

植物生态学报 ›› 2024, Vol. 48 ›› Issue (4): 416-427.DOI: 10.17521/cjpe.2023.0253 cstr: 32100.14.cjpe.2023.0253
曲泽坤1, 朱丽琴1, 姜琦1, 王小红1, 姚晓东1, 蔡世锋2, 罗素珍2, 陈光水1,*( )
)
收稿日期:2023-09-04
接受日期:2023-12-21
出版日期:2024-04-20
发布日期:2024-05-11
通讯作者:
* (gschen@fjnu.edu.cn)
基金资助:
QU Ze-Kun1, ZHU Li-Qin1, JIANG Qi1, WANG Xiao-Hong1, YAO Xiao-Dong1, CAI Shi-Feng2, LUO Su-Zhen2, sCHEN Guang-Shui1,*( )
)
Received:2023-09-04
Accepted:2023-12-21
Online:2024-04-20
Published:2024-05-11
Contact:
* (gschen@fjnu.edu.cn)
Supported by:摘要:
植物细根和丛枝菌根(AM)真菌的养分觅食策略直接影响植物的生产力与碳汇能力, 是森林生态系统稳定性的关键影响因素。养分觅食精度是养分觅食策略的重要方面, 指植物将其根系、菌丝精准地部署到养分相对丰富斑块中的能力。然而, 目前对内生菌根树种细根和菌根菌丝觅食精度间的权衡关系, 以及细根形态能否较好地预测觅食精度仍存在争议。该研究对中亚热带天然常绿阔叶林内17个AM树种进行野外原位根袋磷添加实验, 以模拟土壤中磷养分斑块。施加磷肥4个月后, 对对照组和磷添加组的细根进行形态扫描和分析, 采用膜过滤法提取土壤中的菌丝并用电子显微镜观测, 筛选中部无隔膜且易于染色的菌丝作为AM内生菌根菌丝, 计算其长度。在此基础上, 计算根长觅食精度和菌丝觅食精度, 以研究亚热带AM树种的根长觅食精度与菌丝觅食精度间的权衡及其与细根形态间的相关关系。主要结果有: (1) AM树种根长觅食精度与菌丝觅食精度间相互独立; (2)细根组织密度与根长觅食精度呈显著正相关关系; (3)细根直径与菌丝觅食精度呈显著负相关关系, 比根长与菌丝觅食精度呈显著正相关关系。该研究结果有助于理解亚热带常绿阔叶林AM树种根系养分觅食策略, 表明细根形态等易观测指标可用于评估AM树种细根养分觅食精度。
曲泽坤, 朱丽琴, 姜琦, 王小红, 姚晓东, 蔡世锋, 罗素珍, 陈光水. 亚热带常绿阔叶林丛枝菌根树种养分觅食策略及其与细根形态间的关系. 植物生态学报, 2024, 48(4): 416-427. DOI: 10.17521/cjpe.2023.0253
QU Ze-Kun, ZHU Li-Qin, JIANG Qi, WANG Xiao-Hong, YAO Xiao-Dong, CAI Shi-Feng, LUO Su-Zhen, sCHEN Guang-Shui. Nutrient foraging strategies of arbuscular mycorrhizal tree species in a subtropical evergreen broadleaf forest and their relationship with fine root morphology. Chinese Journal of Plant Ecology, 2024, 48(4): 416-427. DOI: 10.17521/cjpe.2023.0253
| 树种 Species | 科 Family | 属 Genus | 叶习性 Leaf habit | 根样本数 Root sample number | DBH (cm) |
|---|---|---|---|---|---|
| 台湾冬青 Ilex formosana | 冬青科 Aquifoliaceae | 冬青属 Ilex | EB | 2 | 33.1 ± 5.1 |
| 臀果木 Pygeum topengii | 蔷薇科 Rosaceae | 臀果木属 Pygeum | EB | 3 | 20.7 ± 0.8 |
| 新木姜子 Neolitsea aurata | 木兰科 Lauraceae | 新木姜子属 Neolitsea | EB | 3 | 11.6 ± 0.1 |
| 华南桂 Cinnamomum austrosinense | 木兰科 Lauraceae | 肉桂属 Cinnamomum | EB | 3 | 10.9 ± 0.8 |
| 枫香树 Liquidambar formosana | 枫香科 Altingiaceae | 枫香树属 Liquidambar | DB | 3 | 49.6 ± 2.5 |
| 香叶树 Lindera communis | 木兰科 Lauraceae | 山胡椒属 Lindera | EB | 3 | 14.3 ± 0.6 |
| 赤楠 Syzygium buxifolium | 桃金娘科 Myrtaceae | 蒲桃属 Syzygium | EB | 3 | 9.9 ± 0.2 |
| 赤杨叶 Alniphyllum fortunei | 安息香科 Styracaceae | 赤杨叶属 Alniphyllum | DB | 3 | 21.4 ± 1.2 |
| 楠木 Phoebe zhennan | 木兰科 Lauraceae | 楠木属 Phoebe | EB | 3 | 14.7 ± 0.8 |
| 光叶山矾 Symplocos lancifolia | 山矾科 Symplocaceae | 山矾属 Symplocos | EB | 2 | 11.5 ± 0.7 |
| 笔罗子 Meliosma rigida | 清风藤科 Sabiaceae | 泡花树属 Meliosma | EB | 3 | 16.3 ± 1.0 |
| 木荷 Schima superba | 山茶科 Theaceae | 木荷属 Schima | EB | 3 | 26.4 ± 0.5 |
| 茜树 Aidia cochinchinensis | 茜草科 Rubiaceae | 茜树属 Aidia | EB | 3 | 16.7 ± 0.3 |
| 杜英 Elaeocarpus decipiens | 杜英科 Elaeocarpaceae | 杜英属 Elaeocarpus | EB | 3 | 18.6 ± 1.5 |
| 观光木 Michelia odora | 木兰科 Magnoliaceae | 含笑属 Michelia | EB | 3 | 26.5 ± 1.3 |
| 牛矢果 Osmanthus matsumuranus | 木犀科 Oleaceae | 木犀属 Osmanthus | EB | 3 | 19.2 ± 0.1 |
| 木油桐 Vernicia montana | 大戟科 Euphorbiaceae | 油桐属 Vernicia | DB | 3 | 24.0 ± 1.5 |
表1 亚热带常绿阔叶林丛枝菌根树种样树的基本信息
Table 1 Basic information of sample arbuscular mycorrhizal tree species in subtropical evergreen broadleaf forest
| 树种 Species | 科 Family | 属 Genus | 叶习性 Leaf habit | 根样本数 Root sample number | DBH (cm) |
|---|---|---|---|---|---|
| 台湾冬青 Ilex formosana | 冬青科 Aquifoliaceae | 冬青属 Ilex | EB | 2 | 33.1 ± 5.1 |
| 臀果木 Pygeum topengii | 蔷薇科 Rosaceae | 臀果木属 Pygeum | EB | 3 | 20.7 ± 0.8 |
| 新木姜子 Neolitsea aurata | 木兰科 Lauraceae | 新木姜子属 Neolitsea | EB | 3 | 11.6 ± 0.1 |
| 华南桂 Cinnamomum austrosinense | 木兰科 Lauraceae | 肉桂属 Cinnamomum | EB | 3 | 10.9 ± 0.8 |
| 枫香树 Liquidambar formosana | 枫香科 Altingiaceae | 枫香树属 Liquidambar | DB | 3 | 49.6 ± 2.5 |
| 香叶树 Lindera communis | 木兰科 Lauraceae | 山胡椒属 Lindera | EB | 3 | 14.3 ± 0.6 |
| 赤楠 Syzygium buxifolium | 桃金娘科 Myrtaceae | 蒲桃属 Syzygium | EB | 3 | 9.9 ± 0.2 |
| 赤杨叶 Alniphyllum fortunei | 安息香科 Styracaceae | 赤杨叶属 Alniphyllum | DB | 3 | 21.4 ± 1.2 |
| 楠木 Phoebe zhennan | 木兰科 Lauraceae | 楠木属 Phoebe | EB | 3 | 14.7 ± 0.8 |
| 光叶山矾 Symplocos lancifolia | 山矾科 Symplocaceae | 山矾属 Symplocos | EB | 2 | 11.5 ± 0.7 |
| 笔罗子 Meliosma rigida | 清风藤科 Sabiaceae | 泡花树属 Meliosma | EB | 3 | 16.3 ± 1.0 |
| 木荷 Schima superba | 山茶科 Theaceae | 木荷属 Schima | EB | 3 | 26.4 ± 0.5 |
| 茜树 Aidia cochinchinensis | 茜草科 Rubiaceae | 茜树属 Aidia | EB | 3 | 16.7 ± 0.3 |
| 杜英 Elaeocarpus decipiens | 杜英科 Elaeocarpaceae | 杜英属 Elaeocarpus | EB | 3 | 18.6 ± 1.5 |
| 观光木 Michelia odora | 木兰科 Magnoliaceae | 含笑属 Michelia | EB | 3 | 26.5 ± 1.3 |
| 牛矢果 Osmanthus matsumuranus | 木犀科 Oleaceae | 木犀属 Osmanthus | EB | 3 | 19.2 ± 0.1 |
| 木油桐 Vernicia montana | 大戟科 Euphorbiaceae | 油桐属 Vernicia | DB | 3 | 24.0 ± 1.5 |
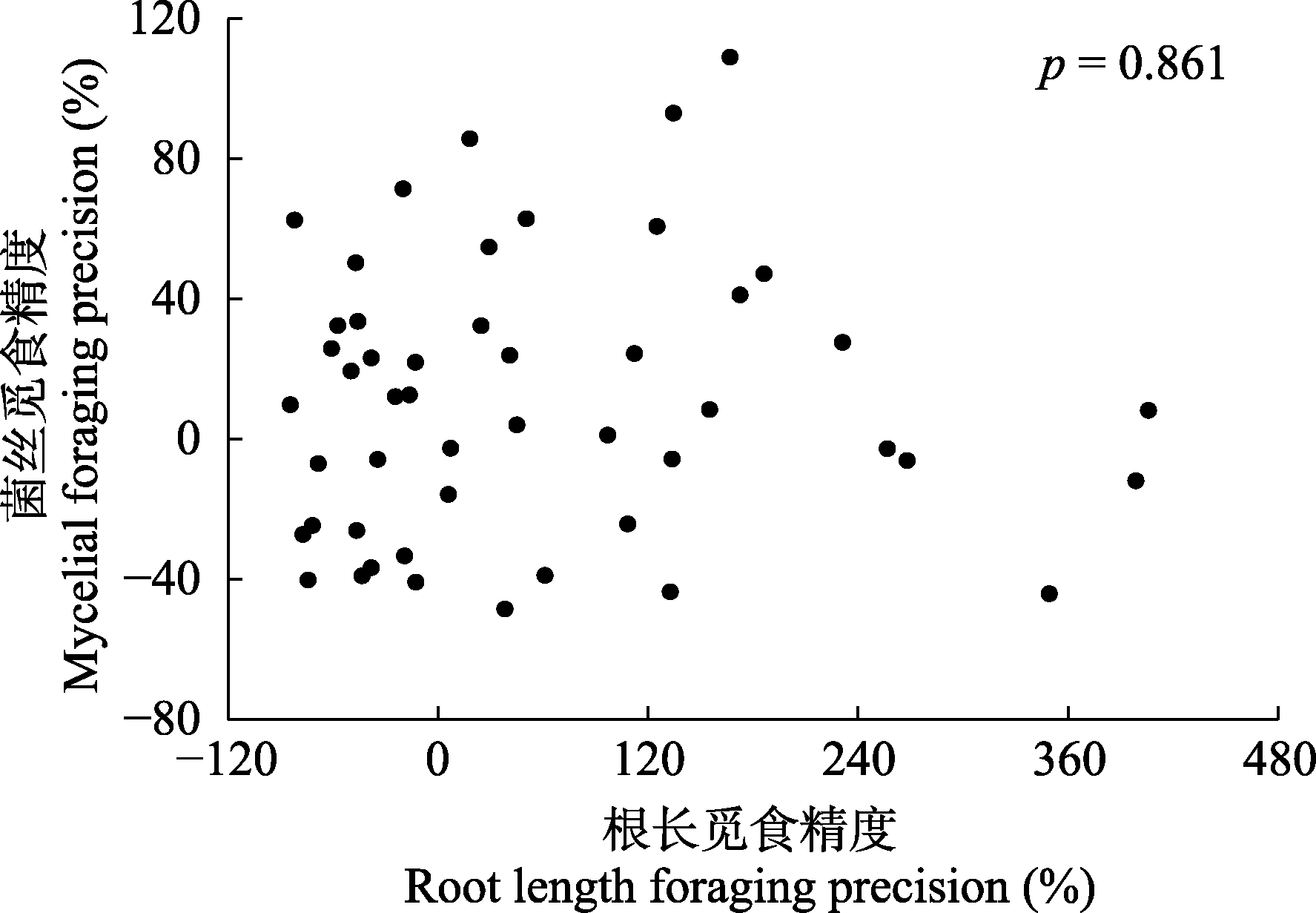
图1 亚热带常绿阔叶林根长觅食精度与菌丝觅食精度的相关性。
Fig. 1 Correlation between root length foraging precision and mycelial foraging precision in subtropical evergreen broadleaf forest.
