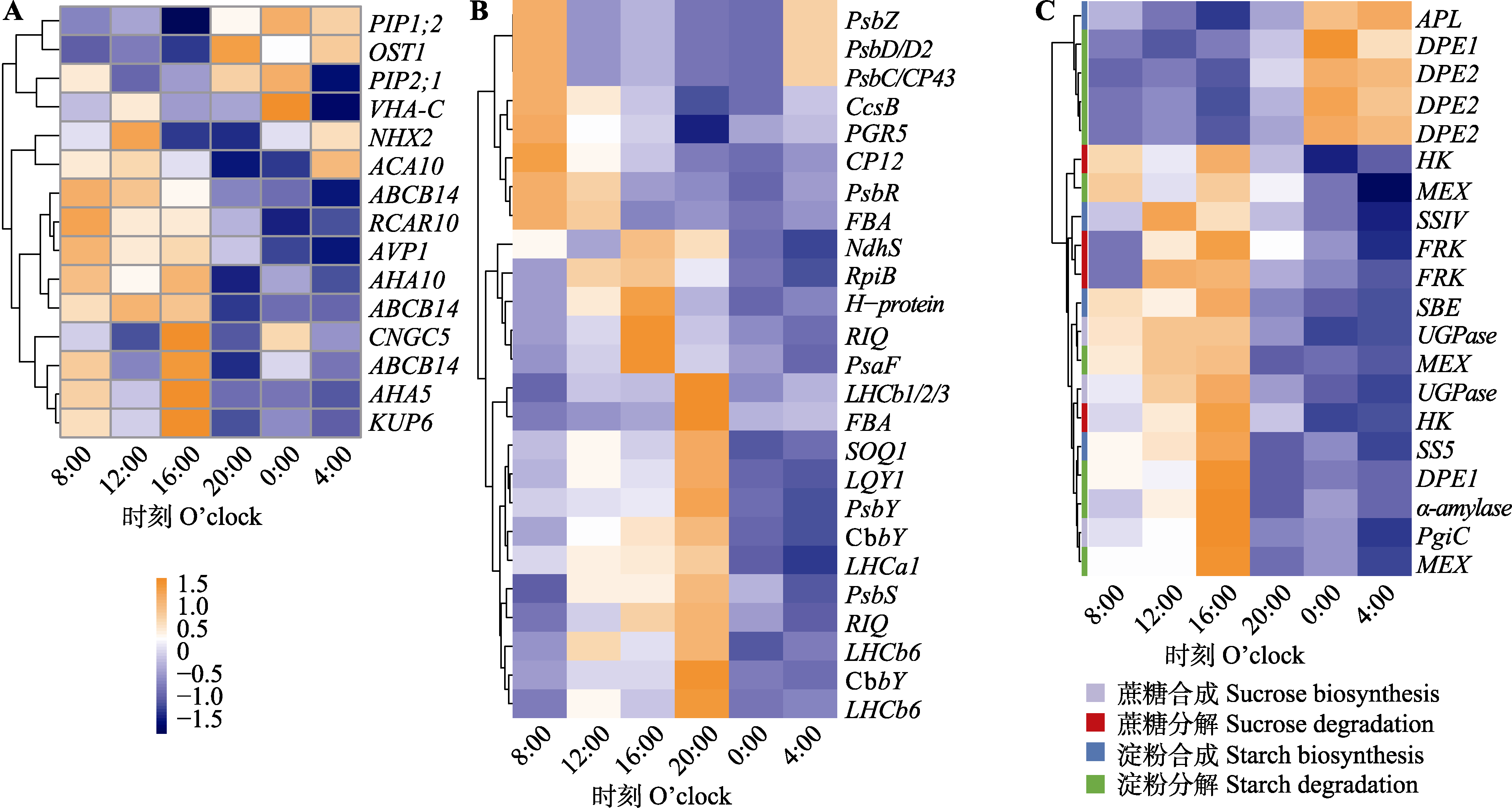
Fig. 2 Expression of significant rhythmic genes related to stomata (A), photosynthesis(B) and non-structural carbohydrates (C) for Brachythecium thraustum under continuous illumination and constant environmental conditions. The heatmap used normalized expression levels, ranging from -1.5 to 1.5.
