

植物生态学报 ›› 2012, Vol. 36 ›› Issue (8): 870-879.DOI: 10.3724/SP.J.1258.2012.00870
收稿日期:2012-02-10
接受日期:2012-05-02
出版日期:2012-02-10
发布日期:2012-08-21
通讯作者:
宋松泉
作者简介:* (E-mail: sqsong@ibcas.ac.cn)
WANG Wei-Qing, CHENG Hong-Yan, LIU Shu-Jun, SONG Song-Quan*( )
)
Received:2012-02-10
Accepted:2012-05-02
Online:2012-02-10
Published:2012-08-21
Contact:
SONG Song-Quan
摘要:
顽拗性种子脱落时具有较高的含水量和代谢活性, 对脱水高度敏感; 但顽拗性种子脱水敏感性的机理至今仍然不清楚。该文以顽拗性黄皮(Clausena lansium)种子为材料, 研究了种子和胚轴对水分丧失的响应, 在脱水过程中胚轴和子叶的呼吸速率, 胚轴和子叶线粒体的细胞色素c氧化酶(CCO)活性、外膜完整性、CCO和交替氧化酶(AOX)途径以及线粒体活性氧清除酶活性的变化。结果表明, 随着水分的丧失, 种子和胚轴的存活率逐渐下降, 种子的脱水敏感性大于胚轴; 胚轴和子叶的呼吸速率以及线粒体外膜的完整性降低。胚轴和子叶线粒体的CCO途径以及胚轴AOX途径的呼吸速率在脱水初期增加, 随着继续脱水下降, 胚轴线粒体AOX途径的呼吸速率则随着脱水显著下降。胚轴线粒体的超氧化物歧化酶(SOD)、抗坏血酸过氧化物酶(APX)和谷胱甘肽还原酶(GR)活性和子叶线粒体的APX活性随着脱水迅速下降; 胚轴线粒体的脱氢抗坏血酸还原酶(DHAR)活性和子叶线粒体的SOD、DHAR和GR活性在脱水初期增加, 然后下降。这些数据表明黄皮种子的脱水敏感性与线粒体的呼吸速率和活性氧清除酶的活性降低密切相关, 也与长期适应热带/亚热带的生境有关。
王伟青, 程红焱, 刘树君, 宋松泉. 黄皮种子线粒体呼吸速率和活性氧清除酶活性对脱水的响应及其生态学意义. 植物生态学报, 2012, 36(8): 870-879. DOI: 10.3724/SP.J.1258.2012.00870
WANG Wei-Qing, CHENG Hong-Yan, LIU Shu-Jun, SONG Song-Quan. Response of respiratory rate and reactive oxygen species scavenging enzyme activity in seed mitochondria of Clausena lansium dehydration and its ecological significance. Chinese Journal of Plant Ecology, 2012, 36(8): 870-879. DOI: 10.3724/SP.J.1258.2012.00870
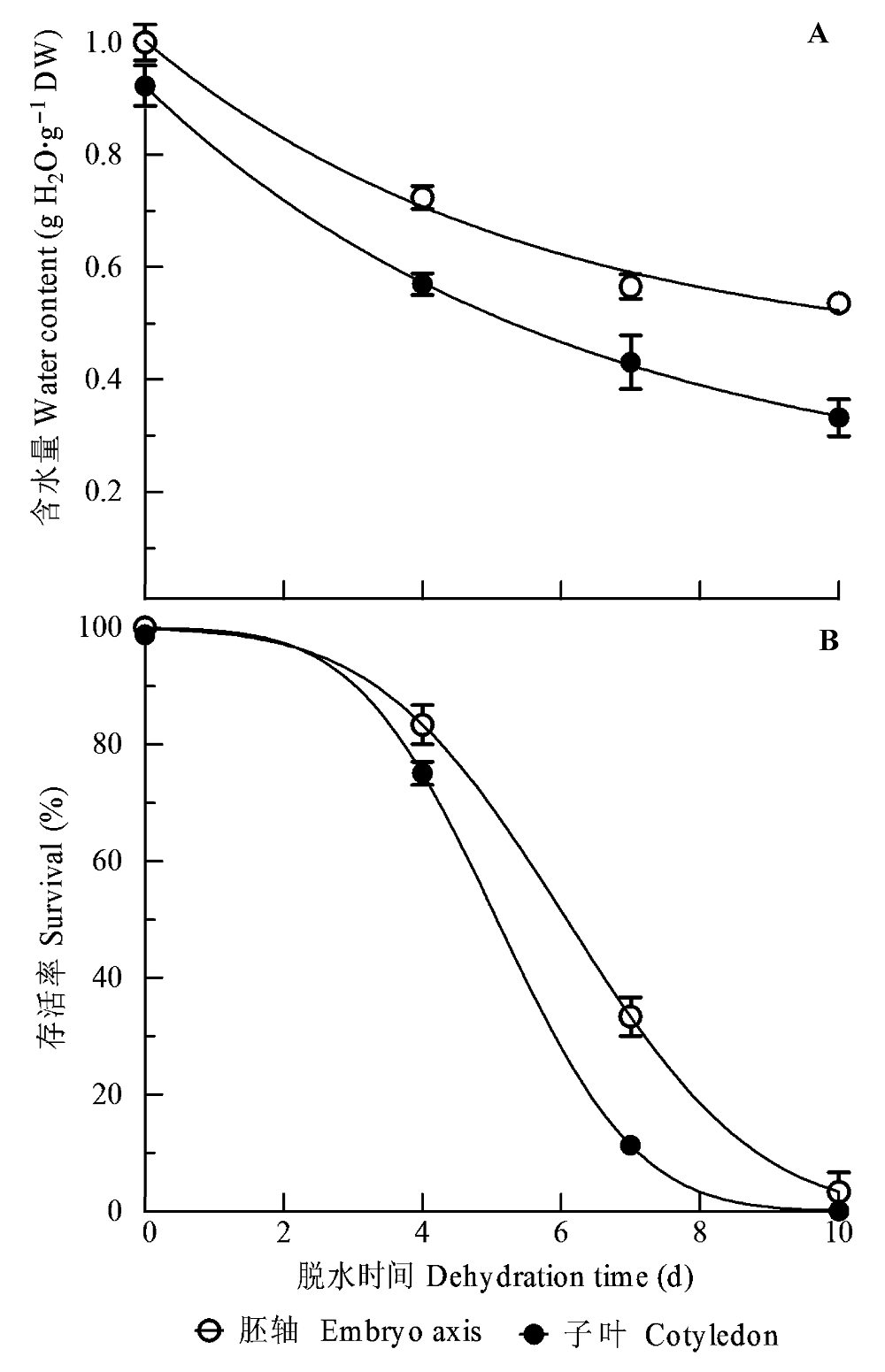
图1 黄皮种子脱水过程中含水量(A)和存活率(B)的变化(平均值±标准误差, n = 25)。种子在25-28 ℃和72%-80%相对湿度中分别脱水0、4、7和10天, 然后测定胚轴、子叶和种子的含水量, 以及胚轴和种子的存活率。种子的含水量与子叶的含水量相同。整粒种子和离体胚轴在30 ℃和黑暗下萌发10天。
Fig. 1 Changes in water content (A) and survival (B) during dehydration of Clausena lansium seeds (mean ± SE, n = 25). Seeds were dehydrated at 25-28 °C and 72%-80% relative humidity for 0, 4, 7 and 10 days, respectively, and then water content of axes, cotyledons and seeds and survival of axes and seeds were immediately measured. The water content of cotyledons and seeds is the same. Whole seed and excised axis were incubated at 30 °C and in darkness.
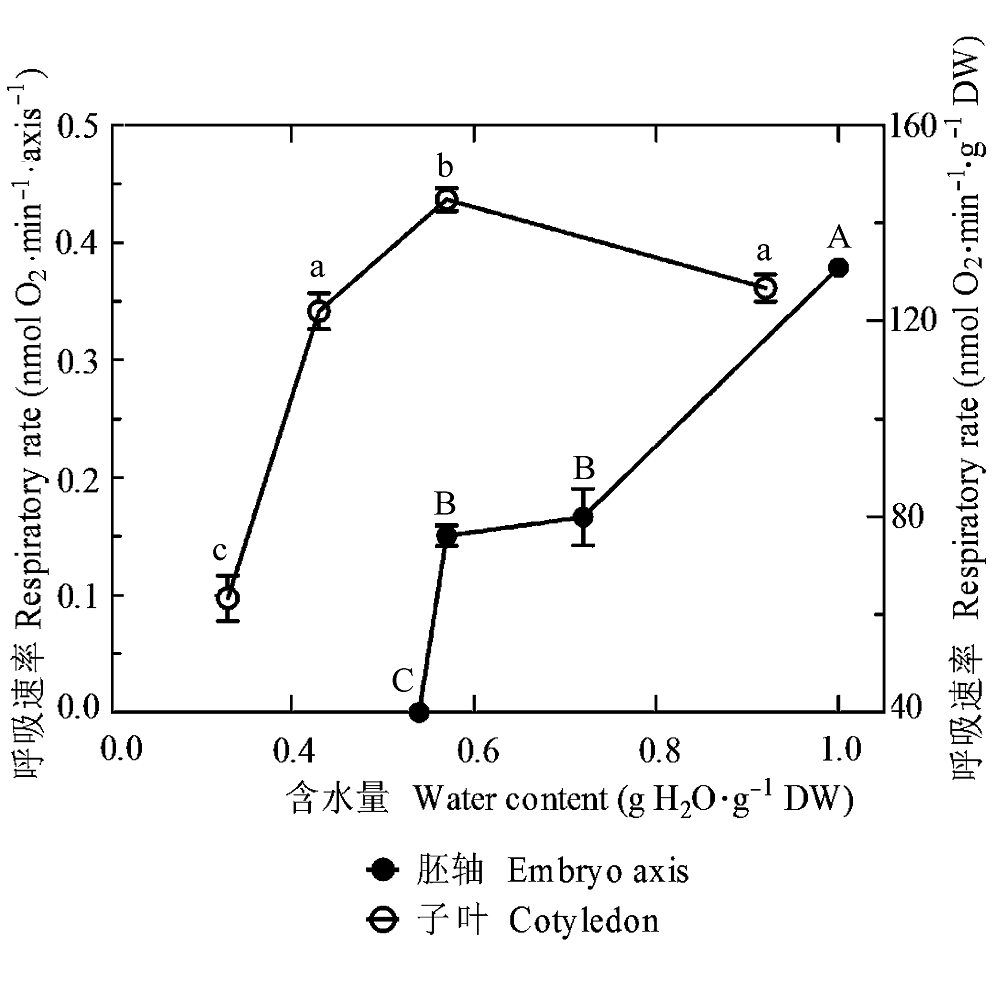
图2 黄皮胚轴和子叶的呼吸速率对脱水的反应。未脱水和脱水的种子在30 ℃和黑暗条件下蒸馏水中吸胀24 h, 然后在25 ℃下分别测定离体胚轴和粉碎子叶的呼吸速率。所有的数据是10个胚轴或者1 g粉碎子叶3次重复的平均值±标准误差。相同的大写和小写字母分别代表胚轴和子叶各处理间无显著性差异(S-N-K, p = 0.05)。
Fig. 2 Response of respiratory rate of Clausena lansium embryo axis and cotyledon to dehydration. The un-dehydrated and dehydrated seeds were imbibed in distilled water at 30 °C in darkness for 24 h, and then respiratory rate of excised embryo axes and grinded cotyledons were measured at 25 °C. All values are mean ± SE of three replicates of 10 axes or 1 g grinded cotyledons. Same uppercase and lowercase letters indicate no significant difference between treatments in embryo axes and cotyledons, respectively (S-N-K, p = 0.05).
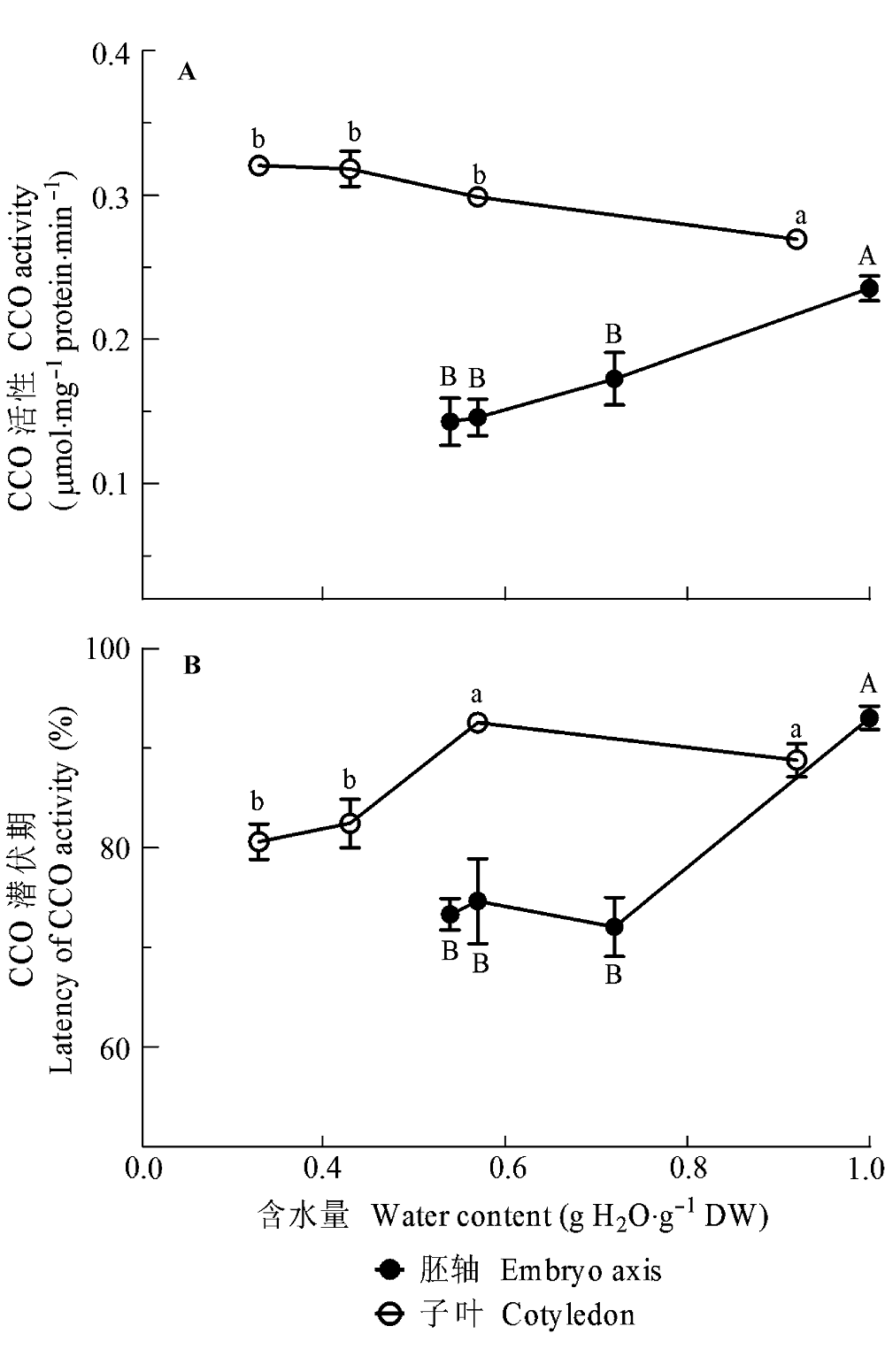
图3 脱水对黄皮胚轴和子叶线粒体细胞色素c氧化酶(CCO)活性(A)和外膜完整性(B)的影响。未脱水和脱水的种子在30 ℃和黑暗条件下吸胀24 h, 然后分别提取胚轴和子叶的线粒体, 并测定CCO活性和计算CCO活性的潜伏期(线粒体外膜的完整性)。所有的数据是200个胚轴或者100 g子叶3次重复的平均值±标准误差。相同的大写和小写字母分别代表胚轴和子叶各处理间无显著性差异 (S-N-K, p = 0.05)。
Fig. 3 Effect of dehydration on cytochrome c oxidase (CCO) activity of mitochondria (A) and integrity of outer mitochondrial membrane (B) in Clausena lansium embryo axis and cotyledon. The un-dehydrated and dehydrated seeds were imbibed in distilled at 30 °C in darkness for 24 h, and the mitochondria of embryo axes and cotyledons were then extracted, respectively. The CCO activity of mitochondria was assayed, and the latency of the CCO activity was calculated to estimate the integrity of outer mitochondrial membrane. All values are mean ± SE of three replicates of 200 axes or 100 g cotyledons. Same uppercase and lowercase letters indicate no significant difference between treatments in embryo axes and cotyledons, respectively (S-N-K, p = 0.05).
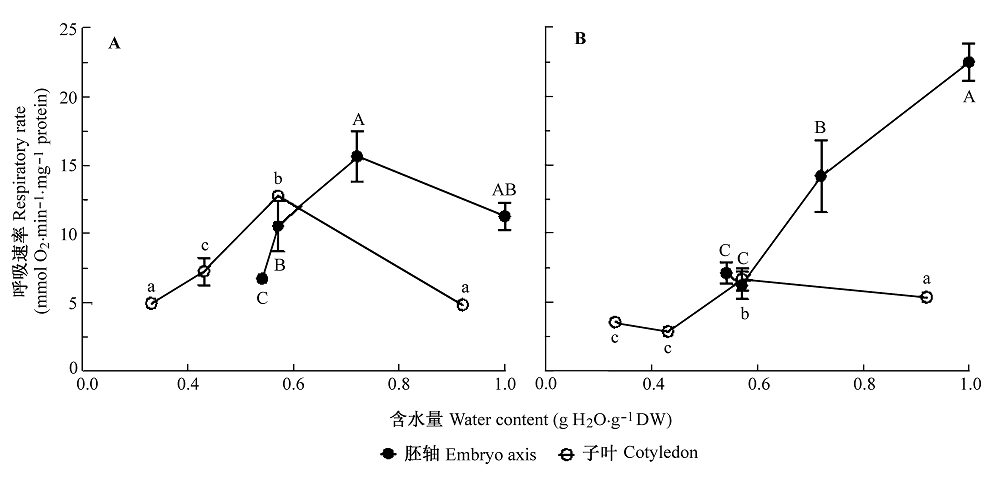
图4 黄皮种子脱水过程中胚轴和子叶线粒体细胞色素c氧化酶途径(A)和交替氧化酶途径(B)呼吸速率的变化。所有的数据是200个胚轴或者100 g子叶3次重复的平均值±标准误差。相同的大写和小写字母分别代表胚轴和子叶各处理间无显著性差异 (S-N-K, p = 0.05)。
Fig. 4 Changes in respiratory rate of cytochrome c oxidase pathway (A) and alternative oxidase pathway (B) in mitochondria of embryo axis and cotyledon during dehydration of Clausena lansium seeds. All values are mean ± SE of three replicates of 200 axes or 100 g cotyledons. Same uppercase and lowercase letters indicate no significant difference between treatments in embryo axes and cotyledons, respectively (S-N-K, p = 0.05)
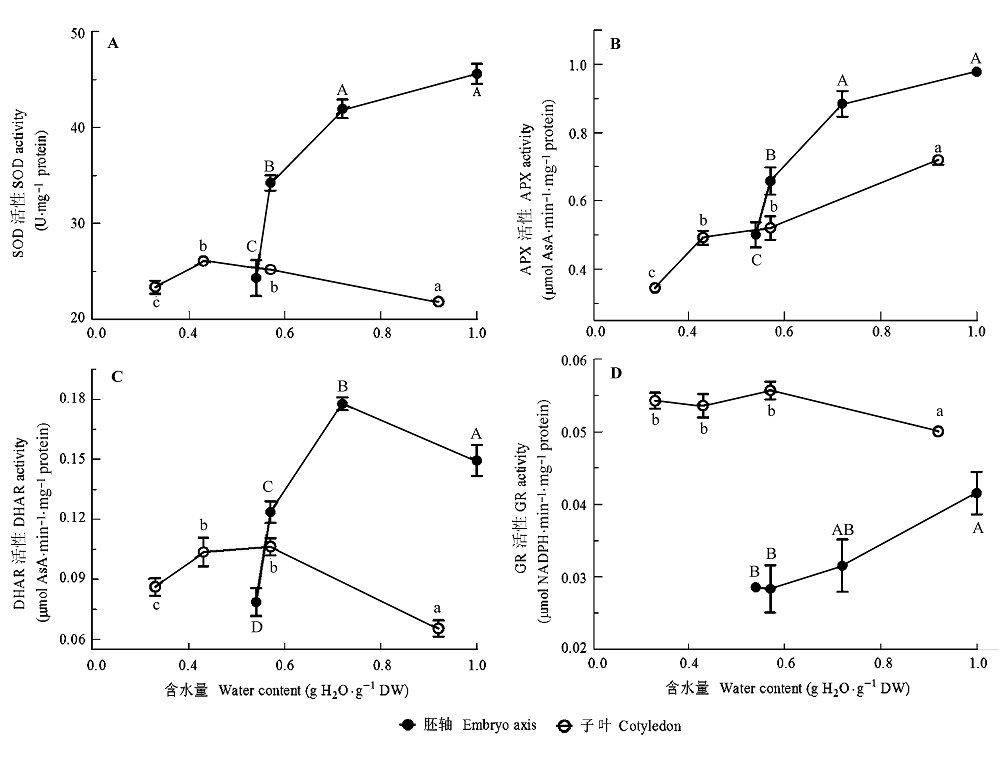
图5 黄皮种子脱水过程中胚轴和子叶线粒体活性氧(ROS)清除酶活性的变化。A, 超氧化物岐化酶(SOD)。B, 抗坏血酸过氧化酶(APX)。C, 脱氢抗坏血酸还原酶(DHAR)。D, 谷胱甘肽还原酶(GR)。所有的数据是200个胚轴或者100 g子叶3次重复的平均值±标准误差。相同的大写和小写字母分别代表胚轴和子叶各处理间无显著性差异(S-N-K, p = 0.05)。
Fig. 5 Changes in activities of reactive oxygen species (ROS) scavenging enzymes in mitochondria of embryo axis and cotyledon during dehydration of Clausena lansium seeds. A, superoxide dismutase (SOD). B, ascorbate peroxidase (APX). C, dehydroascorbate reductase (DHAR). D, glutathione reductase (GR). All values are mean ± SE of three replicates of 200 axes or 100 g cotyledons. Same uppercase and lowercase letters indicate no significant difference between treatments in embryo axes and cotyledons, respectively (S-N-K, p = 0.05).
| [1] | Albury MS, Elliott C, Moore AL (2009). Towards a structural elucidation of the alternative oxidase in plants. Physio- logia Plantarum, 137, 316-327. |
| [2] | Amirsadeghi S, Robson CA, Vanlerberghe GC (2007). The role of the mitochondrion in plant responses to biotic stress. Physiologia Plantarum, 129, 253-266. |
| [3] | Arrigoni O, de Gara L, Tommasi F, Liso R (1992). Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiology, 99, 235-238. |
| [4] | Atkin OK, Macherel D (2009). The crucial role of plant mitochondria in orchestrating drought tolerance. Annals of Botany, 103, 581-597. |
| [5] | Azad AK, Ishikawa T, Ishikawa T, Sawa Y, Shibata H (2008). Intracellular energy depletion triggers programmed cell death during petal senescence in tulip. Journal of Experimental Botany, 59, 2085-2095. |
| [6] | Bailly C (2004). Active oxygen species and antioxidants in seed biology. Seed Science Research, 14, 93-107. |
| [7] | Baskin CC, Baskin JM (1998). Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press, San Diego, USA. |
| [8] | Benamar A, Tallon C, Macherel D (2003). Membrane integrity and oxidative properties of mitochondria isolated from imbibing pea seeds after priming or accelerated ageing. Seed Science Research, 13, 35-45. |
| [9] | Berjak P, Pammenter NW (2004). Biotechnological aspects of non-orthodox seeds: an African perspective. South African Journal of Botany, 70, 102-108. |
| [10] |
Berjak P, Pammenter NW (2008). From avicennia to zizania: seed recalcitrance in perspective. Annals of Botany, 101, 213-228.
DOI URL |
| [11] | Bewley JD (1997). Seed germination and dormancy. The Plant Cell, 9, 1055-1066. |
| [12] | Beyer WF Jr, Fridovich I (1987). Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry, 161, 559-566. |
| [13] | Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254. |
| [14] |
Chaitanya KSK, Naithani SC (1994). Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn F. New Phytologist, 126, 623-627.
DOI URL |
| [15] | Cheng HY, Song SQ (2008). Possible involvement of reactive oxygen species scavenging enzymes in desiccation sensitivity of Antiaris toxicaria seeds and axes. Journal of Integrative Plant Biology, 50, 1549-1556. |
| [16] | Finch-Savage WE, Hendry GAF, Atherton NM (1994). Free radical activity and loss of viability during drying of desiccation-sensitive tree seeds. Proceedings of the Royal Society of Edinburgh Section B: Biological Sciences, 102B, 257-260. |
| [17] |
Hendry GAF (1993). Oxygen, free radical processes and seed longevity. Seed Science Research, 3, 141-153.
DOI URL |
| [18] | Hendry GAF, Finch-Savage WE, Thorpe PC, Atherton NM, Buckland SM, Nilsson KA, Seel WE (1992). Free radical processes and loss of seed viability during desiccation in the recalcitrant species Quercus robur L. New Phytologist, 122, 273-279. |
| [19] | Howell KA, Millar AH, Whelan J (2006). Ordered assembly of mitochondria during rice germination begins with promitochondrial structures rich in components of the protein import apparatus. Plant Molecular Biology, 60, 201-223. |
| [20] | Huang H, Song SQ, Wu XJ (2009). Response of Chinese wampee axes and maize embryos to dehydration at different rates. Journal of Integrative Plant Biology, 51, 67-74. |
| [21] | Huang XM (黄雪梅), Fu JR (傅家瑞), Song SQ (宋松泉) (2004). Changes in desiccation tolerance and cell ultrastructures of wampee axes induced by progressively raising sucrose concentration of culture medium. Acta Phytophysiologica Sinica (植物生理与分子生物学学报), 30, 625-630. (in Chinese with English abstract) |
| [22] | International Seed Testing Association (1999). International rules for seed testing. Seed Science and Technology, 27(Suppl.), 47-50. |
| [23] | Kermode AR, Finch-Savage BE (2002). Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In: Black M, Pritchard HW eds. Desiccation and Survival in Plants: Drying without Dying. CAB International, Wallingford. 149-184. |
| [24] | Lardy HA, Ferguson SM (1969). Oxidative phosphorylation in mitochondria. Annual Review of Biochemistry, 38, 991-1034. |
| [25] |
Leprince O, Buitink J (2010). Desiccation tolerance: from genomics to the field. Plant Science, 179, 554-564.
DOI URL |
| [26] | Leprince O, Hendry GAF, McKersie BD (1993). The mechanisms of desiccation tolerance in developing seeds. Seed Science Research, 3, 231-246. |
| [27] | Li CR, Sun WQ (1999). Desiccation sensitivity and activities of free radical-scavenging enzymes in recalcitrant Theo- broma cacao seeds. Seed Science Research, 9, 209-217. |
| [28] | Møller IM (2001). Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology, 52, 561-591. |
| [29] | Møller IM, Jensen PE, Hansson A (2007). Oxidative modi- fications to cellular components in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 58, 459-481. |
| [30] | Møller IM, Lidén AC, Ericson I, Gardestrom P (1987). Isolation of submitochondrial particles with different polarities. Methods in Enzymology, 148, 442-453. |
| [31] | Møller IM, Sweetlove LJ (2010). ROS signalling—Specificity is required. Trends in Plant Science, 15, 370-374. |
| [32] | Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007). Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiologia Plantarum, 129, 185-195. |
| [33] | Noctor G, Foyer CH (1998). Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 249-279. |
| [34] | Oliver MJ, Bewley JD (1997). Desiccation-tolerance of plant tissues: a mechanistic overview. Horticultural Reviews, 18, 171-213. |
| [35] | Pammenter NW, Greggains V, Kioko JI, Wesley-Smith J, Berjak P, Finch-Savage WE (1998). Effects of differential drying rates on viability retention of recalcitrant seeds of Ekebergia capensis. Seed Science Research, 8, 463-471. |
| [36] | Peixoto F, Vicente J, Madeira VMC (2004). A comparative study of plant and animal mitochondria exposed to paraquat reveals that hydrogen peroxide is not related to the observed toxicity. Toxicology in Vitro, 18, 733-739. |
| [37] | Roach T, Beckett RP, Minibayeva FV, Colville L, Whitaker C, Chen HY, Bailly C, Kranner I (2010). Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant, Cell & Environment, 33, 59-75. |
| [38] | Roach T, Ivanova M, Beckett RP, Minibayeva FV, Green I, Pritchard HW, Kranner I (2008). An oxidative burst of superoxide in embryonic axes of recalcitrant sweet chestnut seeds as induced by excision and desiccation. Physiologia Plantarum, 133, 131-139. |
| [39] | Roberts EH (1973). Predicting the storage life of seeds. Seed Science and Technology, 1, 499-514. |
| [40] | Shi QH, Ding F, Wang XF, Wei M (2007). Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiology and Biochemistry, 45, 542-550. |
| [41] | Smith MT, Berjak P (1995). Deteriorative changes associated with the loss of viability of stored desiccation-tolerant and -sensitive seeds. In: Kigel J, Galili G eds. Seed Development and Germination. Marcel Dekker Inc., New York. 701-704. |
| [42] | Song SQ, Berjak P, Pammenter N (2004). Desiccation sensitivity of Trichilia dregeana axes and antioxidant role of ascorbic acid. Acta Botanica Sinica, 46, 803-810. |
| [43] | Song SQ, Berjak P, Pammenter N, Ntuli TM, Fu JR (2003). Seed recalcitrance: a current assessment. Acta Botanica Sinica, 45, 638-643. |
| [44] | Song SQ (宋松泉), Cheng HY (程红焱), Jiang XC (姜孝成), Long CL (龙春林), Huang ZY (黄振英) (2008). Seed Biology (种子生物学). Science Press, Beijing. 359-370. (in Chinese) |
| [45] | Song SQ, Fu JR (1992). Studies on desiccation-sensitivity and peroxidation of membrane lipids in lychee (Litchi chinensis Sonn.) seeds. Chinese Science Bulletin, 37, 1470-1473. |
| [46] | Song SQ (宋松泉), Fu JR (傅家瑞) (1992). Studies on desiccation-sensitivity and germinative events in Chinese wampee (Clausena lansium [Lour.] Skeels) seeds. Journal of Tropical and Subtropical Botany (热带亚热带植物学报), ( 1), 48-52. (in Chinese with English abstract) |
| [47] | Song SQ (宋松泉), Fu JR (傅家瑞) (1997). Desiccation- sensitivity and lipid peroxidation in Chinese wampee [Clausena lansium (Lour.) Skeels] seeds. Acta Photophysiologica Sinica (植物生理学报), 23, 163-168. (in Chinese with English abstract) |
| [48] | Song SQ (宋松泉), Long CL (龙春林), Yin SH (殷寿华), Lan QY (兰芹英) (2003). Desiccation behavior of seeds and their molecular mechanisms. Acta Botanica Yunnanica (云南植物研究), 25, 465-479. (in Chinese with English abstract) |
| [49] | Struglics A, Fredlund KM, Rasmusson AG, Møller IM (1993). The presence of a short redox chain in the membrane of intact potato tuber peroxisomes and the association of malate dehydrogenase with the peroxisomal membrane. Physiologia Plantarum, 88, 19-28. |
| [50] | Taylor NL, Tan YF, Jacoby RP, Millar AH (2009). Abiotic environmental stress induced changes in the Arabidopsis thaliana chloroplast, mitochondria and peroxisome proteomes. Journal of Proteomics, 72, 367-378. |
| [51] | Tweddle JC, Dickie JB, Baskin CC, Baskin JM (2003). Ecological aspects of seed desiccation sensitivity. Journal of Ecology, 91, 294-304. |
| [52] |
Tweddle JC, Turner RM, Dickie JB (2002). Seed Information Database (Release 3.0, July 2002). http://www.rbgkew.org.uk/data/sid. cited 10 Feb. 2012.
DOI URL PMID |
| [53] | Vertucci CW, Farrant JM (1995). Acquisition and loss of desiccation tolerance. In: Kigel J, Galili G eds. Seed Development and Germination. Marcel Dekker Inc., New York. 237-271. |
| [54] | Wang Y, Li SS, He JX, FU JR (2001). Changes in activity of reactive oxygen scavenging enzymes in recalcitrant wampee (Clausena lansium) seeds during desiccation. Acta Phytophysiologica Sinica, 2001,27, 81-86. |
| [55] | Wang WQ, Cheng HY, Møller IM, Song SQ (2012). The role of recovery of mitochondrial structure and function in desiccation tolerance of pea seeds. Physiologia Planta- rum, 144, 20-34. |
| [1] | 沈健, 何宗明, 董强, 郜士垒, 林宇. 轻度火烧对滨海沙地人工林土壤呼吸速率和非生物因子的影响[J]. 植物生态学报, 2023, 47(7): 1032-1042. |
| [2] | 王祥, 朱亚琼, 郑伟, 关正翾, 盛建东. 昭苏山地草甸4种典型土地利用方式下的土壤呼吸特征[J]. 植物生态学报, 2018, 42(3): 382-396. |
| [3] | 马骏, 唐海萍. 内蒙古农牧交错区不同土地利用方式下土壤呼吸速率及其温度敏感性变化[J]. 植物生态学报, 2011, 35(2): 167-175. |
| [4] | 王文杰, 祖元刚, 王辉民, 杨逢建, 三枝信子, 小池孝良, 山本晋. 基于涡度协方差法和生理生态法对落叶松林CO2通量的初步研究[J]. 植物生态学报, 2007, 31(1): 118-128. |
| [5] | 房秋兰, 沙丽清. 西双版纳热带季节雨林与橡胶林土壤呼吸[J]. 植物生态学报, 2006, 30(1): 97-103. |
| [6] | 张其德, 蒋高明, 朱新广, 王强, 卢从明, 白克智, 匡廷云, 魏其克, 李振声. 12个不同基因型冬小麦的光合能力[J]. 植物生态学报, 2001, 25(5): 532-536. |
| [7] | 李凌浩, 王其兵, 白永飞, 周广胜. 锡林河流域羊草草原群落土壤呼吸及其影响因子的研究[J]. 植物生态学报, 2000, 24(6): 680-686. |
| [8] | 曾小平, 彭少麟, 赵平. 广东南亚热带马占相思林呼吸量的测定[J]. 植物生态学报, 2000, 24(4): 420-424. |
| [9] | 曾小平, 赵平, 彭少麟, 余作岳. 5种木本豆科植物的光合特性研究[J]. 植物生态学报, 1997, 21(6): 539-544. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19