| 树种 Species | 对照斑块细根长度 Fine root length in control patch (cm) | 富磷斑块细根长度 Fine root length in phosphorus-rich patch (cm) | 根长觅食精度 Root length foraging precision (%) | 对照斑块菌丝长度 Mycelium length in control patch (cm) | 富磷斑块菌丝长度 Mycelium length in phosphorus-rich patch (mm) | 菌丝觅食精度 Mycelial foraging precision (%) |
|---|---|---|---|---|---|---|
| 台湾冬青 Ilex formosana | 3.9 ± 2.1 | 7.8 ± 4.9 | 86.7 ± 25.6 | 433.9 ± 96.5 | 372.0 ± 47.9 | -7.2 ± 31.7 |
| 臀果木 Pygeum topengii | 28.8 ± 3.4 | 9.0 ± 2.7 | -67.5 ± 12.0 | 514.9 ±17.4 | 633.5 ± 69.3 | 22.7 ± 9.5 |
| 新木姜子 Neolitsea aurata | 3.3 ± 1.4 | 2.8 ± 1.7 | 35.3 ± 139.4 | 289.7 ± 57.0 | 317.2 ± 77.1 | 17.0 ± 42.1 |
| 华南桂 Cinnamomu austrosinense | 7.7 ± 1.3 | 18.0 ± 0.6 | 138.7 ± 36.7 | 354.5 ± 90.4 | 327.8 ± 47.5 | 1.6 ± 37.0 |
| 枫香树 Liquidambar formosana | 3.3 ± 0.2 | 5.3 ± 2.5 | 64.3 ± 78.7 | 343.3 ± 13.2 | 559.1 ± 157.0 | 61.4 ± 39.6 |
| 香叶树 Lindera communis | 5.5 ± 2.9 | 4.5 ± 1.3 | -2.2 ± 31.7 | 682.6 ± 143.2 | 789.6 ± 234.8 | 13.4 ± 13.6 |
| 赤楠 Syzygium buxifolium | 3.7 ± 0.8 | 10.0 ± 0.5 | 184.8 ± 54.4 | 535.3 ± 161.2 | 671.8 ± 64.6 | 33.0 ± 26.5 |
| 赤杨叶 Alniphyllum fortunei | 4.7 ± 1.3 | 3.3 ± 0.3 | -22.3 ± 28.9 | 213.6 ± 22.9 | 321.2 ± 19.2 | 53.1 ± 25.6 |
| 楠木 Phoebe zhennan | 3.5 ± 0.2 | 3.5 ± 1.1 | -1.1 ± 27.8 | 522.5 ± 72.4 | 546.1 ± 278.7 | 0.8 ± 39.2 |
| 光叶山矾 Symplocos lancifolia | 15.8 ± 3.2 | 24.1 ± 8.2 | 69.4 ± 85.8 | 457.4 ± 21.4 | 506.0 ± 33.1 | 10.5 ± 2.1 |
| 笔罗子 Meliosma rigida | 6.9 ± 3.7 | 6.8 ± 3.0 | 21.6 ± 80.8 | 277.9 ± 49.9 | 434.1 ± 43.3 | 61.3 ± 30.9 |
| 木荷 Schima superba | 11.3 ± 5.8 | 13.9 ± 2.1 | 105.8 ± 172.2 | 339.9 ± 11.2 | 206.2 ± 20.9 | -39.4 ± 4.5 |
| 茜树 Aidia cochinchinensis | 4.8 ± 2.5 | 6.0 ± 2.0 | 82.0 ± 134.7 | 237.7 ± 29.0 | 230.8 ± 26.6 | 0.1 ± 24.3 |
| 杜英 Elaeocarpus decipiens | 5.9 ± 1.2 | 8.5 ± 2.5 | 53.6 ± 41.7 | 265.2 ± 62.7 | 237.7 ± 21.6 | -7.6 ± 12.1 |
| 观光木 Michelia odora | 3.9 ± 2.7 | 4.0 ± 1.3 | 125.3 ± 203.2 | 423.3 ± 70.4 | 343.7 ± 75.9 | -15.7 ± 23.9 |
| 牛矢果 Osmanthus matsumuranus | 1.9 ± 2.0 | 2.0 ± 0.7 | 162.2 ± 182.6 | 405.9 ± 12.6 | 425.3 ± 68.2 | 5.4 ± 20.1 |
| 木油桐 Vernicia montana | 4.7 ± 0.2 | 1.2 ± 0.1 | -74.4 ± 2.2 | 378.1 ± 45.0 | 265.2 ± 55.0 | -30.7 ± 6.8 |
表2 亚热带常绿阔叶林17个丛枝菌根树种对照与富磷斑块中细根、菌丝长度以及根长觅食精度、菌丝觅食精度(平均值±标准误)
Table 2 Fine root length and mycelium length in the control and phosphorus-rich patches, and root length foraging precision and mycelial foraging precision of 17 arbuscular mycorrhizal tree species in a subtropical evergreen broadleaf forest (mean ± SE)
| 树种 Species | 对照斑块细根长度 Fine root length in control patch (cm) | 富磷斑块细根长度 Fine root length in phosphorus-rich patch (cm) | 根长觅食精度 Root length foraging precision (%) | 对照斑块菌丝长度 Mycelium length in control patch (cm) | 富磷斑块菌丝长度 Mycelium length in phosphorus-rich patch (mm) | 菌丝觅食精度 Mycelial foraging precision (%) |
|---|---|---|---|---|---|---|
| 台湾冬青 Ilex formosana | 3.9 ± 2.1 | 7.8 ± 4.9 | 86.7 ± 25.6 | 433.9 ± 96.5 | 372.0 ± 47.9 | -7.2 ± 31.7 |
| 臀果木 Pygeum topengii | 28.8 ± 3.4 | 9.0 ± 2.7 | -67.5 ± 12.0 | 514.9 ±17.4 | 633.5 ± 69.3 | 22.7 ± 9.5 |
| 新木姜子 Neolitsea aurata | 3.3 ± 1.4 | 2.8 ± 1.7 | 35.3 ± 139.4 | 289.7 ± 57.0 | 317.2 ± 77.1 | 17.0 ± 42.1 |
| 华南桂 Cinnamomu austrosinense | 7.7 ± 1.3 | 18.0 ± 0.6 | 138.7 ± 36.7 | 354.5 ± 90.4 | 327.8 ± 47.5 | 1.6 ± 37.0 |
| 枫香树 Liquidambar formosana | 3.3 ± 0.2 | 5.3 ± 2.5 | 64.3 ± 78.7 | 343.3 ± 13.2 | 559.1 ± 157.0 | 61.4 ± 39.6 |
| 香叶树 Lindera communis | 5.5 ± 2.9 | 4.5 ± 1.3 | -2.2 ± 31.7 | 682.6 ± 143.2 | 789.6 ± 234.8 | 13.4 ± 13.6 |
| 赤楠 Syzygium buxifolium | 3.7 ± 0.8 | 10.0 ± 0.5 | 184.8 ± 54.4 | 535.3 ± 161.2 | 671.8 ± 64.6 | 33.0 ± 26.5 |
| 赤杨叶 Alniphyllum fortunei | 4.7 ± 1.3 | 3.3 ± 0.3 | -22.3 ± 28.9 | 213.6 ± 22.9 | 321.2 ± 19.2 | 53.1 ± 25.6 |
| 楠木 Phoebe zhennan | 3.5 ± 0.2 | 3.5 ± 1.1 | -1.1 ± 27.8 | 522.5 ± 72.4 | 546.1 ± 278.7 | 0.8 ± 39.2 |
| 光叶山矾 Symplocos lancifolia | 15.8 ± 3.2 | 24.1 ± 8.2 | 69.4 ± 85.8 | 457.4 ± 21.4 | 506.0 ± 33.1 | 10.5 ± 2.1 |
| 笔罗子 Meliosma rigida | 6.9 ± 3.7 | 6.8 ± 3.0 | 21.6 ± 80.8 | 277.9 ± 49.9 | 434.1 ± 43.3 | 61.3 ± 30.9 |
| 木荷 Schima superba | 11.3 ± 5.8 | 13.9 ± 2.1 | 105.8 ± 172.2 | 339.9 ± 11.2 | 206.2 ± 20.9 | -39.4 ± 4.5 |
| 茜树 Aidia cochinchinensis | 4.8 ± 2.5 | 6.0 ± 2.0 | 82.0 ± 134.7 | 237.7 ± 29.0 | 230.8 ± 26.6 | 0.1 ± 24.3 |
| 杜英 Elaeocarpus decipiens | 5.9 ± 1.2 | 8.5 ± 2.5 | 53.6 ± 41.7 | 265.2 ± 62.7 | 237.7 ± 21.6 | -7.6 ± 12.1 |
| 观光木 Michelia odora | 3.9 ± 2.7 | 4.0 ± 1.3 | 125.3 ± 203.2 | 423.3 ± 70.4 | 343.7 ± 75.9 | -15.7 ± 23.9 |
| 牛矢果 Osmanthus matsumuranus | 1.9 ± 2.0 | 2.0 ± 0.7 | 162.2 ± 182.6 | 405.9 ± 12.6 | 425.3 ± 68.2 | 5.4 ± 20.1 |
| 木油桐 Vernicia montana | 4.7 ± 0.2 | 1.2 ± 0.1 | -74.4 ± 2.2 | 378.1 ± 45.0 | 265.2 ± 55.0 | -30.7 ± 6.8 |
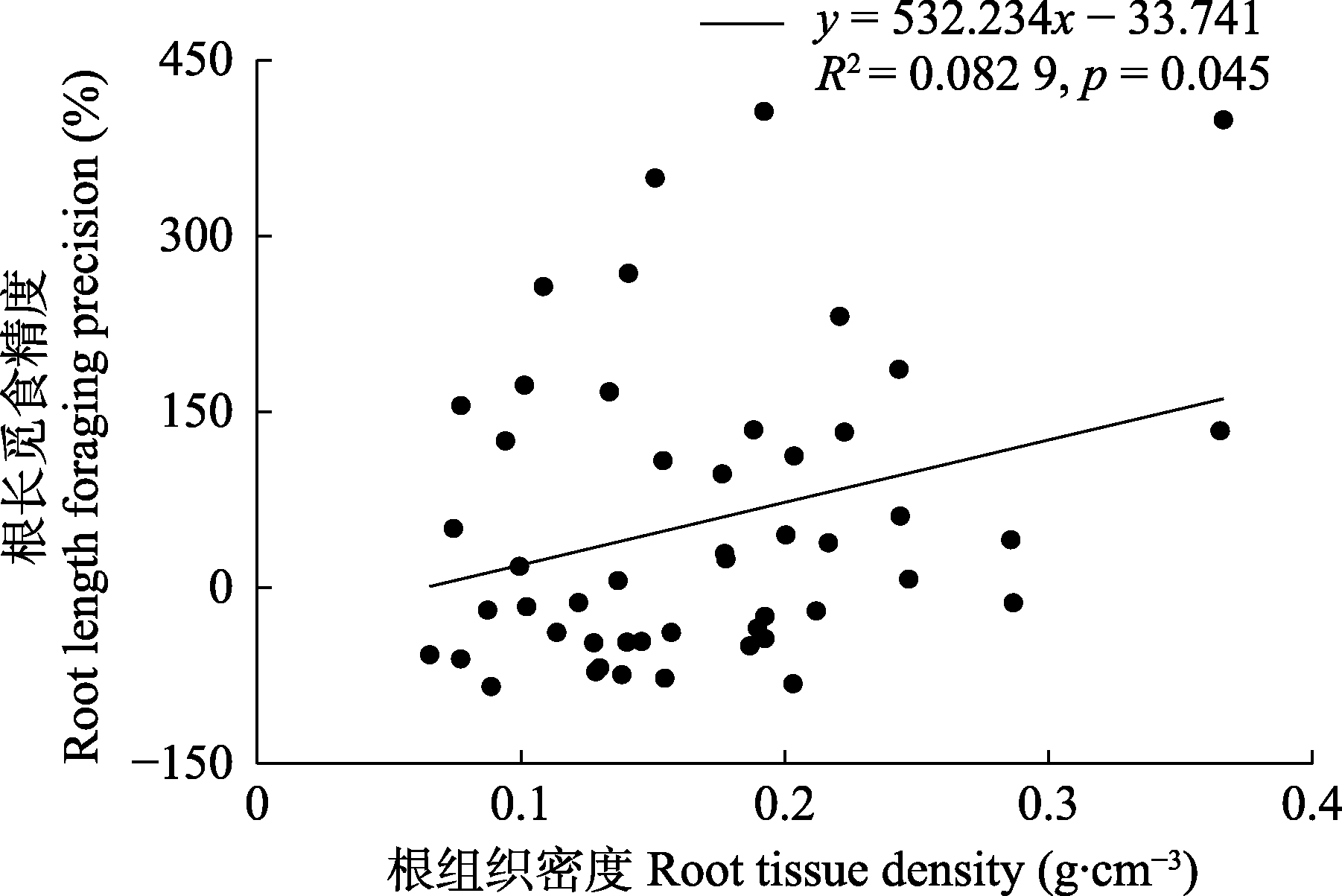
图2 亚热带常绿阔叶林根长觅食精度与根组织密度的线性关系。
Fig. 2 Relationship between root length foraging precision and root tissue density in a subtropical evergreen broadleaf forest.
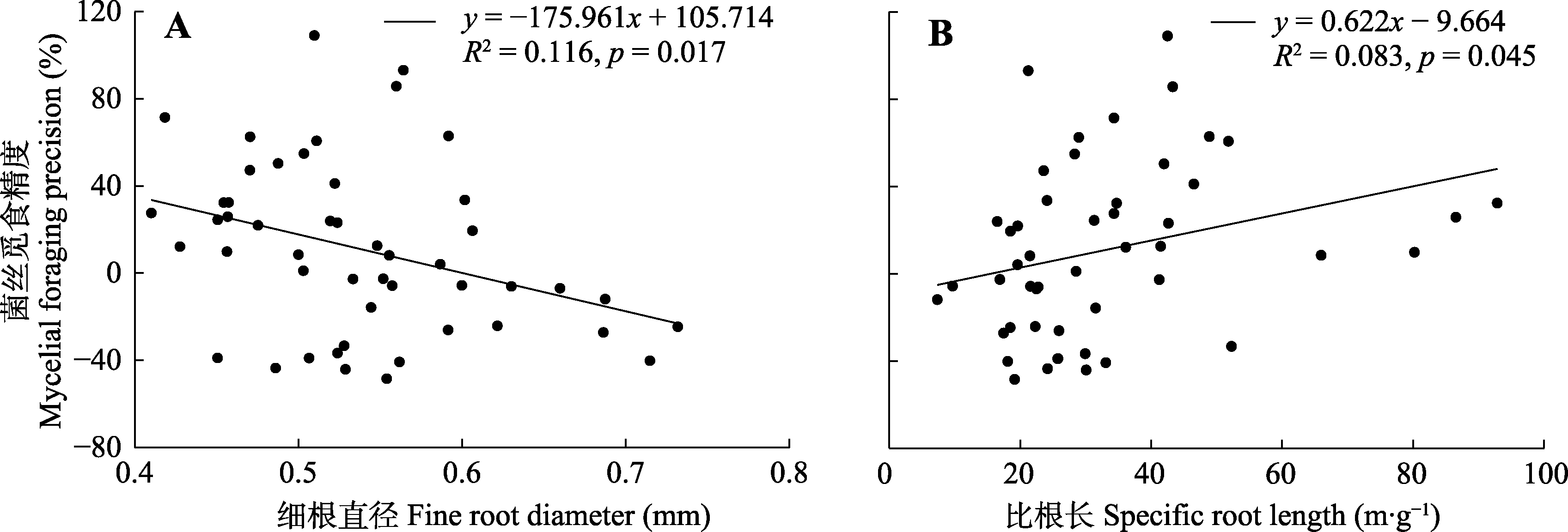
图3 亚热带常绿阔叶林菌丝觅食精度与细根直径(A)和比根长(B)的线性关系。
Fig. 3 Relationships of mycelial foraging precision with fine root diameter (A) and specific root length (B) in a subtropical evergreen broadleaf forest.
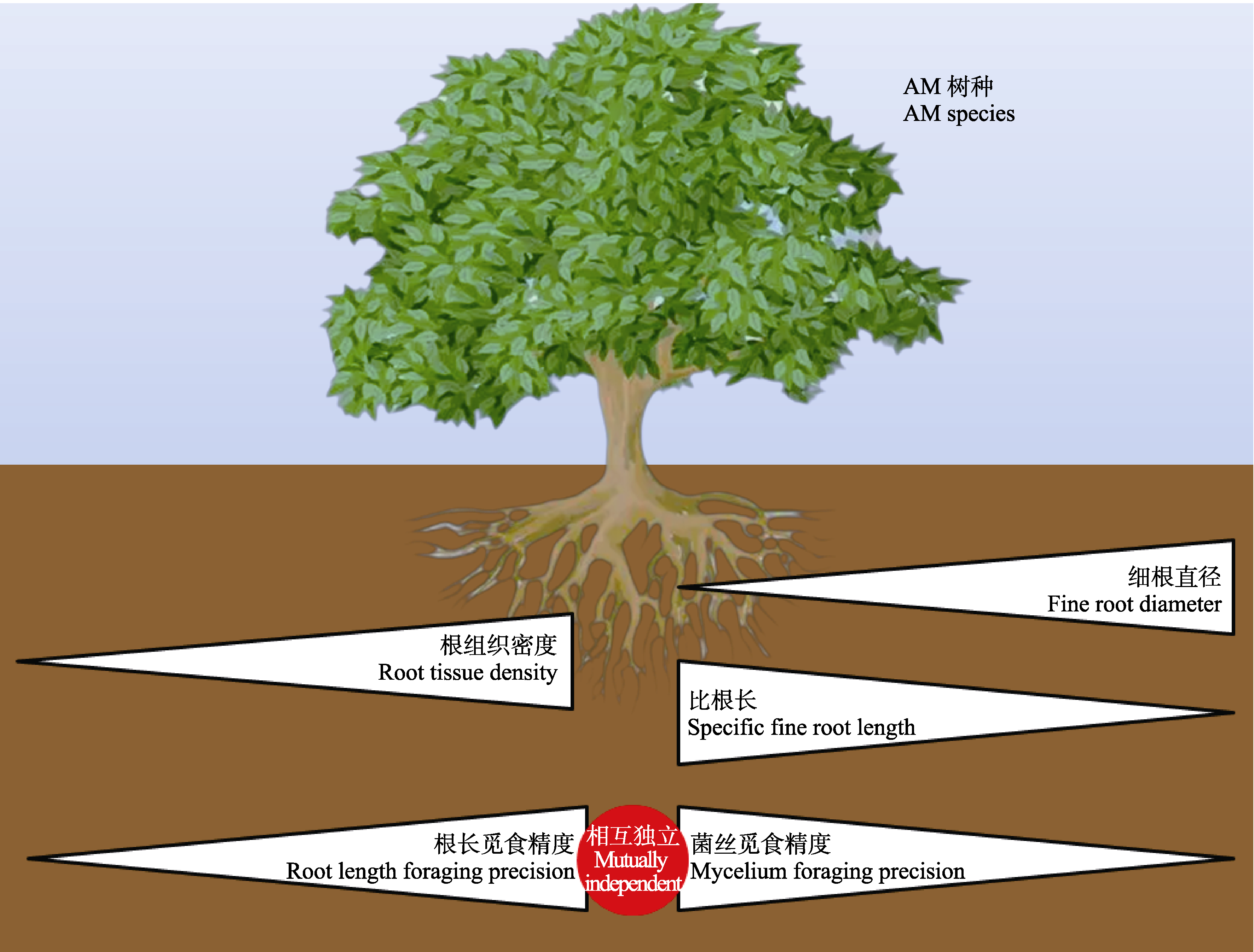
图4 细根形态与根长觅食精度、菌丝觅食精度的关系。AM, 丛枝菌根。
Fig. 4 Relationships of fine root morphology with root length foraging precision and mycelial foraging precision. AM, arbuscular mycorrhiza.
| 协变量 Covariate | 根长觅食精度 Root length foraging precision | 菌丝觅食精度 Mycelium foraging precision | ||||
|---|---|---|---|---|---|---|
| t | F | p | t | F | p | |
| 细根直径 Fine root diameter | 0.003 | <0.001 | 0.998 | -2.486 | 6.178 | 0.017* |
| 比根长 Specific root length | -1.337 | 1.789 | 0.188 | 2.058 | 4.235 | 0.045* |
| 比表面积 Specific root surface area | -1.440 | 2.075 | 0.156 | 1.829 | 3.344 | 0.074 |
| 组织密度 Root tissue density | 2.061 | 4.247 | 0.045* | -0.883 | 0.780 | 0.382 |
表3 亚热带常绿阔叶林细根形态性状对根长觅食精度、菌丝觅食精度影响的混合线性模型分析结果
Table 3 Results of linear mixed models for the effect of fine root morphological traits on root length foraging precision and mycelial foraging precision in a subtropical evergreen broadleaf forest
| 协变量 Covariate | 根长觅食精度 Root length foraging precision | 菌丝觅食精度 Mycelium foraging precision | ||||
|---|---|---|---|---|---|---|
| t | F | p | t | F | p | |
| 细根直径 Fine root diameter | 0.003 | <0.001 | 0.998 | -2.486 | 6.178 | 0.017* |
| 比根长 Specific root length | -1.337 | 1.789 | 0.188 | 2.058 | 4.235 | 0.045* |
| 比表面积 Specific root surface area | -1.440 | 2.075 | 0.156 | 1.829 | 3.344 | 0.074 |
| 组织密度 Root tissue density | 2.061 | 4.247 | 0.045* | -0.883 | 0.780 | 0.382 |
| [1] | Adomako MO, Roiloa S, Yu FH (2022). Potential roles of soil microorganisms in regulating the effect of soil nutrient heterogeneity on plant performance. Microorganisms, 10, 2399. DOI: 10.3390/microorganisms10122399. |
| [2] | Adomako MO, Xue W, Roiloa S, Zhang Q, Du DL, Yu FH (2021). Earthworms modulate impacts of soil heterogeneity on plant growth at different spatial scales. Frontiers in Plant Science, 12, 735495. DOI: 10.3389/fpls.2021.735495. |
| [3] | Almario J, Jeena G, Wunder J, Langen G, Zuccaro A, Coupland G, Bucher M (2017). Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proceedings of the National Academy of Sciences of the United States of America, 114, E9403- E9412. |
| [4] | Bergmann J, Weigelt A, van der Plas F, Laughlin DC, Kuyper TW, Guerrero-Ramirez N, Valverde-Barrantes OJ, Bruelheide H, Freschet GT, Iversen CM, Kattge J, McCormack ML, Meier IC, Rillig MC, Roumet C, et al. (2020). The fungal collaboration gradient dominates the root economics space in plants. Science Advances, 6, eaba3756. DOI: 10.1126/sciadv.aba3756. |
| [5] | Berntson GM (1994). Modelling root architecture: are there tradeoffs between efficiency and potential of resource acquisition? New Phytologist, 127, 483-493. |
| [6] |
Campbell BD, Grime JP, MacKey JM (1991). A trade-off between scale and precision in resource foraging. Oecologia, 87, 532-538.
DOI PMID |
| [7] |
Chen WL, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016). Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proceedings of the National Academy of Sciences of the United States of America, 113, 8741-8746.
DOI PMID |
| [8] | Chen WL, Koide RT, Eissenstat DM (2018). Root morphology and mycorrhizal type strongly influence root production in nutrient hot spots of mixed forests. Journal of Ecology, 106, 148-156. |
| [9] |
Cheng L, Chen WL, Adams TS, Wei X, Li L, McCormack ML, DeForest JL, Koide RT, Eissenstat DM (2016). Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology, 97, 2815-2823.
DOI PMID |
| [10] |
Cleveland CC, Townsend AR, Taylor P, Alvarez-Clare S, Bustamante MMC, Chuyong G, Dobrowski SZ, Grierson P, Harms KE, Houlton BZ, Marklein A, Parton W, Porder S, Reed SC, Sierra CA, et al. (2011). Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecology Letters, 14, 939-947.
DOI PMID |
| [11] |
Comas LH, Eissenstat DM (2009). Patterns in root trait variation among 25 co-existing North American forest species. New Phytologist, 182, 919-928.
DOI PMID |
| [12] | Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS (2001). The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos, 93, 274-285. |
| [13] |
Cui EQ, Lu RL, Xu XN, Sun HF, Qiao Y, Ping JY, Qiu SY, Lin YH, Bao JH, Yong YT, Zheng ZM, Yan ER, Xia JY (2022). Soil phosphorus drives plant trait variations in a mature subtropical forest. Global Change Biology, 28, 3310-3320.
DOI PMID |
| [14] | de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009). A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant, Cell & Environment, 32, 704-712. |
| [15] | Deng M, Hu S, Guo L, Jiang L, Huang Y, Schmid B, Liu C, Chang P, Li S, Liu X, Ma K, Liu L (2023). Tree mycorrhizal association types control biodiversity- productivity relationship in a subtropical forest. Science Advances, 9, eadd4468. DOI: 10.1126/sciadv.add4468. |
| [16] |
Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT (2015). Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytologist, 208, 114-124.
DOI PMID |
| [17] | Farley RA, Fitter AH (1999). The responses of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. Journal of Ecology, 87, 849-859. |
| [18] | Fitter AH (1991). Costs and benefits of mycorrhizas: implications for functioning under natural conditions. Experientia, 47, 350-355. |
| [19] | Freschet GT, Roumet C (2017). Sampling roots to capture plant and soil functions. Functional Ecology, 31, 1506-1518. |
| [20] | Freschet GT, Roumet C, Comas LH, Weemstra M, Rewald B, Bardgett RD, de Deyn GB, Johnson D, Klimešová J, Lukac M, Meier IC, Pagès L, Poorter H, Prieto I, Wurzburger N, et al. (2021). Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytologist, 232, 1123-1158. |
| [21] | Freschet GT, Violle C, Bourget MY, Scherer-Lorenzen M, Fort F (2018a). Allocation, morphology, physiology, architecture: the multiple facets of plant above- and below-ground responses to resource stress. New Phytologist, 219, 1338-1352. |
| [22] | Freschet GT, Violle C, Bourget MY, Scherer-Lorenzen M, Fort F (2018b). Allocation, morphology, physiology, architecture: the multiple facets of plant above- and below-ground responses to resource stress. New Phytologist, 219, 1338-1352. |
| [23] | Grime JP (1979). Plant Strategies and Vegetation Processes. John Wiley and Sons, Chichester, UK, |
| [24] |
Grime JP, Crick JC, Rincon JE (1986). The ecological significance of plasticity. Symposia of the Society for Experimental Biology, 40, 5-29.
PMID |
| [25] |
Gross KL, Peters A, Pregitzer KS (1993). Fine root growth and demographic responses to nutrient patches in four old-field plant species. Oecologia, 95, 61-64.
DOI PMID |
| [26] |
Han WX, Fang JY, Guo DL, Zhang Y (2005). Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytologist, 168, 377-385.
DOI PMID |
| [27] | Hodge A, Storer K (2015). Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant and Soil, 386, 1-19. |
| [28] | Honvault N, Houben D, Nobile C, Firmin S, Lambers H, Faucon MP (2021). Tradeoffs among phosphorus- acquisition root traits of crop species for agroecological intensification. Plant and Soil, 461, 137-150. |
| [29] | Hutchings MJ, Wijesinghe DK (2008). Performance of a clonal species in patchy environments: effects of environmental context on yield at local and whole-plant scales. Evolutionary Ecology, 22, 313-324. |
| [30] | Jackson RB, Caldwell MM (1993). The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology, 74, 612-614. |
| [31] | Jackson RB, Caldwell MM (1996). Integrating resource heterogeneity and plant plasticity: modelling nitrate and phosphate uptake in a patchy soil environment. Journal of Ecology, 84, 891-894. |
| [32] |
Johnson NC (2010). Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist, 185, 631-647.
DOI PMID |
| [33] | Kitayama K (2013). The activities of soil and root acid phosphatase in the nine tropical rain forests that differ in phosphorus availability on Mount Kinabalu, Borneo. Plant and Soil, 367, 215-224. |
| [34] |
Koch AM, Antunes PM, Maherali H, Hart MM, Klironomos JN (2017). Evolutionary asymmetry in the arbuscular mycorrhizal symbiosis: conservatism in fungal morphology does not predict host plant growth. New Phytologist, 214, 1330-1337.
DOI PMID |
| [35] | Kong DL, Wang JJ, Kardol P, Wu HF, Zeng H, Deng XB, Deng Y (2016). Economic strategies of plant absorptive roots vary with root diameter. Biogeosciences, 13, 415-424. |
| [36] | Lambers H (2022). Phosphorus acquisition and utilization in plants. Annual Review of Plant Biology, 73, 17-42. |
| [37] | Laughlin DC (2014). The intrinsic dimensionality of plant traits and its relevance to community assembly. Journal of Ecology, 102, 186-193. |
| [38] | Li HB, Ma QH, Li HG, Zhang FS, Rengel Z, Shen JB (2014). Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant and Soil, 376, 151-163. |
| [39] |
Liu BT, Li HB, Zhu B, Koide RT, Eissenstat DM, Guo DL (2015). Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytologist, 208, 125-136.
DOI PMID |
| [40] | Liu BT, Li L, Rengel Z, Tian J, Li HB, Lu MZ (2019a). Roots and arbuscular mycorrhizal fungi are independent in nutrient foraging across subtropical tree species. Plant and Soil, 442, 97-112. |
| [41] | Liu L, Alpert P, Dong BC, Yu FH (2019b). Modification by earthworms of effects of soil heterogeneity and root foraging in eight species of grass. Science of the Total Environment, 708, 134941. DOI: 10.1016/j.scitotenv.2019.134941. |
| [42] | Ma ZQ, Guo DL, Xu XL, Lu MZ, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO (2018). Erratum: Evolutionary history resolves global organization of root functional traits. Nature, 556, 135. DOI: 10.1038/nature26163. |
| [43] |
Millar NS, Bennett AE (2016). Stressed out symbiotes: hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia, 182, 625-641.
DOI PMID |
| [44] |
Miller RM, Jastrow JD, Reinhardt DR (1995). External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia, 103, 17-23.
DOI PMID |
| [45] | Qian YQ, Luo D, Gong G, Han L, Ju GS, Sun ZY (2014). Effects of spatial scale of soil heterogeneity on the growth of a clonal plant producing both spreading and clumping ramets. Journal of Plant Growth Regulation, 33, 214-221. |
| [46] |
Richardson AE, Simpson RJ (2011). Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiology, 156, 989-996.
DOI PMID |
| [47] | Rillig MC, Field CB, Allen MF (1999). Soil biota responses to long-term atmospheric CO2 enrichment in two California annual grasslands. Oecologia, 119, 572-577. |
| [48] |
Shipley B, de Bello F, Cornelissen JHC, Laliberté E, Laughlin DC, Reich PB (2016). Reinforcing loose foundation stones in trait-based plant ecology. Oecologia, 180, 923-931.
DOI PMID |
| [49] | Smith SE, Read DJ (2008). Mycorrhizal Symbiosis. Academic Press, Cambridge, UK. |
| [50] |
Smithwick EAH, Eissenstat DM, Lovett GM, Bowden RD, Rustad LE, Driscoll CT (2013). Root stress and nitrogen deposition: consequences and research priorities. New Phytologist, 197, 712-719.
PMID |
| [51] | Souza LFT, Billings SA (2022). Temperature and pH mediate stoichiometric constraints of organically derived soil nutrients. Global Change Biology, 28, 1630-1642. |
| [52] |
Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH (2003). Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science, 300, 1138-1140.
DOI PMID |
| [53] | Stiblíková P, Klimeš A, Cahill JF, Koubek T, Weiser M (2023). Interspecific differences in root foraging precision cannot be directly inferred from species’ mycorrhizal status or fine root economics. Oikos, 1, e08995. DOI: 10.1111/oik.08995. |
| [54] | Sui YY, Du F, Zhang XC (2011). Spatial heterogeneity of available soil nutrients in abandoned ole-field communities in the Loess Hilly Region. Acta Prataculturae Sinica, 20(2), 76-84. |
| [隋媛媛, 杜峰, 张兴昌 (2011). 黄土丘陵区撂荒群落土壤速效养分空间变异性研究. 草业学报, 20(2), 76-84.] | |
| [55] | Tennant D (1975). A test of a modified line intersect method of estimating root length. Journal of Ecology, 63, 995-1001. |
| [56] | Tibbett M (2000). Roots, foraging and the exploitation of soil nutrient patches: the role of mycorrhizal symbiosis. Functional Ecology, 14, 397-399. |
| [57] | Tinker PB, Nye PH (2000). Solute Movement in the Rhizosphere. Oxford University Press, New York. |
| [58] | Toju H, Sato H (2018). Root-associated fungi shared between arbuscular mycorrhizal and ectomycorrhizal conifers in a temperate forest. Frontiers in Microbiology, 9, 433. DOI: 10.3389/fmicb.2018.00433. |
| [59] | Toju H, Yamamoto S, Tanabe AS, Hayakawa T, Ishii HS (2016). Network modules and hubs in plant-root fungal biomes. Journal of the Royal Society Interface, 13, 20151097. DOI: 10.1098/rsif.2015.1097. |
| [60] |
Valverde-Barrantes OJ, Freschet GT, Roumet C, Blackwood CB (2017). A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytologist, 215, 1562-1573.
DOI PMID |
| [61] |
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist, 205, 1406-1423.
DOI PMID |
| [62] | Wang P, Diao F, Yin L, Huo C (2016). Absorptive roots trait plasticity explains the variation of root foraging strategies in Cunninghamia lanceolata. Environmental and Experimental Botany, 129, 127-135. |
| [63] |
Wang P, Shu M, Mou P, Weiner J (2018). Fine root responses to temporal nutrient heterogeneity and competition in seedlings of two tree species with different rooting strategies. Ecology and Evolution, 8, 3367-3375.
DOI PMID |
| [64] |
Wen ZH, Li HB, Shen XM, Tang XM, Xiong CY, Li HG, Pang JY, Ryan MH, Lambers H, Shen JB (2019). Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytologist, 223, 882-895.
DOI PMID |
| [65] | Yan XL, Wang C, Ma XQ, Wu PF (2019). Root morphology and seedling growth of three tree species in Southern China in response to homogeneous and heterogeneous phosphorus supplies. Trees, 33, 1283-1297. |
| [66] | Zhu L, Yao X, Chen W, Robinson D, Wang X, Chen T, Jiang Q, Jia L, Fan A, Wu D, Chen G (2023). Plastic responses of below-ground foraging traits to soil phosphorus-rich patches across 17 coexisting AM tree species in a subtropical forest. Journal of Ecology, 111, 830-844. |
| [1] | 张鑫, 邢亚娟, 闫国永, 王庆贵. 细根对降水变化响应的meta分析[J]. 植物生态学报, 2018, 42(2): 164-172. |
| [2] | 周玮, 李洪波, 曾辉. 西藏高寒草原群落植物根系属性在降水梯度下的变异格局[J]. 植物生态学报, 2018, 42(11): 1094-1102. |
| [3] | 陈冠陶, 彭勇, 郑军, 李顺, 彭天驰, 邱细容, 涂利华. 氮添加对亚热带次生常绿阔叶林扁刺栲细根生物量、寿命和形态的短期影响[J]. 植物生态学报, 2017, 41(10): 1041-1050. |
| [4] | 朱婉芮, 汪其同, 刘梦玲, 王华田, 王延平, 张光灿, 李传荣. 酚酸和氮素交互作用下欧美杨107细根形态特征[J]. 植物生态学报, 2015, 39(12): 1198-1208. |
| [5] | 王强,王茜,董梅,王晓娟,张亮,金樑. 分室培养装置在丛枝菌根真菌研究中的应用及其发展[J]. 植物生态学报, 2014, 38(11): 1250-1260. |
| [6] | 刘佳, 项文化, 徐晓, 陈瑞, 田大伦, 彭长辉, 方晰. 湖南会同5个亚热带树种的细根构型及功能特征分析[J]. 植物生态学报, 2010, 34(8): 938-945. |
| [7] | 周鹏, 耿燕, 马文红, 贺金生. 温带草地主要优势植物不同器官间功能性状的关联[J]. 植物生态学报, 2010, 34(1): 7-16. |
| [8] | 徐冰, 程雨曦, 甘慧洁, 周文嘉, 贺金生. 内蒙古锡林河流域典型草原植物叶片与细根性状在种间及种内水平上的关联[J]. 植物生态学报, 2010, 34(1): 29-38. |
| [9] | 郑玲, 吴小芹. 黑松菌根共生体中真菌液泡形态构架及其活力[J]. 植物生态学报, 2008, 32(4): 932-937. |
| [10] | 刘建福, 杨道茂, 欧阳明安, 王丽娜, 张勇, 曾明. 澳洲坚果根系溶提物对AM真菌孢子萌发和菌丝生长的影响[J]. 植物生态学报, 2005, 29(6): 1038-1042. |
| [11] | 程云环, 韩有志, 王庆成, 王政权. 落叶松人工林细根动态与土壤资源有效性关系研究[J]. 植物生态学报, 2005, 29(3): 403-410. |
| [12] | 李芳, 徐冰, 冯固, 潘家荣, 李晓林. 菌丝桥在日本落叶松幼苗间磷传递和植株生长中的作用[J]. 植物生态学报, 2004, 28(2): 218-224. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2026 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()