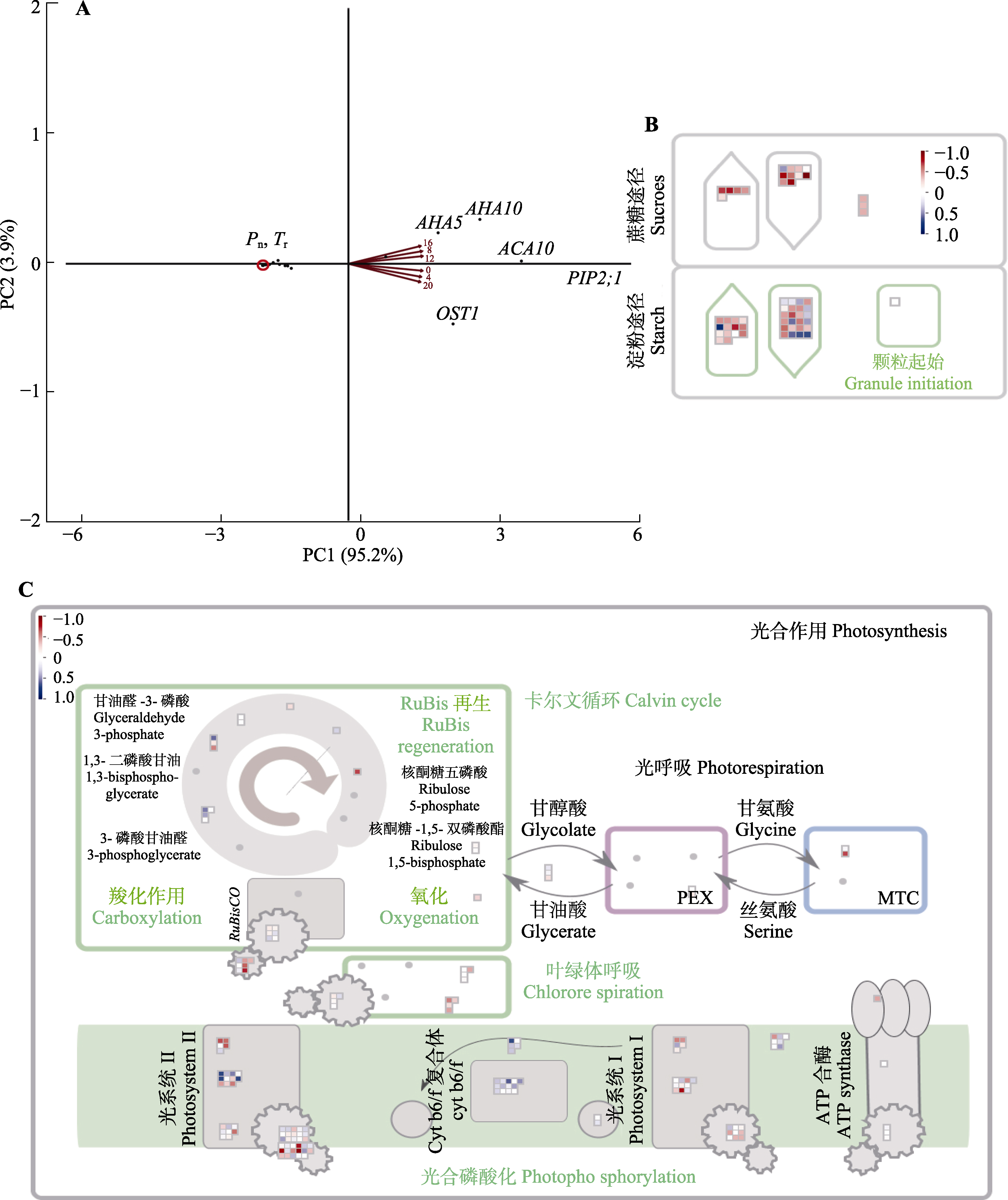
Fig. 3 Correlation analysis of genes related to Brachythecium thraustum. Principal Component (PC) analysis of 15 significant stomatal-related rhythmicity genes (A), 57 significant carbon fixation-related rhythmicity genes correlation plot (B) and 159 significant photosynthesis-related rhythmicity genes correlation plot (C) of Brachythecium thraustum. Red color indicates positive correlation, blue color indicates negative correlation. The heatmap used normalized expression levels, ranging from -1 to 1. In this scheme, blue regions represent high expression levels, while red regions represent low expression levels. ACA10, Ca2+-ATPase 10; AHA5, plasma membrane H+-ATPase 5; AHA10, H+-ATPase 10; MTC, membrane-intrinsic light-harvesting complexes associated with PSII reaction centers; OST1, open stomata 1; PEX, photosystem II (PSII) extrinsic proteins; PIP2;1, aquaporin PIP2-1; Pn, photosynthetic rate; Tr, transpiration rate.
| [1] |
Brodribb TJ, McAdam SAM (2017). Evolution of the stomatal regulation of plant water content. Plant Physiology, 174, 639-649.
