

Chin J Plan Ecolo ›› 2018, Vol. 42 ›› Issue (7): 713-722.DOI: 10.17521/cjpe.2018.0029
• Research Articles • Previous Articles Next Articles
LIU Yuan-Yuan1,2,3, MA Jin-Ze1,2,3, BU Zhao-Jun1,2,3,*( ), WANG Sheng-Zhong1,2,3,*(
), WANG Sheng-Zhong1,2,3,*( ), ZHANG Xue-Bing1, ZHANG Ting-Yu1, LIU Sha-Sha1,2,3, FU Biao1, KANG Yuan1,2,3
), ZHANG Xue-Bing1, ZHANG Ting-Yu1, LIU Sha-Sha1,2,3, FU Biao1, KANG Yuan1,2,3
Online:2018-07-20
Published:2018-11-03
Contact:
Zhao-Jun BU,Sheng-Zhong WANG
Supported by:LIU Yuan-Yuan, MA Jin-Ze, BU Zhao-Jun, WANG Sheng-Zhong, ZHANG Xue-Bing, ZHANG Ting-Yu, LIU Sha-Sha, FU Biao, KANG Yuan. Effect of geographical sources and biochemical traits on plant litter decomposition in a peatland[J]. Chin J Plan Ecolo, 2018, 42(7): 713-722.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2018.0029
| 埋放地 Site for decomposition | 来源地 Site for collection | 物种 Species |
|---|---|---|
| 哈泥 Hani | 大九湖 Dajiuhu | 泥炭藓 Sphagnum palustre |
| 签草 Carex doniana | ||
| 红桦 Betula albosinensis | ||
| 哈泥 Hani | 中央泥炭藓 S. centrale | |
| 中位泥炭藓 S. magellanicum | ||
| 毛薹草 C. lasiocarpa | ||
| 油桦 B. fruticosa var. ruprechtiana | ||
| 满归 Mangui | 中位泥炭藓 S. magellanicum | |
| 锈色泥炭藓S. fuscum | ||
| 瘤囊薹草 C. schmidtii | ||
| 柴桦 B. fruticosa |
Table 1 The sites for litter collection and litter decomposition
| 埋放地 Site for decomposition | 来源地 Site for collection | 物种 Species |
|---|---|---|
| 哈泥 Hani | 大九湖 Dajiuhu | 泥炭藓 Sphagnum palustre |
| 签草 Carex doniana | ||
| 红桦 Betula albosinensis | ||
| 哈泥 Hani | 中央泥炭藓 S. centrale | |
| 中位泥炭藓 S. magellanicum | ||
| 毛薹草 C. lasiocarpa | ||
| 油桦 B. fruticosa var. ruprechtiana | ||
| 满归 Mangui | 中位泥炭藓 S. magellanicum | |
| 锈色泥炭藓S. fuscum | ||
| 瘤囊薹草 C. schmidtii | ||
| 柴桦 B. fruticosa |
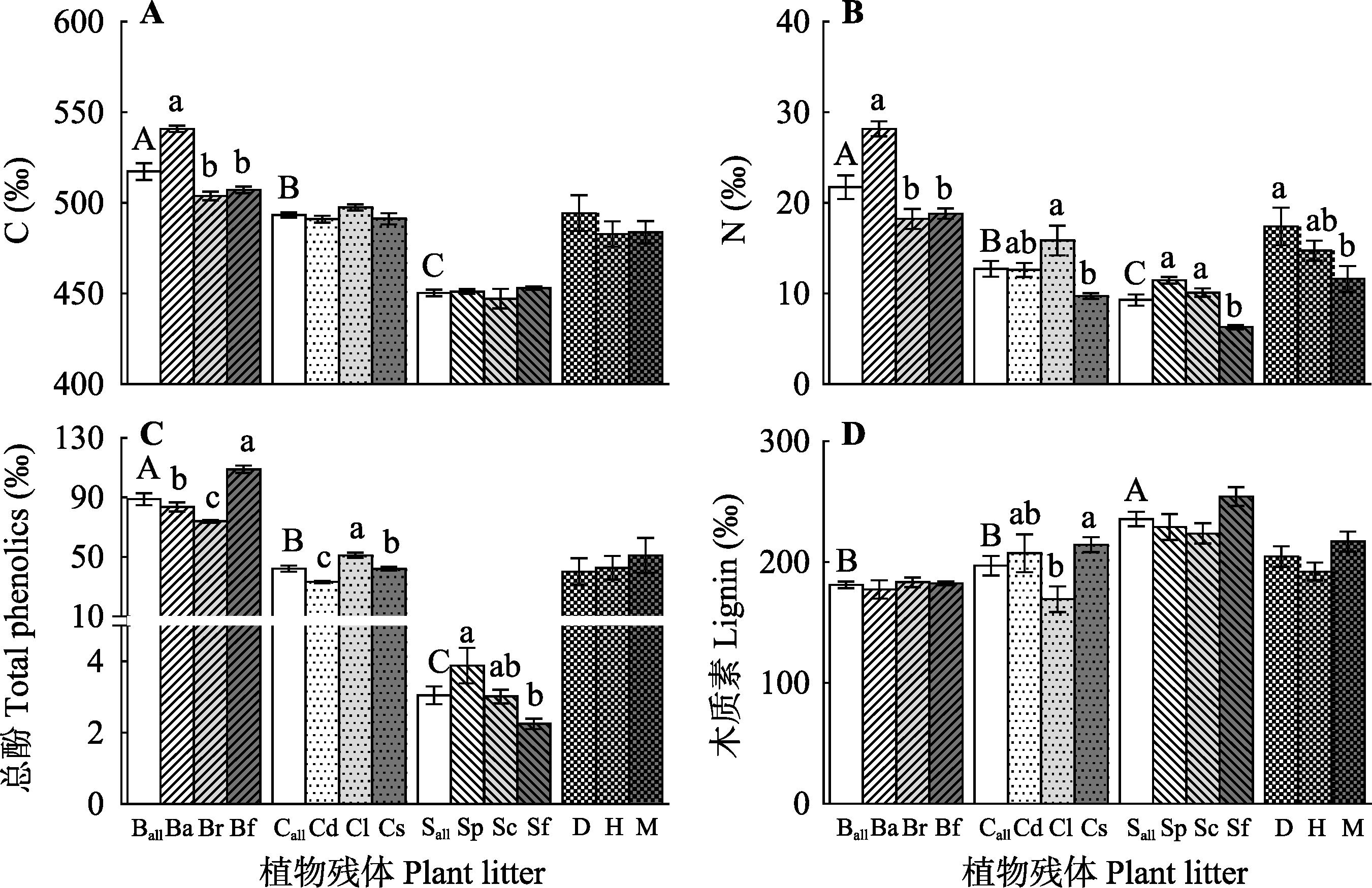
Fig. 1 Initial chemical composition of each plant litter in a peatland and initial chemical composition of all the plant litters from each peatland (mean ± SE, n = 5). Ball, the mean of Betula; Ba, B. albosinensis; Br, B. fruticosa var. ruprechtiana; Bf, B. fruticosa; Call, the mean of Carex; Cd, C. doniana; Cl, C. lasiocarpa; Cs, C. schmidtii; Sall, the mean of Sphagnum; Sp, S. palustre; Sc, S. centrale; Sm, S. magellanicum; Sf, S. fuscum. D, the mean of Dajiuhu; H, the mean of Hani; M, the mean of Mangui. Different capital letters indicate significant differences in initial chemical composition among genera (p < 0.05), and different lowercase letters indicate significant differences in initial chemical composition between both species in a genus or average of all the species among three sites (p < 0.05).
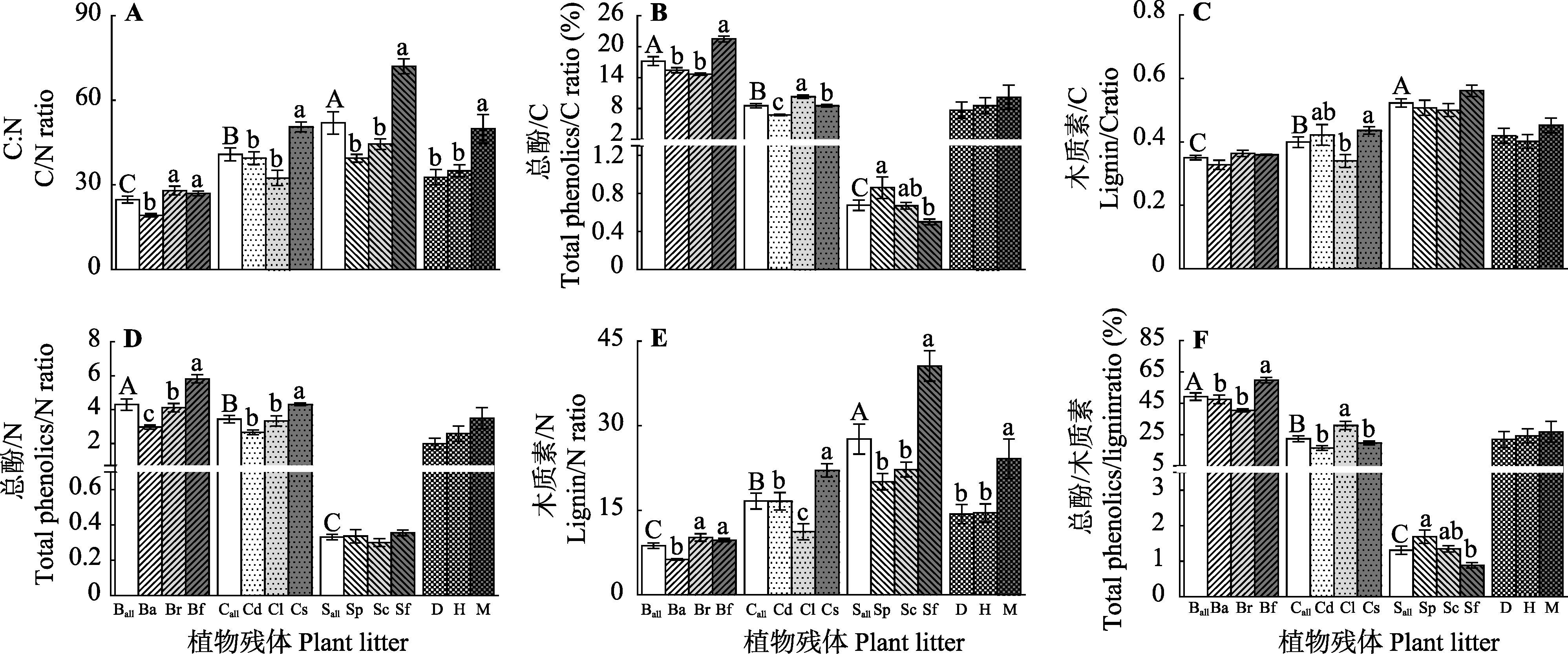
Fig. 2 Initial stoichiometric ratio of each plant litter in a peatland and average initial stoichiometric ratios of all the plant litters from each peatland (mean ± SE, n = 5). Different capital letters indicate significant differences in initial stoichiometric ratios among genera (p < 0.05). Different lowercase letters indicate significant differences in initial stoichiometric ratios between both species in a genus and average of all the species from three sources (p < 0.05). See Fig. 1 for notes.
| 因素 Factor | 相关系数 Correlation coefficient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | 总酚 Total phenolics | 木质素 Lignin | C/N | 总酚/C Total phenolics /C | 木质素/C Lignin/C | 总酚/N Total phenolics/N | 木质素/N Lignin/N | 总酚/木质素Total phenolics/Lignin | ||
| 种 Species | B | 106.293*** | 41.509*** | 62.296*** | 0.400 | 19.628*** | 69.213*** | 3.821 | 45.356*** | 24.485*** | 25.207*** |
| <0.001 | <0.001 | <0.001 | 0.679 | <0.001 | <0.001 | 0.052 | <0.001 | <0.001 | <0.001 | ||
| C | 2.657 | 8.308** | 45.047*** | 4.476* | 16.272*** | 40.042*** | 5.066* | 18.049*** | 15.195** | 16.497*** | |
| 0.111 | 0.005 | <0.001 | 0.035 | <0.001 | <0.001 | 0.025 | <0.001 | 0.001 | <0.001 | ||
| S | 0.827 | 52.314*** | 6.552* | 3.242* | 73.627*** | 6.765** | 2.544 | 1.109 | 34.728*** | 9.823** | |
| 0.461 | <0.001 | 0.012 | 0.075 | <0.001 | 0.011 | 0.120 | 0.361 | <0.001 | 0.003 | ||
| 属 Genus | - | 129.384*** | 43.542*** | 257.553*** | 21.628*** | 24.742*** | 229.423*** | 47.086*** | 84.932*** | 28.998*** | 184.473*** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| 来源地 Source | - | 0.660 | 3.341* | 0.346 | 2.324 | 7.034** | 0.462 | 1.294 | 2.473 | 5.306** | 0.200 |
| 0.522 | 0.045 | 0.709 | 0.110 | 0.002 | 0.633 | 0.285 | 0.097 | 0.009 | 0.819 | ||
Table 2 One-way analysis of variance for the effect of species, genus and source of plants on initial chemical index and stoichiometric ratios of litters
| 因素 Factor | 相关系数 Correlation coefficient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | 总酚 Total phenolics | 木质素 Lignin | C/N | 总酚/C Total phenolics /C | 木质素/C Lignin/C | 总酚/N Total phenolics/N | 木质素/N Lignin/N | 总酚/木质素Total phenolics/Lignin | ||
| 种 Species | B | 106.293*** | 41.509*** | 62.296*** | 0.400 | 19.628*** | 69.213*** | 3.821 | 45.356*** | 24.485*** | 25.207*** |
| <0.001 | <0.001 | <0.001 | 0.679 | <0.001 | <0.001 | 0.052 | <0.001 | <0.001 | <0.001 | ||
| C | 2.657 | 8.308** | 45.047*** | 4.476* | 16.272*** | 40.042*** | 5.066* | 18.049*** | 15.195** | 16.497*** | |
| 0.111 | 0.005 | <0.001 | 0.035 | <0.001 | <0.001 | 0.025 | <0.001 | 0.001 | <0.001 | ||
| S | 0.827 | 52.314*** | 6.552* | 3.242* | 73.627*** | 6.765** | 2.544 | 1.109 | 34.728*** | 9.823** | |
| 0.461 | <0.001 | 0.012 | 0.075 | <0.001 | 0.011 | 0.120 | 0.361 | <0.001 | 0.003 | ||
| 属 Genus | - | 129.384*** | 43.542*** | 257.553*** | 21.628*** | 24.742*** | 229.423*** | 47.086*** | 84.932*** | 28.998*** | 184.473*** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| 来源地 Source | - | 0.660 | 3.341* | 0.346 | 2.324 | 7.034** | 0.462 | 1.294 | 2.473 | 5.306** | 0.200 |
| 0.522 | 0.045 | 0.709 | 0.110 | 0.002 | 0.633 | 0.285 | 0.097 | 0.009 | 0.819 | ||
| 因素 Factor | 干质量损失 Dry mass loss (%) | C损失 Carbon loss (%) | N损失 Nitrogen loss (%) | 总酚损失 Total phenolics loss (%) | 木质素损失 Lignin loss (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | ||
| 种 Species | B | 1.411 | 0.282 | 0.920 | 0.425 | 0.990 | 0.400 | 0.020 | 0.980 | 4.726* | 0.031 |
| C | 16.381*** | <0.001 | 14.883** | 0.001 | 15.432*** | <0.001 | 8.005** | 0.006 | 2.229 | 0.150 | |
| S | 2.524 | 0.122 | 2.902 | 0.094 | 38.145*** | <0.001 | 4.842* | 0.029 | 18.463*** | <0.001 | |
| 属 Genus | - | 57.069*** | <0.001 | 50.719*** | <0.001 | 0.387 | 0.681 | 417.741*** | <0.001 | 3.235* | 0.049 |
| 来源地 Source | - | 0.046 | 0.995 | 0.025 | 0.976 | 1.598 | 0.214 | 0.201 | 0.818 | 1.804 | 0.177 |
Table 3 One-way analysis of variance for the effect of species, genus and source of plant litters on decomposition in a peatland
| 因素 Factor | 干质量损失 Dry mass loss (%) | C损失 Carbon loss (%) | N损失 Nitrogen loss (%) | 总酚损失 Total phenolics loss (%) | 木质素损失 Lignin loss (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | ||
| 种 Species | B | 1.411 | 0.282 | 0.920 | 0.425 | 0.990 | 0.400 | 0.020 | 0.980 | 4.726* | 0.031 |
| C | 16.381*** | <0.001 | 14.883** | 0.001 | 15.432*** | <0.001 | 8.005** | 0.006 | 2.229 | 0.150 | |
| S | 2.524 | 0.122 | 2.902 | 0.094 | 38.145*** | <0.001 | 4.842* | 0.029 | 18.463*** | <0.001 | |
| 属 Genus | - | 57.069*** | <0.001 | 50.719*** | <0.001 | 0.387 | 0.681 | 417.741*** | <0.001 | 3.235* | 0.049 |
| 来源地 Source | - | 0.046 | 0.995 | 0.025 | 0.976 | 1.598 | 0.214 | 0.201 | 0.818 | 1.804 | 0.177 |
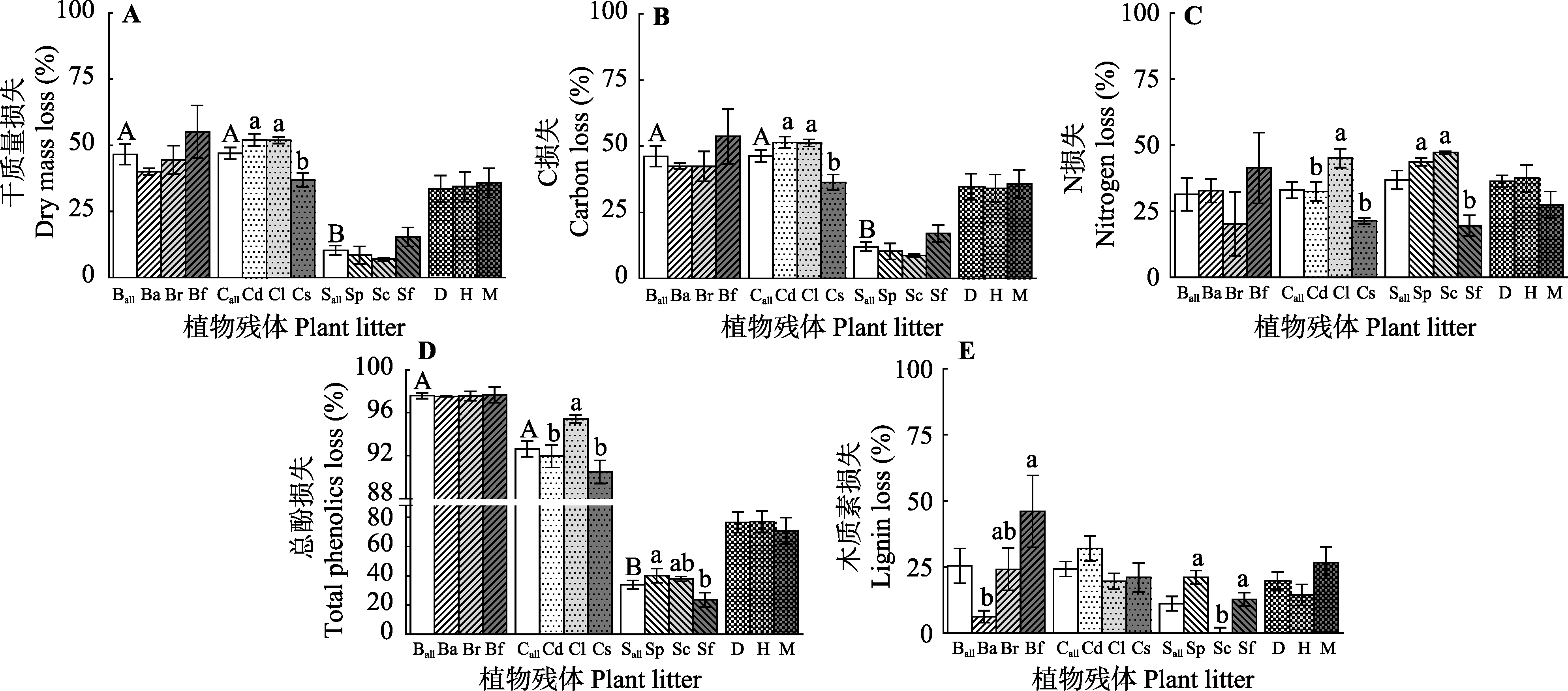
Fig. 3 The effects of species and source on losses of litter dry mass (A), C (B), N (C), total phenolics (D) and lignin (E)(mean ± SE, n = 5). Different capital letters indicate significant differences in the effects of different genera on dry mass, C, N, total phenolics and lignin loss (p < 0.05). Different lowercase letters indicate significant differences in dry mass, C, N, total phenolics and lignin losses between different species in a same genus and among all species from different sources (p < 0.05). See Fig. 1 for notes.
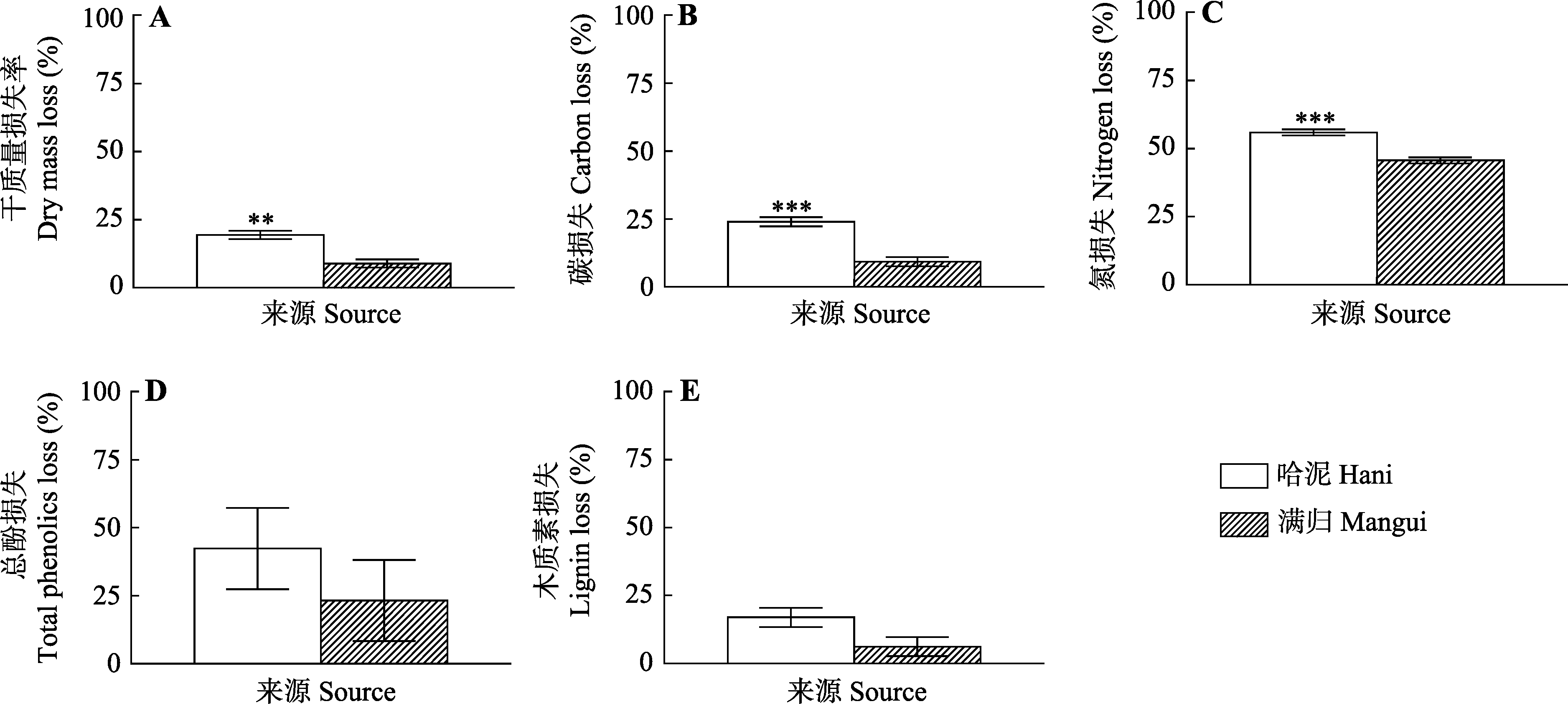
Fig. 4 Effect of plant litter source on the losses of dry mass (A), C (B), N (C), total phenolics (D) and lignin (E) of Sphagnum magellanicum litters (mean ± SE, n = 5). **, p < 0.01; ***, p < 0.001.
| 相关系数 Correlation coefficient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | 总酚 Total phenolics | 木质素 Lignin | C/N | 总酚/C Total phenolics/C | 木质素/C Lignin/C | 多酚/N Total phenolics/N | 木质素/N Lignin/N | 多酚/木质素 Total phenolics/Lignin | |
| B | -0.291 | -0.301 | 0.370 | 0.264 | 0.286 | 0.404 | 0.280 | 0.433 | 0.281 | 0.345 |
| C | 0.400 | 0.720** | 0.002 | -0.526* | -0.787** | -0.014 | -0.518* | -0.784** | -0.740** | 0.251 |
| S | 0.469 | -0.493 | -0.403 | 0.544* | 0.516* | -0.403 | 0.543* | 0.472 | 0.523* | -0.444 |
| 总体 Total | 0.796* | 0.531 | 0.801** | -0.793** | -0.555 | 0.815** | -0.840** | 0.870** | -0.655 | 0.793* |
Table 4 Correlation analysis between dry mass loss and initial chemical traits in plant litters
| 相关系数 Correlation coefficient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | 总酚 Total phenolics | 木质素 Lignin | C/N | 总酚/C Total phenolics/C | 木质素/C Lignin/C | 多酚/N Total phenolics/N | 木质素/N Lignin/N | 多酚/木质素 Total phenolics/Lignin | |
| B | -0.291 | -0.301 | 0.370 | 0.264 | 0.286 | 0.404 | 0.280 | 0.433 | 0.281 | 0.345 |
| C | 0.400 | 0.720** | 0.002 | -0.526* | -0.787** | -0.014 | -0.518* | -0.784** | -0.740** | 0.251 |
| S | 0.469 | -0.493 | -0.403 | 0.544* | 0.516* | -0.403 | 0.543* | 0.472 | 0.523* | -0.444 |
| 总体 Total | 0.796* | 0.531 | 0.801** | -0.793** | -0.555 | 0.815** | -0.840** | 0.870** | -0.655 | 0.793* |
| [1] |
Aerts R ( 1997). Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos, 79, 439-449.
DOI URL |
| [2] |
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH ( 2009). Home-field advantage accelerates leaf litter decomposition in forests. Soil Biology & Biochemistry, 41, 606-610.
DOI URL |
| [3] | Bai GR, Wang SZ, Gao J, Yu JL ( 2004). Liquid-heat conditions and microbic decomposition on the forming of turf deposits. Journal of Shanghai Normal University (Natural Sciences), 33(3), 91-97. |
| [ 白光润, 王淑珍, 高峻, 于金莲 ( 2004). 中国亚热带、热带泥炭形成的水热条件与微生物分解相关性. 上海师范大学学报(自然科学版), 33(3), 91-97.] | |
| [4] |
Berg B, Berg MP, Bottner P, Box E, Breymeyer A, Anta RCD, Couteaux M, Escudero A, Gallardo A, Kratz WR, Madeira M, M?lk?nen E, McClaugherty CA, Meentemeyer V, Mu?oz F, Piussi P, Remacle JA, Santo AVD ( 1993). Litter mass loss rates in pine forest of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry, 20(3), 127-159.
DOI URL |
| [5] |
Bragazza L, Freeman C, Jones T, Rydin H, Limpens J, Fenner N, Ellis T, Gerdol R, Hájek M, Hájek T, Iacumin P, Kutnar L, Tahvanainen T, Toberman H ( 2006). Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proceedings of the National Academy of Sciences of the United States of America, 103, 19386-19389.
DOI URL PMID |
| [6] |
Bragazza L, Siffi C, Iacumin P, Gerdol R ( 2007). Mass loss and nutrient release during litter decay in peatland: The role of microbial adaptability to litter chemistry. Soil Biology & Biochemistry, 39, 257-267.
DOI URL |
| [7] |
Breeuwer A, Heijmans M, Robroek BJM, Limpens J, Berendse F ( 2008). The effect of increased temperature and nitrogen deposition on decomposition in bogs. Oikos, 117, 1258-1268.
DOI URL |
| [8] |
Bubier JL, Moore TR, Bledzki LA ( 2007). Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Global Change Biology, 13, 1168-1186.
DOI URL |
| [9] |
Bu ZJ, Joosten H, Li HK, Zhao GL, Zheng XX, Ma JZ, Zeng J ( 2011 a). The response of peatlands to climate warming: A review. Acta Ecologica Sinica, 31, 157-162.
DOI URL |
| [10] |
Bu ZJ, Rydin H, Chen X ( 2011 b). Direct and interaction-mediated effects of environmental changes on peatland bryophytes. Oecologia, 166, 555-563.
DOI URL PMID |
| [11] |
Carvalhais N, Forkel M, Khomik M, Bellarby J, Jung M, Migliavacca M, Mu M, Saatchi S, Santoro M, Thurner M, Weber U, Ahrens B, Beer C, Cescatti A, Randerson JT, Reichstein M ( 2014). Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature, 514, 213-217.
DOI URL PMID |
| [12] |
Chen YH, Han WX, Tang LY, Tang ZY, Fang JY ( 2013). Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography, 36, 178-184.
DOI URL |
| [13] |
Coq S, Weigel J, Butenschoen O, Bonal D, H?ttenschwiler S ( 2011). Litter composition rather than plant presence affects decomposition of tropical litter mixtures. Plant and Soil, 343, 273-286.
DOI URL |
| [14] |
Dorrepaal E, Cornelissen JHC, Aerts R, Wallén B, van Logtestijn RSP ( 2005). Are growth forms consistent predictors of leaf litter quality and decomposability across peatlands along a latitudinal gradient? Journal of Ecology, 93, 817-828.
DOI URL |
| [15] |
Dyer ML, Meentemeyer V, Berg B ( 1990). Apparent controls of mass loss rate of leaf litter on a regional scale. Scandinavian Journal of Forest Research, 5, 311-323.
DOI URL |
| [16] | Gogo S, Laggoun-Défarge F, Merzouki F, Mounier S, Guirimand-Dufour A, Jozja N, Huguet A, Delarue F, Défarge C ( 2016). In situ and laboratory non-additive litter mixture effect on C dynamics of Sphagnum rubellum and Molinia caerulea litters. Journal of Soils & Sediments, 16, 13-27. |
| [17] |
Hobbie SE ( 2008). Nitrogen effects on decomposition: A five-year experiment in eight temperate sites. Ecology, 89, 2633-2644.
DOI URL PMID |
| [18] |
Irons III JG, Oswood MW, Stout RJ, Pringle CM ( 1994). Latitudinal patterns in leaf litter breakdown: Is temperature really important? Freshwater Biology, 32, 401-411.
DOI URL |
| [19] | Johnson LC, Damman AWH ( 1993). Decay and its regulation in Sphagnum peatlands. Advances in Bryology, 5, 249-296. |
| [20] |
Johnson LC, Damman AWH ( 1991). Species-controlled Sphagnum decay on a South Swedish raised bog. Oikos, 61, 234-242.
DOI URL |
| [21] |
K?rner C ( 1989). The nutritional status of plants from high altitudes. Oecologia, 81, 379-391.
DOI URL |
| [22] | Li W, Bu ZJ, Zhang BJ, Long C, Tang RJ, Cui QW ( 2013). Decomposition of Sphagnum litter in 4 peatlands of the Changbai Mountains along an altitudinal gradient. Journal of Mountain Science , 31, 442-447. |
| [ 李伟, 卜兆君, 张兵将, 龙川, 唐瑞江, 崔钱王 ( 2013). 长白山不同海拔泥炭地泥炭藓残体的分解. 山地学报, 31, 442-447.] | |
| [23] |
Medvedeff CA, Bridgham SD, Pfeifer-Meister L, Keller JK ( 2015). Can Sphagnum leachate chemistry explain differences in anaerobic decomposition in peatlands? Soil Biology & Biochemistry, 86, 34-41.
DOI URL |
| [24] |
Melillo JM, Aber JD, Muratore JF ( 1982). Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology, 63, 621-626.
DOI URL |
| [25] |
Moore TR, Basiliko N ( 2006). Decomposition in boreal peatlands. In: Wieder RK, Vitt DH eds. Boreal Peatland Ecosystems. Springer-Verlag, Berlin.
DOI URL |
| [26] |
Moore TR, Trofymow JA, Siltanen M, Prescott C, Group CW ( 2005). Patterns of decomposition and carbon, nitrogen, and phosphorus dynamic. Canadian Journal of Forest Research, 35, 133-142.
DOI URL |
| [27] |
Müller T, Magid J, Jensen LS, Nielsen NE ( 2003). Decomposition of plant residues of different quality in soil—DAISY model calibration and simulation based on experimental data. Ecological Modelling, 166, 3-18.
DOI URL |
| [28] |
Ouyang LM, Wang C, Wang WQ, Tong C ( 2013). Carbon, nitrogen and phosphorus stoichiometric characteristics during the decomposition of Spartina alterniflora and Cyperus malaccensis var. brevifolius litters. Acta Ecologica Sinica, 33, 389-394.
DOI URL |
|
[ 欧阳林梅, 王纯, 王维奇, 仝川 ( 2013). 互花米草与短叶茳芏枯落物分解过程中碳氮磷化学计量学特征. 生态学报, 33, 389-394.]
DOI URL |
|
| [29] | Palozzi JE, Lindo Z ( 2017). Pure and mixed litters of Sphagnum and Carex exhibit a home-field advantage in Boreal peatlands. Soil Biology & Biochemistry, 115, 161-168. |
| [30] | Rydin H, Jeglum JK ( 2013). The Biology of Peatlands. Oxford University Press, Oxford. |
| [31] |
Singleton VL, Orthofer R, Lamuela-Raventós RM ( 1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152-178.
DOI URL |
| [32] |
Straková P, Anttila J, Spetz P, Kitunen V, Tapanila T, Laiho R ( 2010). Litter quality and its response to water level drawdown in boreal peatlands at plant species and community level. Plant and Soil, 335, 501-520.
DOI URL |
| [33] |
Stubbs TL, Kennedy AC, Reisenauer PE, Burns JW ( 2009). Chemical composition of residue from cereal crops and cultivars in dryland ecosystems. Agronomy Journal, 101, 538-545.
DOI URL |
| [34] | Tahvanainen T, Haraguchi A ( 2013). Effect of pH on phenol oxidase activity on decaying Sphagnum mosses. European Journal of Soil Biology, 54, 41-47. |
| [35] |
Wang HJ, Richardson CJ, Ho MC ( 2015). Dual controls on carbon loss during drought in peatlands. Nature Climate Change, 5, 584-587.
DOI URL |
| [36] | Wang H, Yan PF, Zhan PF, Zhang XN, Liu ZY, Guo YJ, Xiao DR ( 2018). The relative contributions of litter quality, simulated rising temperature, and habitat to litter decomposition. Chinese Journal of Applied Ecology, 29, 474-482. |
| [ 王行, 闫鹏飞, 展鹏飞, 张晓宁, 刘振亚, 郭玉静, 肖德荣 ( 2018). 凋落物植物质量、模拟增温及生境对凋落物分解的相对贡献. 应用生态学报, 29, 474-482.] | |
| [37] |
Wang J, Huang JH ( 2001). Comparison of major nutrient release patterns in leaf litter decomposition in warm temperate zone of China. Acta Phytoecologica Sinica, 25, 375-380.
DOI URL |
|
[ 王瑾, 黄建辉 ( 2001). 暖温带地区主要树种叶片凋落物分解过程中主要元素释放的比较. 植物生态学报, 25, 375-380.]
DOI URL |
|
| [38] | Zeng J, Bu ZJ, Wang M, Ma JZ, Zhao HY, Li HK, Wang SZ ( 2013). Effects of nitrogen deposition on peatland: A review. Chinese Journal of Ecology, 32, 473-481. |
| [ 曾竞, 卜兆君, 王猛, 马进泽, 赵红艳, 李鸿凯, 王升忠 ( 2013). 氮沉降对泥炭地影响的研究进展. 生态学杂志, 32, 473-481.] | |
| [39] | Zhang JE (2007). Commonly Used Experimental Research Methods and Techniques in Ecology. Chemical Industry Press, Beijing. |
| [ 章家恩 (2007). 生态学常用实验研究方法与技术. 化学工业出版社, 北京.] | |
| [40] |
Zhang LH, Zhang SJ, Ye GF, Shao HB, Lin GH, Brestic M ( 2013). Changes of tannin and nutrients during decomposition of branchlets of Casuarina equisetifolia plantation in subtropical coastal areas of China. Plant Soil & Environment, 59, 74-79.
DOI URL |
| [1] | Kangwei Jiang Qing-Qing QINGZHANG Wang Yafei Li Hong Ding Yu Yang Yongqiang Tuerxunnayi Reyimu. Characteristics of plant functional groups and the relationships with soil environmental factors in the middle part of the northern slope of Tianshan Mountain under different grazing intensities [J]. Chin J Plant Ecol, 2024, 48(预发表): 0-0. |
| [2] | LI Wei, ZHANG Rong. Case verification of community structure determining community productivity in subalpine meadow [J]. Chin J Plant Ecol, 2023, 47(5): 713-723. |
| [3] | ZHAO Zhen-Xian, CHEN Yin-Ping, WANG Li-Long, WANG Tong-Tong, LI Yu-Qiang. Comparison on leaf construction cost of different plant groups in the desert area of the Hexi Corridor [J]. Chin J Plant Ecol, 2023, 47(11): 1551-1560. |
| [4] | LUO Ming-Mo, CHEN Yue, YANG Gang, HU Bin, LI Wei, CHEN Huai. Short-term response of soil prokaryotic community structure to water level restoration in degraded peatland of the Zoigê Plateau [J]. Chin J Plant Ecol, 2021, 45(5): 552-561. |
| [5] | ZONG Ning, SHI Pei-Li, ZHAO Guang-Shuai, ZHENG Li-Li, NIU Ben, ZHOU Tian-Cai, HOU Ge. Variations of nitrogen and phosphorus limitation along the environmental gradient in alpine grasslands on the Northern Xizang Plateau [J]. Chin J Plant Ecol, 2021, 45(5): 444-455. |
| [6] | GAMADAERJI , YANG Ze, TAN Xing-Ru, WANG Shan-Shan, LI Wei-Jing, YOU Cui-Hai, WANG Yan-Bing, ZHANG Bing-Wei, REN Ting-Ting, CHEN Shi-Ping. Effect of altered litter input and nitrogen addition on ecosystem aboveground primary productivity and plant functional group composition in a semiarid grassland [J]. Chin J Plant Ecol, 2020, 44(8): 791-806. |
| [7] | MIAO Bai-Ling, LIANG Cun-Zhu, SHI Ya-Bo, LIANG Mao-Wei, LIU Zhong-Ling. Temporal changes in precipitation altered aboveground biomass in a typical steppe in Nei Mongol, China [J]. Chin J Plant Ecol, 2019, 43(7): 557-565. |
| [8] | FU Yi-Wen, TIAN Da-Shuan, WANG Jin-Song, NIU Shu-Li, ZHAO Ken-Tian. Patterns and affecting factors of nitrogen use efficiency of plant leaves and roots in Nei Mongol and Qinghai-Xizang Plateau grasslands [J]. Chin J Plant Ecol, 2019, 43(7): 566-575. |
| [9] | FENG Lu, BU Zhao-Jun, WU Yu-Huan, LIU Sha-Sha, LIU Chao. Characteristic environmental factors in peatlands facilitate the formation of persistent Sphagnum spore banks [J]. Chin J Plant Ecol, 2019, 43(6): 512-520. |
| [10] | Qian YANG, Wei WANG, Hui ZENG. Effects of nitrogen addition on the plant diversity and biomass of degraded grasslands of Nei Mongol, China [J]. Chin J Plant Ecol, 2018, 42(4): 430-441. |
| [11] | LI Rui, HU Chao-Chen, XU Shi-Qi, WU Di, DONG Yu-Ping, SUN Xin-Chao, MAO Rong, WANG Xian-Wei, LIU Xue-Yan. Leaf C, N, and P concentrations and their stoichiometry in peatland plants of Da Hinggan Ling, China [J]. Chin J Plant Ecol, 2018, 42(12): 1154-1167. |
| [12] | YANG Lei, SUN Han, FAN Yan-Wen, HAN Wei, ZENG Ling-Bing, LIU Chao, WANG Xiang-Ping. Changes in leaf nitrogen and phosphorus stoichiometry of woody plants along an altitudinal gradient in Changbai Mountain, China [J]. Chin J Plan Ecolo, 2017, 41(12): 1228-1238. |
| [13] | Jing WANG, Shan-Shan WANG, Xian-Guo QIAO, Ang LI, Jian-Guo XUE, Muqier HASI, Xue-Yao ZHANG, Jian-Hui HUANG. Influence of nitrogen addition on the primary production in Nei Mongol degraded grassland [J]. Chin J Plan Ecolo, 2016, 40(10): 980-990. |
| [14] | FAN Da-Yong,XIONG Gao-Ming,ZHANG Ai-Ying,LIU Xi,XIE Zong-Qiang,LI Zhao-Jia. Effect of water-lever regulation on species selection for ecological restoration practice in the water-level fluctuation zone of Three Gorges Reservoir [J]. Chin J Plan Ecolo, 2015, 39(4): 416-432. |
| [15] | SONG Yan-Tao, ZHOU Dao-Wei, LI Qiang, WANG Ping, HUANG Ying-Xin. Leaf nitrogen and phosphorus stoichiometry in 80 herbaceous plant species of Songnen grassland in Northeast China [J]. Chin J Plant Ecol, 2012, 36(3): 222-230. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn