

植物生态学报 ›› 2024, Vol. 48 ›› Issue (3): 287-305.DOI: 10.17521/cjpe.2022.0409 cstr: 32100.14.cjpe.2022.0409
所属专题: 光合作用
收稿日期:2022-10-14
接受日期:2023-06-06
出版日期:2024-03-20
发布日期:2024-04-24
通讯作者:
*(yezp@jgsu.edu.cn)
基金资助:Received:2022-10-14
Accepted:2023-06-06
Online:2024-03-20
Published:2024-04-24
Contact:
*(yezp@jgsu.edu.cn)
Supported by:摘要:
电子传递速率光响应模型是研究植物光合生理和生态学的重要工具, 可为量化原初反应光能的吸收和传递对光的响应提供理论依据。该文综述了目前常用的电子传递速率光响应模型的数学特征, 分析了不同模型的优势及其在实际应用中的潜在问题, 并在此基础上对这些模型可能的发展趋势进行了展望。原初反应包括光能的吸收、光合色素分子的激发和退激发(包括光化学反应、荧光发射和热耗散)、激子共振传递以及光系统II (PSII)反应中心发生电荷分离产生电子传递速率等一系列复杂的物理和生化反应过程。电子传递速率光响应经验模型和半机理模型因不涉及或只涉及部分原初反应过程而难以解释藻类和高等植物的PSII动力学下调、光适应和光保护等现象。电子传递速率光响应机理模型综合考虑了光合色素分子的物理参数(如本征光能吸收截面(σik)、分子处于最低激发态的平均寿命(τmin)、分子的能级简并度和处于激发态的光合色素分子数(Nk))在整个原初反应过程中的重要作用, 不仅可以获得藻类和高等植物叶片的最大电子传递速率以及对应的饱和光强等光合参数, 还可以获得σik和τmin等重要的物理参数, 以及有效光能吸收截面(
王复标, 叶子飘. 植物电子传递速率光响应模型的研究进展. 植物生态学报, 2024, 48(3): 287-305. DOI: 10.17521/cjpe.2022.0409
WANG Fu-Biao, YE Zi-Piao. A review on light response models of electron transport rates of plant. Chinese Journal of Plant Ecology, 2024, 48(3): 287-305. DOI: 10.17521/cjpe.2022.0409
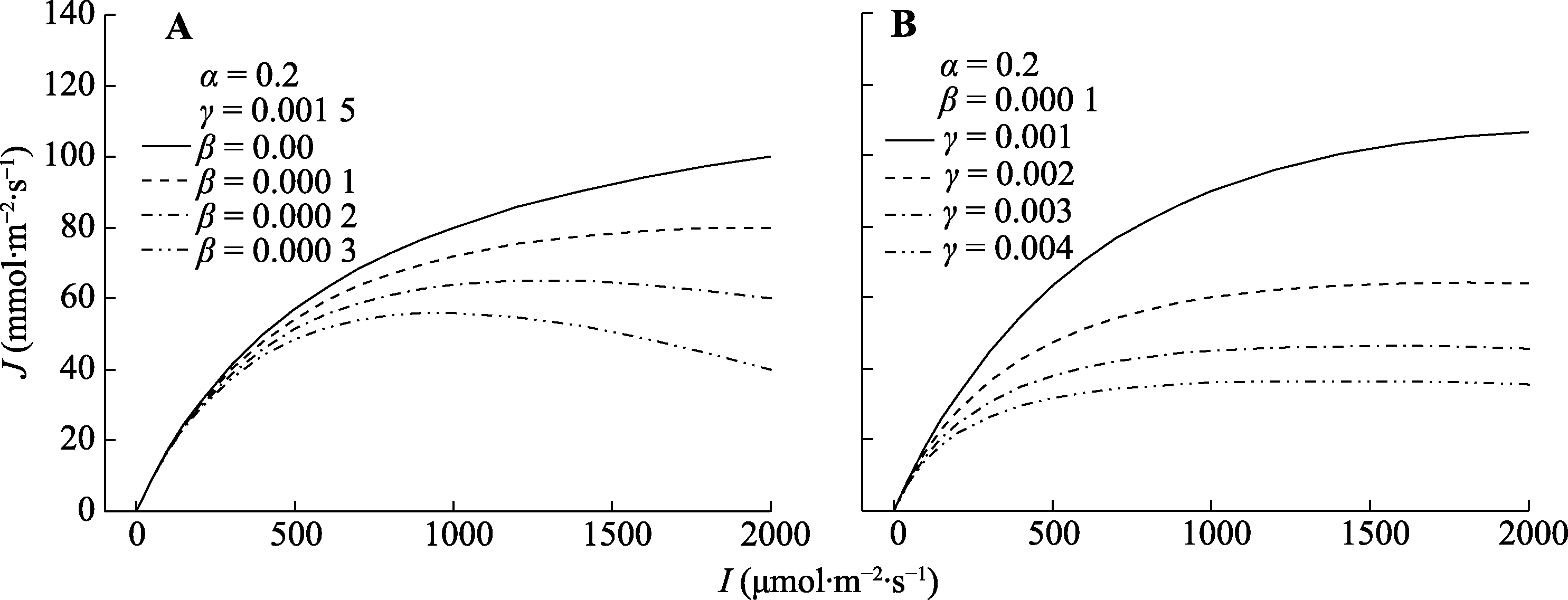
图1 不同光系统II (PSII)动力学下调系数(β)值(A)和饱和系数(γ)值(B)对J/I响应曲线的影响。I, 光强; J, 电子传递速率。α, 初始斜率。
Fig. 1 Effect of different values of β (A) and γ (B) on the J/I curves. I, light intensity; J, electron transport rate. α, initial slope; β, coefficient of dynamic down-regulation for photosystem II (PSII); γ, saturation coefficient.
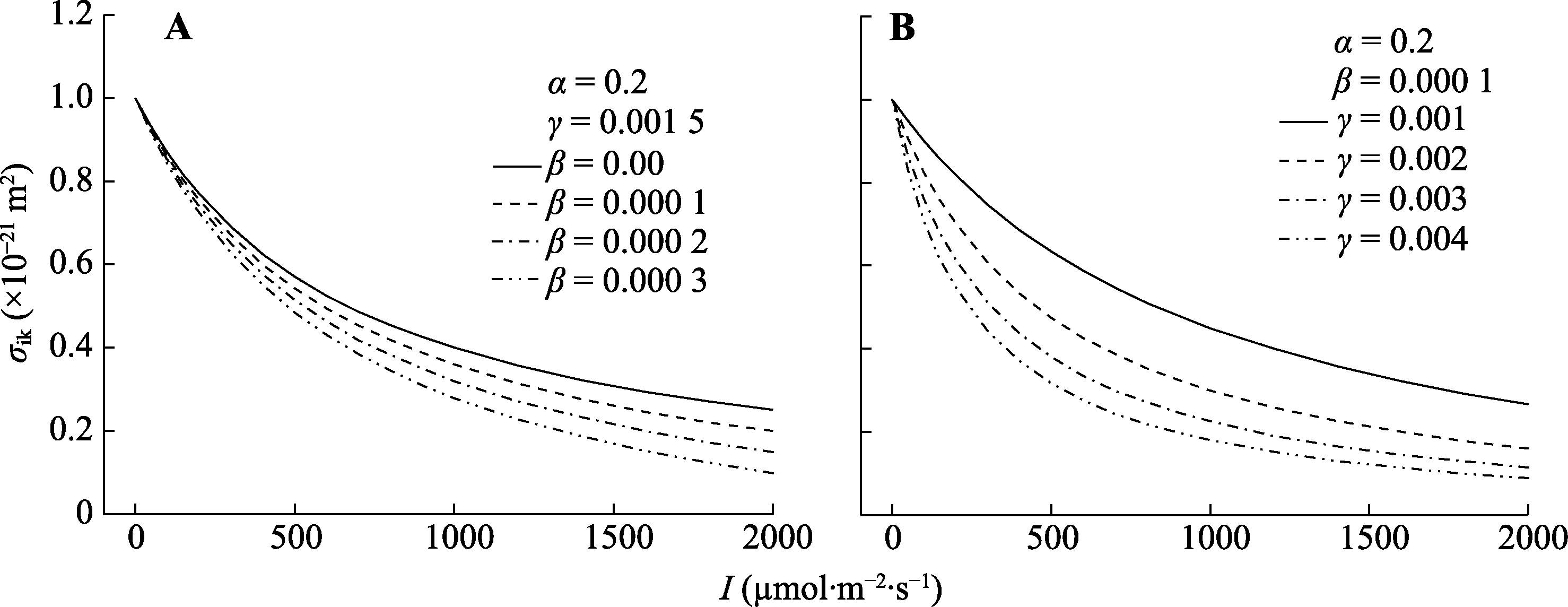
图2 不同光系统II (PSII)动力学下调系数(β)值(A)和饱和系数(γ)值(B)对$\sigma_{\mathrm{ik}}^{\prime}$/I响应曲线的影响。$\sigma_{\mathrm{ik}}^{\prime}$, 有效光能吸收截面; I, 光强。α, 初始斜率。
Fig. 2 Effect of different values of β (A) and γ (B) on the$\sigma_{\mathrm{ik}}^{\prime}$/I curves. $\sigma_{\mathrm{ik}}^{\prime}$, effective light energy absorption cross-section; I, light intensity. α, initial slope; β, coefficient of dynamic down-regulation for photosystem II (PSII); γ, saturation coefficient.
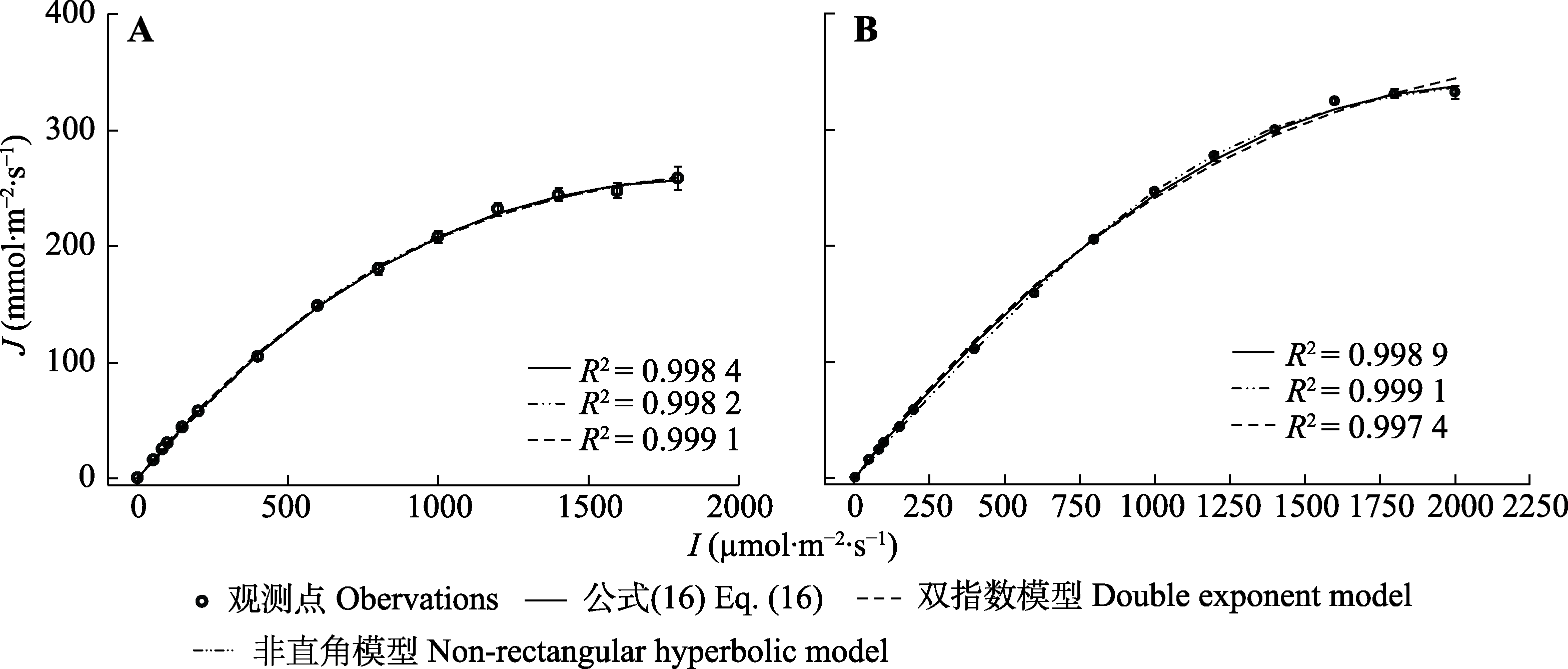
图3 3个模型拟合小麦(A)和大豆(B)的J/I响应曲线(平均值±标准误, n = 3)。I, 光强; J, 电子传递速率; 公式(16),$ J=\alpha \frac{1-\beta I}{1+\gamma I} I$。
Fig. 3 J/I curves fitted by three models for Triticum aestivum (A) and Glycine max (B) (mean ± SE, n = 3). I, light intensity; J, electron transport rate; Eq. (16), $ J=\alpha \frac{1-\beta I}{1+\gamma I} I$.
| 参数 Parameter | 小麦 Triticum aestivum | 大豆 Glycine max | ||||||
|---|---|---|---|---|---|---|---|---|
| 公式(16) Eq. (16) | 非直角双 曲线模型 NRH model | 双指数函数模型 Double exponent model | 观测 Observation | 公式(16) Eq. (16) | 非直角双 曲线模型 NRH | 双指数函数模型 Double exponent model | 观测 Observation | |
| 曲度 Curvature (θ) | - | 0.816 ± 0.009 | - | - | 0.924 ± 0.005 | - | ||
| 初始斜率 Initial slope (α) | 0.295 ± 0.012a | 0.282 ± 0.012a | 0.282 ± 0.012a | - | 0.299 ± 0.006a | 0.282 ± 0.005a | 0.282 ± 0.012a | - |
| 光系统II动力学下调系数 Coefficient of dynamic down-regulation for photosystem II (β) | (2.42 ± 0.28) × 10 -3 | - | 37.98 ± 1.27 | - | (3.07 ± 0.08) × 10-4 | - | (6.13 ± 0.27) × 10 3 | - |
| 饱和系数 Saturation coefficient (γ) | (1.26 ± 0.66) × 10 -4 | - | - | - | (-1.50 ± 0.24) × 10-4 | - | - | - |
| β = 0时最大电子传递速率 Maximum photosynthetic electron flow when β = 0 (Js) | - | - | (8.47± 0.35) × 105 | - | - | - | (1.81 ± 0.23) × 108 | - |
| 最大电子传递速率 Maximum photosynthetic electron flow (Jmax) | 257.23 ± 7.36b | 304.91 ± 7.11a | 264.74 ± 8.45b | 261.56± 7.32b | 332.79 ± 5.16b | 373.87 ± 5.47a | 367.61 ± 8.13a | 332.86 ± 5.01b |
| 饱和光强 Saturation irradiance (PARsat) | 1 873.37 ± 109.46b | - | 2 221.35 ± 125.89a | 1 734.16 ± 66.15b | 1 906.01 ± 19.97b | - | 2 948.74 ± 105.78a | 1 933.23 ± 66.27b |
| 确定系数 Determined coefficient (R2) | 0.998 4 ± 0.000 9 | 0.998 2 ± 0.000 7 | 0.999 4 ± 0.000 6 | - | 0.998 9 ± 0.000 1 | 0.999 2 ± 0.000 1 | 0.998 0 ± 0.000 2 | - |
表1 3个模型拟合小麦和大豆的J/I响应曲线得到的光合参数(平均值±标准误)
Table 1 Photosynthetic parameters of J/I curves fitted by three models for Triticum aestivum and Glycine max (mean ± SE)
| 参数 Parameter | 小麦 Triticum aestivum | 大豆 Glycine max | ||||||
|---|---|---|---|---|---|---|---|---|
| 公式(16) Eq. (16) | 非直角双 曲线模型 NRH model | 双指数函数模型 Double exponent model | 观测 Observation | 公式(16) Eq. (16) | 非直角双 曲线模型 NRH | 双指数函数模型 Double exponent model | 观测 Observation | |
| 曲度 Curvature (θ) | - | 0.816 ± 0.009 | - | - | 0.924 ± 0.005 | - | ||
| 初始斜率 Initial slope (α) | 0.295 ± 0.012a | 0.282 ± 0.012a | 0.282 ± 0.012a | - | 0.299 ± 0.006a | 0.282 ± 0.005a | 0.282 ± 0.012a | - |
| 光系统II动力学下调系数 Coefficient of dynamic down-regulation for photosystem II (β) | (2.42 ± 0.28) × 10 -3 | - | 37.98 ± 1.27 | - | (3.07 ± 0.08) × 10-4 | - | (6.13 ± 0.27) × 10 3 | - |
| 饱和系数 Saturation coefficient (γ) | (1.26 ± 0.66) × 10 -4 | - | - | - | (-1.50 ± 0.24) × 10-4 | - | - | - |
| β = 0时最大电子传递速率 Maximum photosynthetic electron flow when β = 0 (Js) | - | - | (8.47± 0.35) × 105 | - | - | - | (1.81 ± 0.23) × 108 | - |
| 最大电子传递速率 Maximum photosynthetic electron flow (Jmax) | 257.23 ± 7.36b | 304.91 ± 7.11a | 264.74 ± 8.45b | 261.56± 7.32b | 332.79 ± 5.16b | 373.87 ± 5.47a | 367.61 ± 8.13a | 332.86 ± 5.01b |
| 饱和光强 Saturation irradiance (PARsat) | 1 873.37 ± 109.46b | - | 2 221.35 ± 125.89a | 1 734.16 ± 66.15b | 1 906.01 ± 19.97b | - | 2 948.74 ± 105.78a | 1 933.23 ± 66.27b |
| 确定系数 Determined coefficient (R2) | 0.998 4 ± 0.000 9 | 0.998 2 ± 0.000 7 | 0.999 4 ± 0.000 6 | - | 0.998 9 ± 0.000 1 | 0.999 2 ± 0.000 1 | 0.998 0 ± 0.000 2 | - |
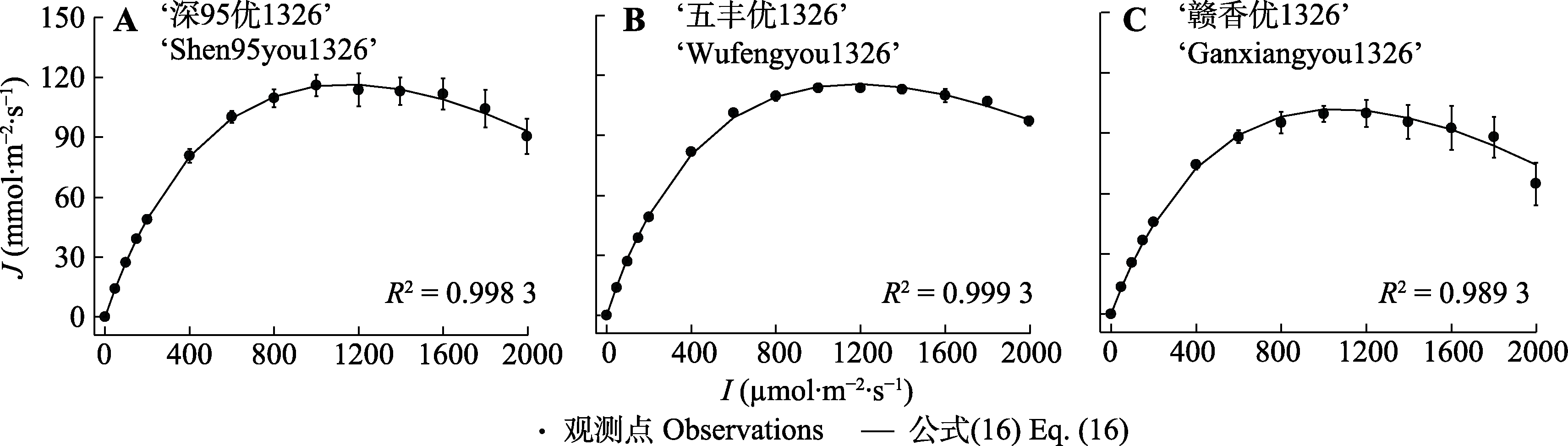
图4 3个水稻品种的电子传递速率光响应曲线(平均值±标准误, n = 3)。I, 光强; J, 电子传递速率; 公式(16), $ J=\alpha \frac{1-\beta I}{1+\gamma I} I$。
Fig. 4 Light response of electron transport rate for three Oryza sativa cultivars (mean ± SE, n = 3). I, light intensity; J, electron transport rate; Eq. (16), $ J=\alpha \frac{1-\beta I}{1+\gamma I} I$.
| 参数 Parameter | 品种 Cultivars | ||
|---|---|---|---|
| ‘深95优1326’ ‘Shen95you1326’ | ‘五丰优1326’ ‘Wufengyou1326’ | ‘赣香优1326’ ‘Ganxiangyou1326’ | |
| 初始斜率 Initial slope (α) (μmol·μmol-1) | 0.308 ± 0.017a | 0.321 ± 0.003a | 0.281 ± 0.005b |
| 最大电子传递速率 Maximum electron transport rate (Jmax) (μmol·m-2·s-1) | 116.99 ± 6.97a | 115.63 ± 2.16a | 102.48 ± 0.58b |
| 饱和光强 Saturation irradiance (PARsat) (μmol·m-2·s-1) | 1 155.84 ± 16.41a | 1 181.05 ± 11.32a | 1 076.22 ± 13.55a |
| 叶绿素含量 Chlorophyll content (mg·dm-2) | 4.58 ± 0.34b | 6.11 ± 0.31a | 5.23 ± 0.25ab |
| 叶绿素a/叶绿素b Chlorophyll a/ chlorophyll b | 2.80 ± 0.54a | 2.23 ± 0.07a | 2.37 ± 0.02a |
| 本征截面 Eign cross-section (σik ) (×10-21 m2) | 2.69 ± 0.27a | 2.09 ± 0.10b | 2.13 ± 0.09b |
| 最小平均寿命 Minimum average life-time (τmin) (×10-3 s) | 1.41 ± 0.21b | 1.62 ± 0.03a | 1.43 ± 0.04b |
| 确定系数 Determined coefficient (R2) | 0.998 3 | 0.999 3 | 0.989 3 |
表2 3个水稻品种电子传递速率光响应的特征参数(平均值±标准误)
Table 2 Characteristic parameters of light response curves of electron transport rate for three Oryza sativa cultivars (mean ± SE)
| 参数 Parameter | 品种 Cultivars | ||
|---|---|---|---|
| ‘深95优1326’ ‘Shen95you1326’ | ‘五丰优1326’ ‘Wufengyou1326’ | ‘赣香优1326’ ‘Ganxiangyou1326’ | |
| 初始斜率 Initial slope (α) (μmol·μmol-1) | 0.308 ± 0.017a | 0.321 ± 0.003a | 0.281 ± 0.005b |
| 最大电子传递速率 Maximum electron transport rate (Jmax) (μmol·m-2·s-1) | 116.99 ± 6.97a | 115.63 ± 2.16a | 102.48 ± 0.58b |
| 饱和光强 Saturation irradiance (PARsat) (μmol·m-2·s-1) | 1 155.84 ± 16.41a | 1 181.05 ± 11.32a | 1 076.22 ± 13.55a |
| 叶绿素含量 Chlorophyll content (mg·dm-2) | 4.58 ± 0.34b | 6.11 ± 0.31a | 5.23 ± 0.25ab |
| 叶绿素a/叶绿素b Chlorophyll a/ chlorophyll b | 2.80 ± 0.54a | 2.23 ± 0.07a | 2.37 ± 0.02a |
| 本征截面 Eign cross-section (σik ) (×10-21 m2) | 2.69 ± 0.27a | 2.09 ± 0.10b | 2.13 ± 0.09b |
| 最小平均寿命 Minimum average life-time (τmin) (×10-3 s) | 1.41 ± 0.21b | 1.62 ± 0.03a | 1.43 ± 0.04b |
| 确定系数 Determined coefficient (R2) | 0.998 3 | 0.999 3 | 0.989 3 |
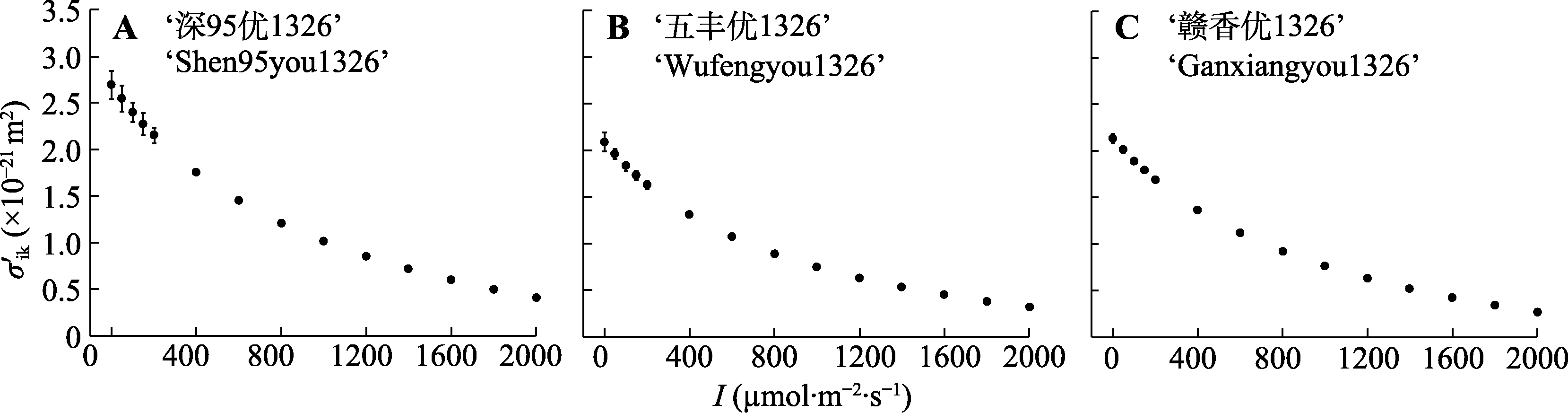
图5 3个水稻品种光合色素分子的有效光能吸收截面($\sigma_{\mathrm{ik}}^{\prime}$)对光的响应(平均值±标准误, n = 3)。I, 光强。
Fig. 5 Light response of the effective light energy absorption cross-section ($\sigma_{\mathrm{ik}}^{\prime}$) for three Oryza sativa cultivars (mean ± SE, n = 3). I, light intensity.
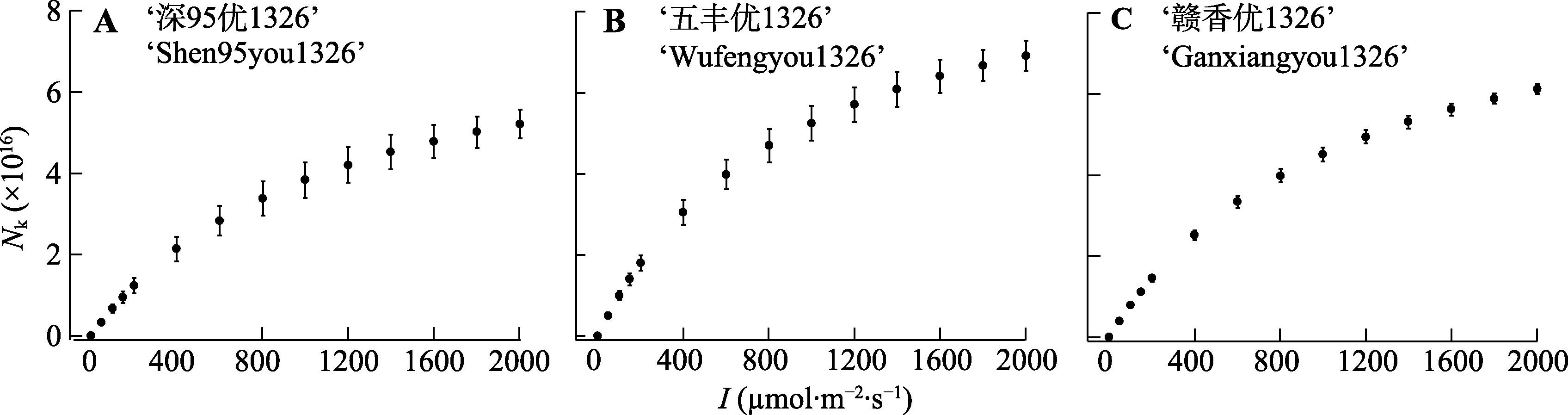
图6 3个水稻品种处于激发态的光合色素分子数(Nk)对光的响应(平均值±标准误, n = 3)。I, 光强。
Fig. 6 Light response of molecules in the excited state (Nk) for three Oryza sativa cultivars (mean ± SE, n = 3). I, light intensity.
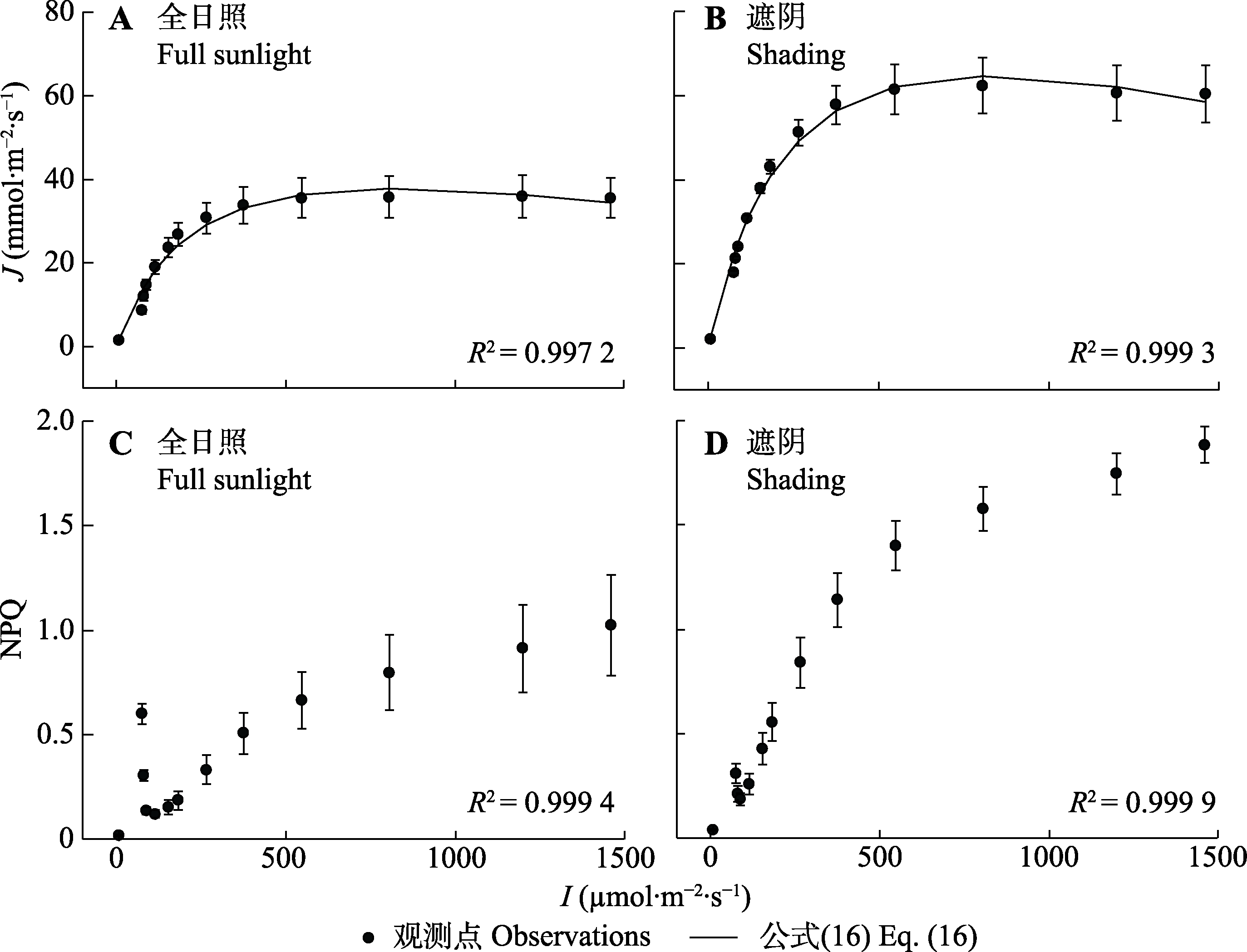
图7 杂种杜鹃在遮阴和全日照条件下的J/I响应曲线和NPQ/I响应曲线(平均值±标准误, n = 5)。I, 光强; J, 电子传递速率; NPQ, 非光化学淬灭; 公式(16),$ J=\alpha \frac{1-\beta I}{1+\gamma I} I$。
Fig. 7 J/I and NPQ/I curves for Rhododendron ‘Hybrida’ under shading and full sunlight conditions (mean ± SE, n = 5). I, light intensity; J, electron transport rate; NPQ, non-photochemical quenching; Eq. (16), $J=\alpha \frac{1-\beta I}{1+\gamma I} I$.
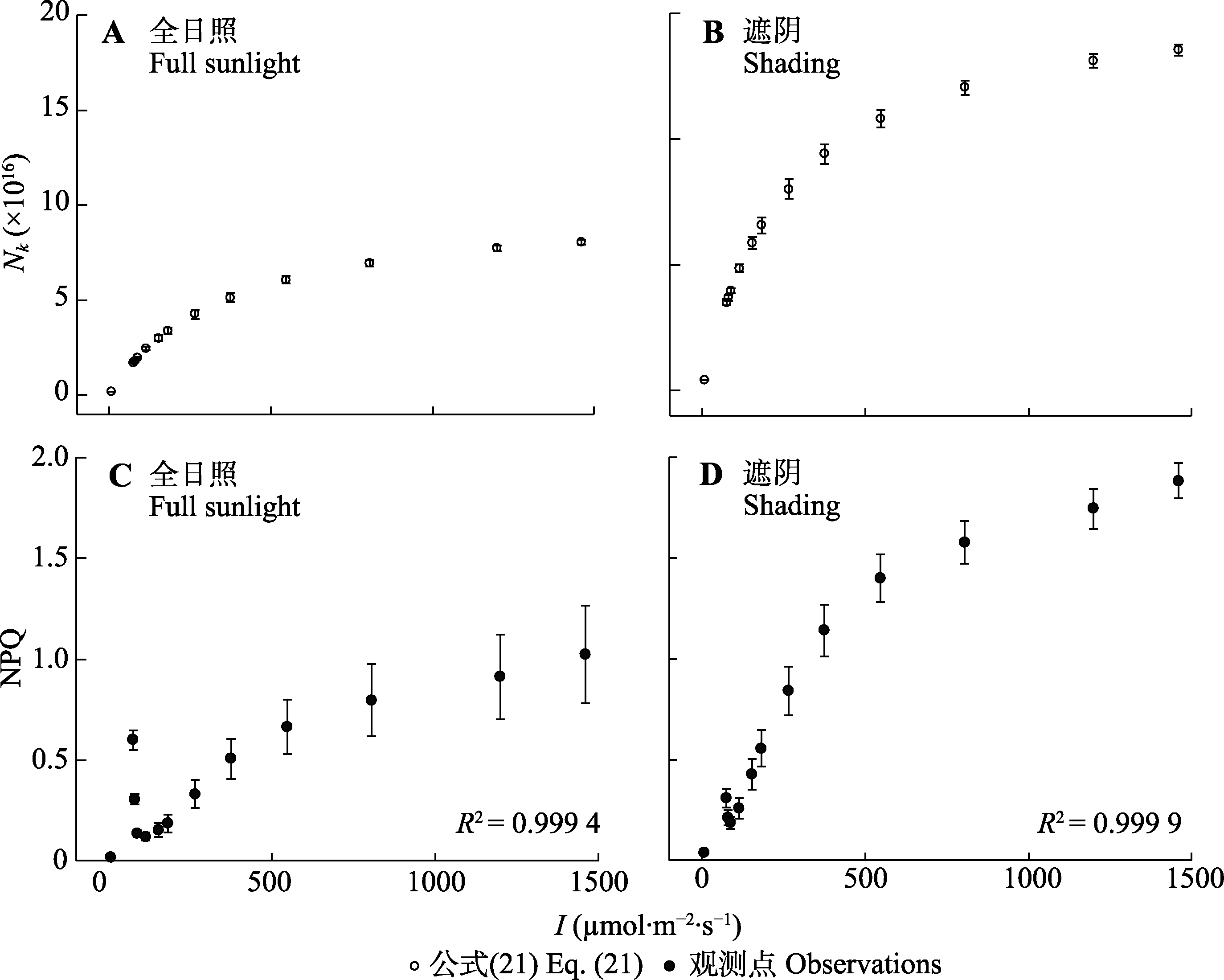
图8 杂种杜鹃在遮阴和全日照条件下的Nk/I和NPQ/I响应曲线(平均值±标准误, n = 5)。I, 光强; Nk, 处于激发态的光合色素分子数; NPQ, 非光化学淬灭; 公式(21),$ N_{\mathrm{k}}=\frac{1}{1+g_{\mathrm{i}} / g_{\mathrm{k}}} \frac{\gamma I}{1+\gamma I} N_{0}$。
Fig. 8 Nk/I and NPQ/I curves for Rhododendron ‘Hybrida’ under shading and full sunlight conditions(mean ± SE, n = 5). I, light intensity; Nk, molecules in the excited state; NPQ, non-photochemical quenching; Eq. (21),$ N_{\mathrm{k}}=\frac{1}{1+g_{\mathrm{i}} / g_{\mathrm{k}}} \frac{\gamma I}{1+\gamma I} N_{0}$.
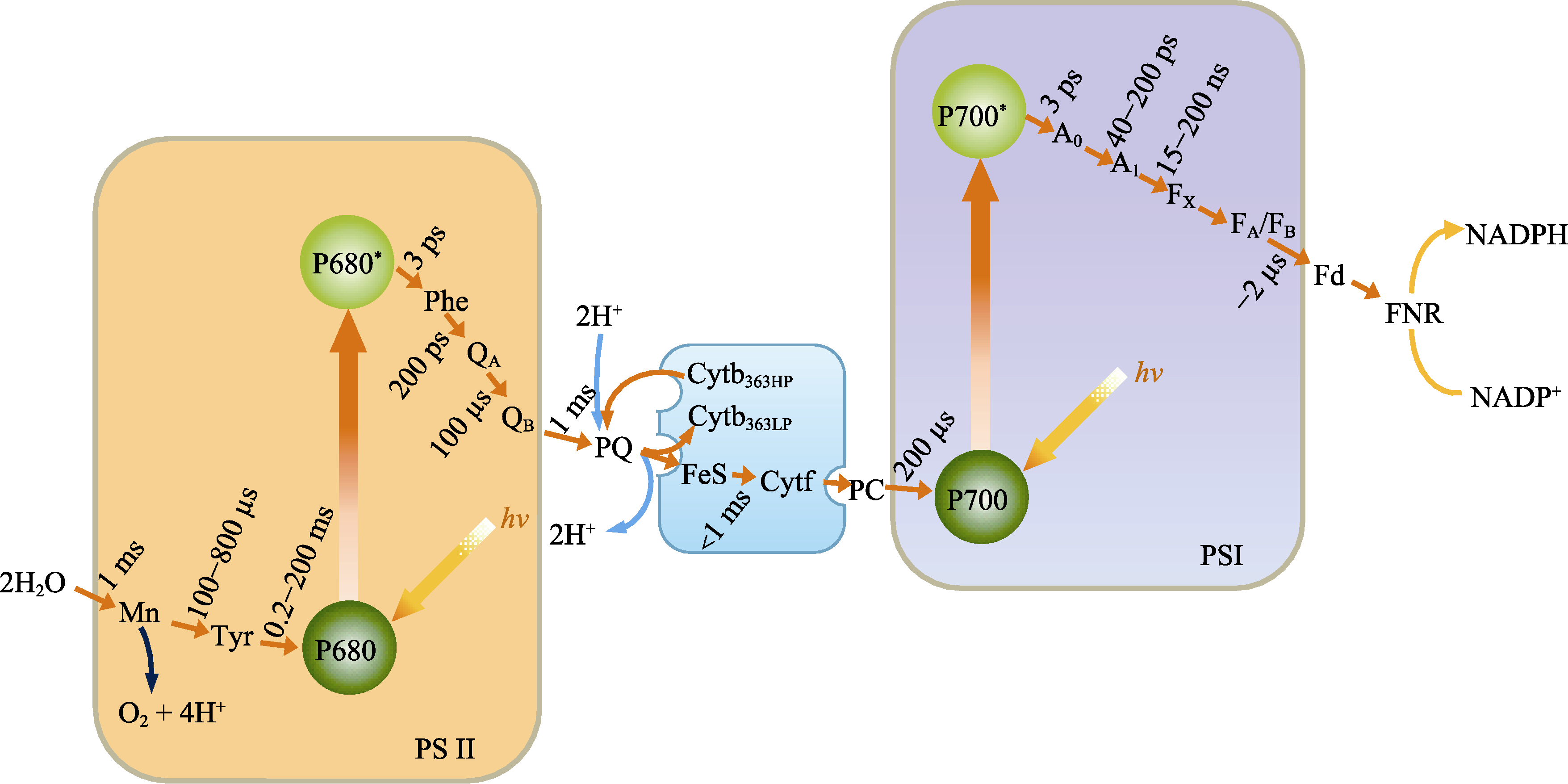
图9 光合作用电子传递的Z链。A0和A1, 光系统I (PSI)原初电子受体; Cytb363HP, 363HP细胞色素b; Cytb363LP, 363LP细胞色素b; Cytf, 细胞色素f; FA/FB, PSI次级电子受体; FeS, 铁硫中心; Fd, 铁氧还蛋白; FNR, Fd-NADP还原酶; FX, 非血红素铁硫蛋白; hv, 光照; Mn, 锰簇; NADP+, 脱氢烟酰胺腺嘌呤二核苷磷酸; NADPH, 烟酰胺腺嘌呤二核苷磷酸; PC, 质蓝素; Phe, 脱镁叶绿素; PQ, 质体醌; P700, PSI反应中心色素; P700*, 激发态的PSI反应中心色素; P680, 光系统II (PSII)反应中心色素; P680*, 激发态的PSII反应中心色素; QA, PSII初级醌受体; QB, PSII次级醌受体; Tyr, 酪氨酸。
Fig. 9 Z chain of electron transport in photosynthesis. A0 and A1, the primary electron acceptor of photosystem I (PSI); Cytb363HP, 363HP cytochrome b; Cytb363LP, 363LP cytochrome b; Cytf, cytochrome f; FA/FB, the secondary electron acceptor of PSI; FeS, iron-sulifide protein; Fd, ferredoxin; FNR, Fd-NADP reductase; Fx, non-heme iron sulfide protein; hv, irradiation with light; Mn, manganese cluster; NADP+, dehydronicotinamide adenine dinucleotide phosphate; NADPH, nicotinamide adenine dinucleotide phosphate; PC, plasto cynin; Phe, phephytin; PQ, plastoquinone; P700, the pigment of PSI reaction center; P700*, the excited pigment of PSI reaction center; P680, the pigment of photosystem II (PSII) reaction center; P680*, the excited pigment of PSII reaction center; QA, the primary quinone electron acceptor of PSII; QB, the secondary quinone electron acceptor of PSII; Tyr, tyrosine.
| [1] | Abramavicius D, Mukamel S (2010). Energy-transfer and charge-separation pathways in the reaction center of photosystem II revealed by coherent two-dimensional optical spectroscopy. The Journal of Chemical Physics, 133, 184501. DOI: 10.1063/1.3493580. |
| [2] | Ahammed GJ, Xu W, Liu A, Chen S (2018). COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Frontiers in Plant Science, 9, 998. DOI: 10.3389/fpls.2018.00998. |
| [3] | Arikan B, Ozfidan-Konakci C, Alp FN, Zengin G, Yildiztugay E (2022). Rosmarinic acid and hesperidin regulate gas exchange, chlorophyll fluorescence, antioxidant system and the fatty acid biosynthesis-related gene expression in Arabidopsis thaliana under heat stress. Phytochemistry, 198, 113157. DOI: 10.1016/j.phytochem.2022.113157. |
| [4] | Asada K (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology & Plant Molecular Biology, 50, 601-639. |
| [5] | Asada K (2000). The water-water cycle as alternative photon and electron sinks. Philosophical Transactions of the Royal Society B: Biological Sciences, 355, 1419-1431. |
| [6] | Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000). Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philosophical Transactions of the Royal Society B: Biological Sciences, 355, 1433-1446. |
| [7] |
Bailey S, Melis A, MacKey KRM, Cardol P, Finazzi G, van Dijken G, Berg GM, Arrigo K, Shrager J, Grossman A (2008). Alternative photosynthetic electron flow to oxygen in marine Synechococcus. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1777, 269-276.
DOI PMID |
| [8] |
Baker NR (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89-113.
DOI PMID |
| [9] |
Borisova-Mubarakshina MM, Naydov IA, Ivanov BN (2018). Oxidation of the plastoquinone pool in chloroplast thylakoid membranes by superoxide anion radicals. FEBS Letters, 592, 3221-3228.
DOI PMID |
| [10] | Brading P, Warner ME, Davey P, Smith DJ, Achterberg EP, Suggett DJ (2011). Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). Limnology & Oceanography, 56, 927-938. |
| [11] | Brody SS, Rabinowitch E (1957). Excitation lifetime of photosynthetic pigments in vitro and in vivo. Science, 125, 555. DOI: 10.1126/science.125.3247.555. |
| [12] |
Buckley TN, Diaz-Espejo A (2015). Reporting estimates of maximum potential electron transport rate. New Phytologist, 205, 14-17.
DOI PMID |
| [13] | Buckley TN, Farquhar GD (2004). A new analytical model for whole-leaf potential electron transport rate. Plant, Cell & Environment, 27, 1487-1502. |
| [14] |
Cheng L, Fuchigami LH, Breen PJ (2001). The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves. Journal of Experimental Botany, 52, 1865-1872.
PMID |
| [15] |
Cruz JA, Avenson TJ, Kanazawa A, Takizawa K, Edwards GE, Kramer DM (2005). Plasticity in light reactions of photosynthesis for energy production and photoprotection. Journal of Experimental Botany, 56, 395-406.
PMID |
| [16] | Ebenhöh O, Fucile G, Finazzi G, Rochaix JD, Goldschmidt- Clermont M (2014). Short-term acclimation of the photosynthetic electron transfer chain to changing light: a mathematical model. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, 20130223. DOI: 10.1098/rstb.2013.0223. |
| [17] |
Ehleringer J (1981). Leaf absorptances of Mohave and Sonoran Deserts plants. Oecologia, 49, 366-370.
DOI PMID |
| [18] |
Ehleringer J, Pearcy RW (1983). Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiology, 73, 555-559.
DOI PMID |
| [19] |
Eichelmann H, Oja V, Peterson RB, Laisk A (2011). The rate of nitrite reduction in leaves as indicated by O2 and CO2 exchange during photosynthesis. Journal of Experimental Botany, 62, 2205-2215.
DOI PMID |
| [20] |
Farquhar GD, Busch FA (2017). Changes in the chloroplastic CO2 concentration explain much of the observed Kok effect: a model. New Phytologist, 214, 570-584.
DOI PMID |
| [21] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
DOI PMID |
| [22] | Fasham MJR, Platt T (1983). Photosynthetic response of phytoplankton to light: a physiological model. Proceedings of the Royal Society B: Biological Sciences, 219, 355-370. |
| [23] |
Fassioli F, Olaya-Castro A, Scheuring S, Sturgis JN, Johnson NF (2009). Energy transfer in light-adapted photosynthetic membranes: from active to saturated photosynthesis. Biophysical Journal, 97, 2464-2473.
DOI PMID |
| [24] | Furutani R, Ohnishi M, Mori Y, Wada S, Miyake C (2022). The difficulty of estimating the electron transport rate at photosystem I. Journal of Plant Research, 135, 565-577. |
| [25] |
Gobets B, van Grondelle R (2001). Energy transfer and trapping in photosystem I. Biochimica et Biophysica Acta (BBA)- Bioenergetics, 1507, 80-99.
PMID |
| [26] |
Govindjee (1990). Photosystem II heterogeneity: the acceptor side. Photosynthesis Research, 25, 151-160.
DOI PMID |
| [27] | Goyal M, Kaur R (2019). Interactive effect of nitrogen nutrition, nitrate reduction and seasonal variation on oxalate synthesis in leaves of Napier-bajra hybrid (Pennisetum purpureum × P. glaucum). Crop & Pasture Science, 70, 669-675. |
| [28] |
Han T, Zhu GF, Ma JZ, Wang ST, Zhang K, Liu XW, Ma T, Shang SS, Huang CL (2020). Sensitivity analysis and estimation using a hierarchical Bayesian method for the parameters of the FvCB biochemical photosynthetic model. Photosynthesis Research, 143, 45-66.
DOI PMID |
| [29] | Harrison WG, Platt T (1986). Photosynthesis-irradiance relationships in polar and temperate phytoplankton populations. Polar Biology, 5, 153-164. |
| [30] |
He L, Luo H, He XP, Bian JM, Zhu CL, Fu JR, Wu Y, He HH (2020). Photosynthetic characteristics of different super early rice based on mechanistic model of light-response of photosynthetic electron flow. Journal of Nuclear Agricultural Sciences, 34, 418-424.
DOI |
|
[贺俐, 罗辉, 贺晓鹏, 边建民, 朱昌兰, 傅军如, 吴杨, 贺浩华 (2020). 基于光合电子流的超级早稻光合特性研究. 核农学报, 34, 418-424.]
DOI |
|
| [31] |
Hemschemeier A, Happe T (2011). Alternative photosynthetic electron transport pathways during anaerobiosis in the green alga Chlamydomonas reinhardtii. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1807, 919-926.
DOI PMID |
| [32] |
Höhner R, Pribil M, Herbstová M, Lopez LS, Kunz HH, Li M, Wood M, Svoboda V, Puthiyaveetil S, Leister D, Kirchhoff H (2020). Plastocyanin is the long-range electron carrier between photosystem II and photosystem I in plants. Proceedings of the National Academy of Sciences of the United States of America, 117, 15354-15362.
DOI PMID |
| [33] | Hu WH, Zhang SS, Xiao YA, Yan XH (2015). Physiological responses and photo-protective mechanisms of two Rhododendron plants to natural sunlight after long term shading. Chinese Journal of Plant Ecology, 39, 1093-1100. |
|
[胡文海, 张斯斯, 肖宜安, 闫小红 (2015). 两种杜鹃花属植物对长期遮阴后全光照环境的生理响应及其光保护机制. 植物生态学报, 39, 1093-1100.]
DOI |
|
| [34] |
Hu X, Ritz T, Damjanović A, Autenrieth F, Schulten K (2002). Photosynthetic apparatus of purple bacteria. Quarterly Reviews of Biophysics, 35, 1-62.
PMID |
| [35] | Jin HL, Fu M, Duan ZK, Duan SJ, Li MS, Dong XX, Liu B, Feng DR, Wang JF, Peng LW, Wang HB (2018). Low photosynthetic efficiency 1 is required for light-regulated photosystem II biogenesis in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 115, 6075-6084. |
| [36] |
Kirchhoff H, Haase W, Wegner S, Danielsson R, Ackermann R, Albertsson PA (2007). Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry, 46, 11169-11176.
PMID |
| [37] |
Kouřil R, Dekker JP, Boekema EJ (2012). Supramolecular organization of photosystem II in green plants. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1817, 2-12.
DOI PMID |
| [38] | Krall JP, Edwards GE (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Physiologia Plantarum, 86, 180-187. |
| [39] |
Krause GH, Weis E (1984). Chlorophyll fluorescence as a tool in plant physiology. Photosynthesis Research, 5, 139-157.
DOI PMID |
| [40] | Kwiatkowski L, Aumont O, Bopp L, Ciais P (2018). The impact of variable phytoplankton stoichiometry on projections of primary production, food quality, and carbon uptake in the global ocean. Global Biogeochemical Cycles, 32, 516-528. |
| [41] | Ley AC, Mauzerall DC (1982). Absolute absorption cross- sections for photosystem II and the minimum quantum requirement for photosynthesis in Chlorella vulgaris. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 680, 95-106. |
| [42] |
Li L, Zhang XX, Zheng R, Guo JQ (2016). Photosynthetic characteristics and photosynthesis-light response curve models of summer maize. Chinese Journal of Plant Ecology, 40, 1310-1318.
DOI |
|
[李力, 张祥星, 郑睿, 郭建青 (2016). 夏玉米光合特性及光响应曲线拟合. 植物生态学报, 40, 1310-1318.]
DOI |
|
| [43] |
Li LY, Li J, Tong XJ, Meng P, Zhang JS, Zhang JR (2018). Simulation on the light-response curves of electron transport rate of Quercus variabilis and Robinia pseudoacacia leaves in the Xiaolangdi area, China. Chinese Journal of Plant Ecology, 42, 1009-1021.
DOI |
|
[李理渊, 李俊, 同小娟, 孟平, 张劲松, 张静茹 (2018). 黄河小浪底栓皮栎、刺槐叶片电子传递速率-光响应的模拟. 植物生态学报, 42, 1009-1021.]
DOI |
|
| [44] |
Li X, Wang HB, Jin HL (2020). Light signaling-dependent regulation of PSII biogenesis and functional maintenance. Plant Physiology, 183, 1855-1868.
DOI PMID |
| [45] | Li XN, Hao CL, Zhong JW, Liu FL, Cai J, Wang X, Zhou Q, Dai TB, Cao WX, Jiang D (2015). Mechano-stimulated modifications in the chloroplast antioxidant system and proteome changes are associated with cold response in wheat. BMC Plant Biology, 15, 219. DOI: 10.1186/s12870-015-0610-6. |
| [46] | Li X, Brestic M, Tan D, Zivcak M, Zhu X, Liu S, Song F, Reiter RJ, Liu F (2018). Melatonin alleviates low PS I-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. Journal of Pineal Research, 64, e12453. DOI: 10.1111/jpi.12453. |
| [47] | Liang XY, Liu SR (2019). In-situ measurement of photosynthetic characteristics of dominant tree species based on canopy crane in a Korean pine broad-leaved forest in Changbai Mountain, northeastern China. Chinese Journal of Applied Ecology, 30, 1494-1502. |
|
[梁星云, 刘世荣 (2019). 基于冠层塔吊原位测定长白山温带阔叶红松原始林群落主要树种的光合特征. 应用生态学报, 30, 1494-1502.]
DOI |
|
| [48] | Liang ZR, Liu FL, Yuan YM, Du XX, Wang WJ, Sun XT, Gao YP, Wang FJ (2018). Effect of different temperatures on growth and photosynthetic characteristic of Laminaria hyperborea young seedling. Marine Sciences, 42(4), 71-78. |
| [梁洲瑞, 刘福利, 袁艳敏, 杜欣欣, 汪文俊, 孙修涛, 高亚平, 王飞久 (2018). 不同温度对极北海带幼苗生长及光合特性的影响. 海洋科学, 42(4), 71-78.] | |
| [49] | Liu J, van Iersel MW (2021). Photosynthetic physiology of blue, green, and red light: light intensity effects and underlying mechanisms. Frontiers in Plant Science, 12, 619987. DOI: 10.3389/fpls.2021.619987. |
| [50] |
Long SP, Bernacchi CJ (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany, 54, 2393-2401.
DOI PMID |
| [51] | Lu JZ, Yin ZP, Lu T, Yang XL, Wang F, Qi MF, Li TL, Liu YF (2020). Cyclic electron flow modulate the linear electron flow and reactive oxygen species in tomato leaves under high temperature. Plant Science, 292, 110387. DOI: 10.1016/j.plantsci.2019.110387. |
| [52] | Major KM, Dunton KH (2002). Variations in light-harvesting characteristics of the seagrass, Thalassia testudinum: evidence for photoacclimation. Journal of Experimental Marine Biology & Ecology, 275, 173-189. |
| [53] | Malkin R, Niyogi K (2000). Photosynthesis//Buchanan BB, Gruissem W, Jones RL. Biochemistry and Molecular Biology of Plants. American Society of Plant Physiology, Rockville. 568-628. |
| [54] |
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence—A practical guide. Journal of Experimental Botany, 51, 659-668.
DOI PMID |
| [55] | Miyake C (2010). Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant & Cell Physiology, 51, 1951-1963. |
| [56] | Moreno-García B, López-Calcagno PE, Raines CA, Sweetlove LJ (2022). Suppression of metabolite shuttles for export of chloroplast and mitochondrial ATP and NADPH increases the cytosolic NADH:NAD+ ratio in tobacco leaves in the dark. Journal of Plant Physiology, 268, 153578. DOI: 10.1016/j.jplph.2021.153578. |
| [57] |
Müller P, Li X, Niyogi KK (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiology, 125, 1558-1566.
DOI PMID |
| [58] |
Nelson N, Yocum CF (2006). Structure and function of photosystems I and II. Annual Review of Plant Biology, 57, 521-565.
PMID |
| [59] |
Niyogi KK, Truong TB (2013). Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Current Opinion in Plant Biology, 16, 307-314.
DOI PMID |
| [60] |
Ochoa-Fernandez R, Abel NB, Wieland FG, Schlegel J, Koch LA, Miller JB, Engesser R, Giuriani G, Brandl SM, Timmer J, Weber W, Ott T, Simon R, Zurbriggen MD (2020). Optogenetic control of gene expression in plants in the presence of ambient white light. Nature Methods, 17, 717-725.
DOI PMID |
| [61] | Ort DR, Baker NR (2002). A photoprotective role for O2 as an alternative electron sink in photosynthesis? Current Opinion in Plant Biology, 5, 193-198. |
| [62] |
Panitchayangkoon G, Voronine DV, Abramavicius D, Caram JR, Lewis NHC, Mukamel S, Engel GS (2011). Direct evidence of quantum transport in photosynthetic light- harvesting complexes. Proceedings of the National Academy of Sciences of the United States of America, 108, 20908-20912.
DOI PMID |
| [63] | Papageorgiou GC, Govindjee G (2004). Chlorophyll a Fluorescence— A Signature of Photosynthesis. Springer, Dordrecht, the Netherlands. 818. |
| [64] | Pavlovič A, Slováková L, Pandolfi C, Mancuso S (2011). On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). Journal of Experimental Botany, 62, 1991-2000. |
| [65] | Platt T, Gallegos CL, Harrison WG (1980). Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research, 38, 687-701. |
| [66] | Platt T, Sathyendranath S, Longhurst A (1995). Remote sensing of primary production in the ocean: promise and fulfilment. Philosophical Transactions of the Royal Society B: Biological Sciences, 348, 191-202. |
| [67] | Ralph PJ, Gademann R (2005). Rapid light curves: a powerful tool to assess photosynthetic activity. Aquatic Botany, 82, 222-237. |
| [68] | Rascher U, Liebig M, Luttge U (2000). Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant, Cell & Environment, 23, 1397-1405. |
| [69] | Renk H, Ochocki S, Kurzyk S (2000). In situ and simulated in situ primary production in the Gulf of Gdañsk. Oceanologia, 42, 263-282. |
| [70] | Ritchie R, Bunthawin S (2010). The use of pulse amplitude modulation (PAM) fluorometry to measure photosynthesis in a CAM orchid, Dendrobium spp. (D. cv. Viravuth Pink). International Journal of Plant Sciences, 171, 575-585. |
| [71] |
Robakowski P (2005). Susceptibility to low-temperature photoinhibition in three conifers differing in successional status. Tree Physiology, 25, 1151-1160.
PMID |
| [72] | Robakowski P, Pers-Kamczyc E, Ratajczak E, Thomas PA, Ye Z, Rabska M, Iszkuło G (2018). Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Frontiers in Plant Science, 9, 742. DOI: 10.3389/fpls.2018.00742. |
| [73] | Rubio FC, Camacho FG, Sevilla JM, Chisti Y, Grima EM (2003). A mechanistic model of photosynthesis in microalgae. Biotechnology & Bioengineering, 81, 459-473. |
| [74] | Sáez PL, Bravo LA, Latsague MI, Toneatti MJ, Sánchez-Olate M, Ríos DG (2013). Light energy management in micropropagated plants of Castanea sativa, effects of photoinhibition. Plant Science, 201-202, 12-24. |
| [75] | Sagun JV, Badger MR, Chow WS, Ghannoum O (2021). Mehler reaction plays a role in C3and C4 photosynthesis under shade and low CO2. Photosynthesis Research, 149, 171-185. |
| [76] | Sathyendranath S, Longhurst A, Caverhill CM, Platt T (1995). Regionally and seasonally differentiated primary production in the North Atlantic. Deep Sea Research Part I: Oceanographic Research Papers, 42, 1773-1802. |
| [77] | Sener M, Strümpfer J, Hsin J, Chandler D, Scheuring S, Hunter CN, Schulten K (2011). Förster energy transfer theory as reflected in the structures of photosynthetic light-harvesting systems. Chemphyschem, 12, 518-531. |
| [78] |
Serodio J, Ezequiel J, Frommlet J, Laviale M, Lavaud J (2013). A method for the rapid generation of nonsequential light-response curves of chlorophyll fluorescence. Plant Physiology, 163, 1089-1102.
DOI PMID |
| [79] |
Shameer S, Ratcliffe RG, Sweetlove LJ (2019). Leaf energy balance requires mitochondrial respiration and export of chloroplast NADPH in the light. Plant Physiology, 180, 1947-1961.
DOI PMID |
| [80] | Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment, 30, 1035-1040. |
| [81] |
Shasmita, Swain H, Ray A, Mohapatra PK, Sarkar RK, Mukherjee AK (2018). Riboflavin (Vitamin B2) mediated defence induction against bacterial leaf blight: probing through chlorophyll a fluorescence induction O-J-I-P transients. Functional Plant Biology, 45, 1251-1261.
DOI PMID |
| [82] | Streb P, Josse EM, Gallouet E, Baptist F, Kuntz M, Cornic G (2005). Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant, Cell & Environment, 28, 1123-1135. |
| [83] | Suggett DJ, Le Floc’H E, Harris GN, Leonardos N, Geider RJ (2007). Different strategies of photoacclimation by two strains of Emiliania huxleyi (Haptophyta). Journal of Phycology, 43, 1209-1222. |
| [84] | Suggett DJ, MacIntyre HL, Geider RJ (2004). Evaluation of biophysical and optical determinations of light absorption by photosystem II in phytoplankton. Limnology & Oceanography Methods, 2, 316-332. |
| [85] | Suggett DJ, Prasil O, Borowitzka MA (2010). Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications (Developments in Applied Phycology). Springer, London. 277-292. |
| [86] |
Sun JS, Sun JD, Feng ZZ (2015). Modelling photosynthesis in flag leaves of winter wheat (Triticum aestivum) considering the variation in photosynthesis parameters during development. Functional Plant Biology, 42, 1036-1044.
DOI PMID |
| [87] |
Takahashi S, Badger MR (2011). Photoprotection in plants: a new light on photosystem II damage. Trends in Plant Science, 16, 53-60.
DOI PMID |
| [88] | Talmy D, Blackford J, Hardman-Mountford NJ, Dumbrell AJ, Geider RJ (2013). An optimality model of photoadaptation in contrasting aquatic light regimes. Limnology & Oceanography, 58, 1802-1818. |
| [89] | Tang XL, Cao YH, Gu LH, Zhou BZ (2017a). Advances in photo-physiological responses of leaves to environmental factors based on the FvCB model. Acta Ecologica Sinica, 37, 6633-6645. |
| [唐星林, 曹永慧, 顾连宏, 周本智 (2017a). 基于FvCB模型的叶片光合生理对环境因子的响应研究进展. 生态学报, 37, 6633-6645.] | |
| [90] | Tang XL, Zhou BZ, Zhou Y, Ni X, Cao YH, Gu LH (2017b). Photo-physiological and photo-biochemical characteristics of several herbaceous and woody species based on FvCB model. Chinese Journal of Applied Ecology, 28, 1482-1488. |
| [唐星林, 周本智, 周燕, 倪霞, 曹永慧, 顾连宏 (2017b). 基于FvCB模型的几种草本和木本植物光合生理生化特性. 应用生态学报, 28, 1482-1488.] | |
| [91] | Thornley JHM (1976). Mathematical Models in Plant Physiology: a Quantitative Approach to Problems in Plant and Crop Physiology. Academic Press, London. 86-110. |
| [92] | Turpin DH, Elrifi IR, Birch DG, Weger HG, Holmes JJ (1988). Interactions between photosynthesis, respiration, and nitrogen assimilation in microalgae. Canadian Journal of Botany, 66, 2083-2097. |
| [93] | Vetoshkina DV, Ivanov BN, Khorobrykh SA, Proskuryakov II, Borisova-Mubarakshina MM (2017). Involvement of the chloroplast plastoquinone pool in the Mehler reaction. Physiologia Plantarum, 161, 45-55. |
| [94] | Wan WB, Hua DX, Le J, Liu MX, Cao N (2013). Laser- induced chlorophyll fluorescence lifetime measurement and characteristic analysis. Acta Physica Sinica, 62, 190601. DOI: 10.7498/aps.62.190601. |
| [万文博, 华灯鑫, 乐静, 刘美霞, 曹宁 (2013). 激光诱导叶绿素荧光寿命的测量及其特性分析. 物理学报, 62, 190601. DOI: 10.7498/aps.62.190601.] | |
| [95] | Wang X, Kang M, Wang SY, Cao WX, Zhu Y, Liu B (2022). Comparison of rapid A-Ci response (RACiR) curve fitted by different photosynthetic carbon assimilation CO2 response models in rice. Journal of Nanjing Agricultural University, 45, 1099-1106. |
| [王雪, 康敏, 王偲媛, 曹卫星, 朱艳, 刘兵 (2022). 不同光合-CO2响应模型对水稻快速A-Ci响应(RACiR)曲线拟合效果的比较研究. 南京农业大学学报, 45, 1099-1106.] | |
| [96] | Wang Y, Yu YT, Zhang HB, Huo YZ, Liu XQ, Che YH, Wang JC, Sun GY, Zhang HH (2022). The phytotoxicity of exposure to two polybrominated diphenyl ethers (BDE47 and BDE209) on photosynthesis and the response of the hormone signaling and ROS scavenging system in tobacco leaves. Journal of Hazardous Materials, 426, 128012. DOI: 10.1016/j.jhazmat.2021.128012. |
| [97] | White AJ, Critchley C (1999). Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynthesis Research, 59, 63-72. |
| [98] | Wu A, Song Y, van Oosterom EJ, Hammer GL (2016). Connecting biochemical photosynthesis models with crop models to support crop improvement. Frontiers in Plant Science, 7, 1518. DOI: 10.3389/fpls.2016.01518. |
| [99] | Xia Q, Tan JL, Cheng SY, Jiang YN, Guo Y (2019). Sensing plant physiology and environmental stress by automatically tracking Fj and Fi features in PSII chlorophyll fluorescence induction. Photochemistry & Photobiology, 95, 1495-1503. |
| [100] | Yang XL, Liu LH, Yin ZK, Wang XY, Wang SB, Ye ZP (2020). Quantifying photosynthetic performance of phytoplankton based on photosynthesis-irradiance response models. Environmental Sciences Europe, 32, 1-13. |
| [101] | Ye ZP (2012). Nonlinear optical absorption of photosynthetic pigment molecules in leaves. Photosynthesis Research, 112, 31-37. |
| [102] | Ye ZP, Duan SH, Chen XM, Duan HL, Gao CP, Kang HJ, An T, Zhou SX (2021). Quantifying light response of photosynthesis: addressing the long-standing limitations of non-rectangular hyperbolic model. Photosynthetica, 59, 185-191. |
| [103] | Ye ZP, Kang HJ, An T, Duan HL, Wang FB, Yang XL, Zhou SX (2020). Modeling light response of electron transport rate and its allocation for ribulose biphosphate carboxylation and oxygenation. Frontiers in Plant Science, 11, 581851. DOI: 10.3389/fpls.2020.581851. |
| [104] | Ye ZP, Liu YG, Kang HJ, Duan HL, Chen XM, Zhou SX (2019). Comparing two measures of leaf photorespiration rate across a wide range of light intensities. Journal of Plant Physiology, 240, 153002. DOI: 10.1016/j.jplph.2019.153002. |
| [105] | Ye ZP, Robakowski P, Suggett DJ (2013a). A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules. Planta, 237, 837-847. |
| [106] | Ye Z, Suggett DJ, Robakowski P, Kang H (2013b). A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytologist, 199, 110-120. |
| [107] | Ye ZP, Zheng Z, Kang HJ, Wang FB, An T, Duan SH (2019). Stomatal and non-stomatal limitations on photosynthesis of flag leaf of medium mature indica rice at early earring stage under natural conditions. Chinese Journal of Ecology, 38, 1004-1012. |
| [叶子飘, 郑卓, 康华靖, 王复标, 安婷, 段世华 (2019). 自然条件下中熟籼稻初穗期剑叶光合的气孔和非气孔限制特征. 生态学杂志, 38, 1004-1012.] | |
| [108] | Yin X, Struik PC, Goudriaan J (2021). On the needs for combining physiological principles and mathematics to improve crop models. Field Crops Research, 271, 108254. DOI: 10.1016/j.fcr.2021.108254. |
| [109] | Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR (2012). A kinetic model of rapidly reversible nonphotochemical quenching. Proceedings of the National Academy of Sciences of the United States of America, 109, 15757-15762. |
| [1] | 叶子飘, 段世华, 安婷, 康华靖. 最大电子传递速率的确定及其对电子流分配的影响[J]. 植物生态学报, 2018, 42(4): 498-507. |
| [2] | 李理渊, 李俊, 同小娟, 孟平, 张劲松, 张静茹. 黄河小浪底栓皮栎、刺槐叶片电子传递速率-光响应的模拟[J]. 植物生态学报, 2018, 42(10): 1009-1021. |
| [3] | 叶子飘, 段世华, 安婷, 康华靖. C4作物电子传递速率对CO2响应模型的构建及应用[J]. 植物生态学报, 2018, 42(10): 1000-1008. |
| [4] | 叶子飘, 胡文海, 闫小红. 光系统II实际光化学量子效率对光的响应模型的比较[J]. 植物生态学报, 2016, 40(11): 1208-1217. |
| [5] | 叶子飘,胡文海,肖宜安,樊大勇,尹建华,段世华,闫小红,贺俐,张斯斯. 光合电子流对光响应的机理模型及其应用[J]. 植物生态学报, 2014, 38(11): 1241-1249. |
| [6] | 鱼腾飞, 冯起, 司建华. 极端干旱区多枝柽柳叶片气孔导度的环境响应模拟[J]. 植物生态学报, 2012, 36(6): 483-490. |
| [7] | 张绪成, 于显枫, 高世铭. 高大气CO2浓度下氮素对小麦叶片光能利用的影响[J]. 植物生态学报, 2010, 34(10): 1196-1203. |
| [8] | 韩广轩, 周广胜. 土壤呼吸作用时空动态变化及其影响机制研究与展望[J]. 植物生态学报, 2009, 33(1): 197-205. |
| [9] | 张云霞, 李晓兵, 张云飞. 基于数字相机、ASTER和MODIS影像综合测量植被盖度[J]. 植物生态学报, 2007, 31(5): 842-849. |
| [10] | 祖元刚, 张衷华, 王文杰, 杨逢建, 贺海升. 薇甘菊叶和茎的光合特性[J]. 植物生态学报, 2006, 30(6): 998-1004. |
| [11] | 米湘成, 马克平, 邹应斌. 人工神经网络模型及其在农业和生态学研究中的应用[J]. 植物生态学报, 2005, 29(5): 863-870. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2026 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()