DOI PMID |
| [2] |
Bruce VG (1972). Mutants of the biological clock in Chlamydomonas reinhardi. Genetics, 70, 537-548.
DOI PMID |
| [3] |
Buchfink B, Xie C, Huson DH (2015). Fast and sensitive protein alignment using DIAMOND. Nature Methods, 12, 59-60.
DOI PMID |
| [4] |
Byrne TE, Wells MR, Johnson CH (1992). Circadian rhythms of chemotaxis to ammonium and of methylammonium uptake in chlamydomonas. Plant Physiology, 98, 879-886.
DOI PMID |
| [5] |
Chaves MM, Flexas J, Pinheiro C (2008). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551-560.
DOI URL |
| [6] |
Chen ZH, Chen G, Dai F, Wang YZ, Hills A, Ruan YL, Zhang GP, Franks PJ, Nevo E, Blatt MR (2017). Molecular evolution of grass stomata. Trends in Plant Science, 22, 124-139.
DOI URL |
| [7] |
Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004). A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. The Plant Journal, 37, 853-863.
DOI URL |
| [8] |
Dodd AN, Kusakina J, Hall A, Gould PD, Hanaoka M (2014). The circadian regulation of photosynthesis. Photosynthesis Research, 119, 181-190.
DOI PMID |
| [9] |
Emms DM, Kelly S (2019). OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biology, 20, 238. DOI: 10.1186/s13059-019-1832-y.
DOI PMID |
| [10] |
Endo M (2016). Tissue-specific circadian clocks in plants. Current Opinion in Plant Biology, 29, 44-49.
DOI PMID |
| [11] |
Ferrari C, Proost S, Janowski M, Becker J, Nikoloski Z, Bhattacharya D, Price D, Tohge T, Bar-Even A, Fernie A, Stitt M, Mutwil M (2019). Kingdom-wide comparison reveals the evolution of diurnal gene expression in Archaeplastida. Nature Communications, 10, 737. DOI: 10.1038/s41467-019-08703-2.
DOI PMID |
| [12] |
Fogelmark K, Troein C (2014). Rethinking transcriptional activation in the Arabidopsis circadian clock. PLoS Computational Biology, 10, e1003705. DOI: 10.1371/journal.pcbi.1003705.
DOI URL |
| [13] | Glime JM (2007). Chapter 2—Life cycles and morphology// Glime JM. Bryophyte Ecology Volume 1: Physiological Ecology. [2022-04-18]. https://digitalcommons.mtu.edu/bryophyte-ecology1/1. |
| [14] |
Goto K, Johnson CH (1995). Is the cell division cycle gated by a circadian clock? The case of Chlamydomonas reinhardtii. Journal of Cell Biology, 129, 1061-1069.
DOI PMID |
| [15] |
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng QD, Chen ZH, Mauceli E, Hacohen N, Gnirke A, Rhind N, et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology, 29, 644-652.
DOI PMID |
| [16] |
Greenham K, McClung CR (2015). Integrating circadian dynamics with physiological processes in plants. Nature Reviews Genetics, 16, 598-610.
DOI PMID |
| [17] |
Hauser F, Waadt R, Schroeder JI (2011). Evolution of abscisic acid synthesis and signaling mechanisms. Current Biology, 21, R346-R355.
DOI URL |
| [18] |
Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AAR (2013). Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature, 502, 689-692.
DOI |
| [19] | Jones MA (2017). Interplay of circadian rhythms and light in the regulation of photosynthesis-derived metabolism. Progress in Botany, 79, 147-171. |
| [20] |
Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han LQ, David K, Putterill J, Nam HG, Somers DE (2007). ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature, 449, 356-360.
DOI |
| [21] |
Li B, Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12, 323. DOI: 10.1186/1471-2105-12-323.
DOI PMID |
| [22] |
Linde AM, Eklund DM, Kubota A, Pederson ERA, Holm K, Gyllenstrand N, Nishihama R, Cronberg N, Muranaka T, Oyama T, Kohchi T, Lagercrantz U (2017). Early evolution of the land plant circadian clock. New Phytologist, 216, 576-590.
DOI URL |
| [23] |
Más P, Kim WY, Somers DE, Kay SA (2003). Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature, 426, 567-570.
DOI |
| [24] |
Moulager M, Monnier A, Jesson B, Bouvet R, Mosser J, Schwartz C, Garnier L, Corellou F, Bouget FY (2007). Light-dependent regulation of cell division in Ostreococcus: evidence for a major transcriptional input. Plant Physiology, 144, 1360-1369.
DOI PMID |
| [25] |
Noordally ZB, Ishii K, Atkins KA, Wetherill SJ, Kusakina J, Walton EJ, Kato M, Azuma M, Tanaka K, Hanaoka M, Dodd AN (2013). Circadian control of chloroplast transcription by a nuclear-encoded timing signal. Science, 339, 1316-1319.
DOI PMID |
| [26] |
Okada R, Kondo S, Satbhai SB, Yamaguchi N, Tsukuda M, Aoki S (2009). Functional characterization of CCA1/LHY homolog genes, PpCCA1a and PpCCA1b, in the moss Physcomitrella patens. The Plant Journal, 60, 551-563.
DOI PMID |
| [27] |
Pilgrim ML, McClung CR (1993). Differential involvement of the circadian clock in the expression of genes required for ribulose-1,5-bisphosphate carboxylase/oxygenase synthesis, assembly, and activation in Arabidopsis thaliana. Plant Physiology, 103, 553-564.
PMID |
| [28] |
Pokhilko A, Fernández AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ (2012). The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Molecular Systems Biology, 8, 574. DOI: 10.1038/msb.2012.6.
DOI URL |
| [29] |
Resco de Dios V, Arthur G, Pedro FJ, Alday JG, Michael B, Jorge DC, Sébastien D, Sonia GM, Zachary K, Damien L, Paula MG, Alexandru M, Clément P, Karin PW, Olivier R, et al. (2016). Circadian rhythms have significant effects on leaf-to-canopy scale gas exchange under field conditions. GigaScience, 5, 43. DOI: 10.1186/s13742-016-0149-y.
DOI PMID |
| [30] |
Resco de Dios V, Gessler A (2018). Circadian regulation of photosynthesis and transpiration from genes to ecosystems. Environmental and Experimental Botany, 152, 37-48.
DOI URL |
| [31] |
Resco de Dios V, Turnbull MH, Barbour MM, Ontedhu J, Ghannoum O, Tissue DT (2013). Soil phosphorous and endogenous rhythms exert a larger impact than CO2 or temperature on nocturnal stomatal conductance in Eucalyptus tereticornis. Tree Physiology, 33, 1206-1215.
DOI URL |
| [32] |
Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011). Land plants acquired active stomatal control early in their evolutionary history. Current Biology, 21, 1030-1035.
DOI PMID |
| [33] |
Schwacke R, Ponce-Soto GY, Krause K, Bolger AM, Arsova B, Hallab A, Gruden K, Stitt M, Bolger ME, Usadel B (2019). MapMan4: a refined protein classification and annotation framework applicable to multi-omics data analysis. Molecular Plant, 12, 879-892.
DOI PMID |
| [1] | YANG Shang-Jin, FAN Yun-Xiang, ZHANG Yu-Wen, HAN Qiao-Ling, ZHAO Yue, DUAN Jie, DI Nan, XI Ben-Ye. Comparison of methods for dividing nighttime sap flow components in Populus tomentosa trees [J]. Chin J Plant Ecol, 2024, 48(4): 496-507. |
| [2] | LI Wei-Bin, ZHANG Hong-Xia, ZHANG Yu-Shu, CHEN Ni-Na. Influence of diurnal asymmetric warming on carbon sink capacity in a broadleaf Korean pine forest in Changbai Mountains, China [J]. Chin J Plant Ecol, 2023, 47(9): 1225-1233. |
| [3] | WANG Jia-Yi, WANG Xiang-Ping, XU Cheng-Yang, XIA Xin-Li, XIE Zong-Qiang, FENG Fei, FAN Da-Yong. Response of hydraulic architecture in Fraxinus velutina street trees to the percentage of impervious pavement in Beijing [J]. Chin J Plant Ecol, 2023, 47(7): 998-1009. |
| [4] | WANG Xiu-Ying, CHEN Qi, DU Hua-Li, ZHANG Rui, MA Hong-Lu. Evapotranspiration interpolation in alpine marshes wetland on the Qingzang Plateau based on machine learning [J]. Chin J Plant Ecol, 2023, 47(7): 912-921. |
| [5] | ZHANG Min, SANG Ying, SONG Jin-Feng. Root pressure of hydroponic Dracaena sanderiana and its determinants [J]. Chin J Plant Ecol, 2023, 47(7): 1010-1019. |
| [6] | BAI Xue, LI Yu-Jing, JING Xiu-Qing, ZHAO Xiao-Dong, CHANG Sha-Sha, JING Tao-Yu, LIU Jin-Ru, ZHAO Peng-Yu. Response mechanisms of millet and its rhizosphere soil microbial communities to chromium stress [J]. Chin J Plant Ecol, 2023, 47(3): 418-433. |
| [7] | ZHAO Xiao-Ning, TIAN Xiao-Nan, LI Xin, LI Guang-De, GUO You-Zheng, JIA Li-Ming, DUAN Jie, XI Ben-Ye. Analysis of applicability of Granier’s original equation for calculating the stem sap flux density—Take Populus tomentosa as an example [J]. Chin J Plant Ecol, 2023, 47(3): 404-417. |
| [8] | LIU Hai-Yan, ZANG Sha-Sha, ZHANG Chun-Xia, ZUO Jin-Cheng, RUAN Zuo-Xi, WU Hong-Yan. Photochemical reaction of photosystem II in diatoms under phosphorus starvation and its response to high light intensity [J]. Chin J Plant Ecol, 2023, 47(12): 1718-1727. |
| [9] | ZHU Yu-Ying, ZHANG Hua-Min, DING Ming-Jun, YU Zi-Ping. Changes of vegetation greenness and its response to drought-wet variation on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(1): 51-64. |
| [10] | FENG Yin-Cheng, WANG Yun-Qi, WANG Yu-Jie, WANG Kai, WANG Song-Nian, WANG Jie-Shuai. Water vapor fluxes and their relationship with environmental factors in a conifer-broadleaf mixed forest ecosystem in Jinyun Mountain, Chongqing, China [J]. Chin J Plant Ecol, 2022, 46(8): 890-903. |
| [11] | XIONG Bo-Wen, LI Tong, HUANG Ying, YAN Chun-Hua, QIU Guo-Yu. Effects of different reference temperature values on the accuracy of vegetation transpiration estimation by three-temperature model [J]. Chin J Plant Ecol, 2022, 46(4): 383-393. |
| [12] | MA Yan-Ze, YANG Xi-Lai, XU Yan-Sen, FENG Zhao-Zhong. Response of key parameters of leaf photosynthetic models to increased ozone concentration in four common trees [J]. Chin J Plant Ecol, 2022, 46(3): 321-329. |
| [13] | HUANG Ying, CHEN Zhi, SHI Zhe, XIONG Bo-Wen, YAN Chun-Hua, QIU Guo-Yu. Temporal and spatial variation characteristics and different calculation methods for the key parameter αe in the generalized complementary principle of evapotranspiration [J]. Chin J Plant Ecol, 2022, 46(3): 300-310. |
| [14] | YUAN Yuan, MU Yan-Mei, DENG Yu-Jie, LI Xin-Hao, JIANG Xiao-Yan, GAO Sheng-Jie, ZHA Tian- Shan, JIA Xin. Effects of land cover and phenology changes on the gross primary productivity in an Artemisia ordosica shrubland [J]. Chin J Plant Ecol, 2022, 46(2): 162-175. |
| [15] | WANG Li-Shuang, TONG Xiao-Juan, MENG Ping, ZHANG Jin-Song, LIU Pei-Rong, LI Jun, ZHANG Jing-Ru, ZHOU Yu. Energy flux and evapotranspiration of two typical plantations in semi-arid area of western Liaoning, China [J]. Chin J Plant Ecol, 2022, 46(12): 1508-1522. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn