

Chin J Plant Ecol ›› 2019, Vol. 43 ›› Issue (12): 1036-1047.DOI: 10.17521/cjpe.2019.0104
Special Issue: 微生物生态学
• Reviews • Previous Articles Next Articles
WANG Zhao-Guo,WANG Chuan-Kuan( )
)
Received:2019-05-09
Accepted:2019-11-08
Online:2019-12-20
Published:2020-02-24
Contact:
WANG Chuan-Kuan ORCID:0000-0003-3513-5426
Supported by:WANG Zhao-Guo, WANG Chuan-Kuan. Mechanisms of carbon source-sink limitations to tree growth[J]. Chin J Plant Ecol, 2019, 43(12): 1036-1047.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2019.0104
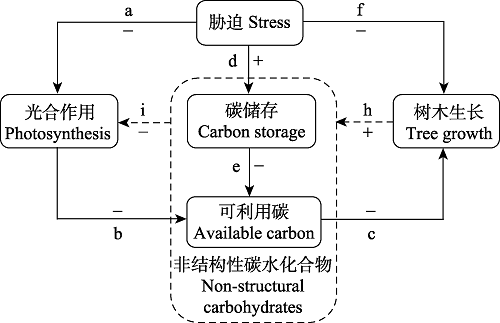
Fig. 1 A conceptual framework of the mechanisms of carbon source-sink limitations to tree growth. From a-b-c pathway, carbon assimilation is reduced by biotic and abiotic stresses (such as defoliation, drought and low temperature), hence tree growth is limited by available carbon (i.e. carbon source limitation). From d-e-c pathway, the storage of non-structural carbohydrates (NSC) is an active process, which decreases available carbon for tree growth (carbon source limitation). From f-h-i pathway, tree growth is constrained by biotic and abiotic stresses directly, leading to NSC accumulation and thus limitation to photosynthesis (i.e. carbon sink limitation). Solid lines represent direct effects, and dotted lines represent feedbacks. + and - represent positive and negative effects, respectively.
| [1] |
Aber J, Neilson RP, McNulty S, Lenihan JM, Bachelet D, Drapek RJ ( 2001). Forest processes and global environmental change: Predicting the effects of individual and multiple stressors. BioScience, 51, 735-751.
DOI URL |
| [2] |
Ågren GI ( 2008). Stoichiometry and nutrition of plant growth in natural communities. Annual Review of Ecology, Evolution, and Systematics, 39, 153-170.
DOI URL |
| [3] |
Ainsworth EA, Rogers A ( 2007). The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant, Cell & Environment, 30, 258-270.
DOI URL PMID |
| [4] |
Alvarez-Uria P, Körner C ( 2007). Low temperature limits of root growth in deciduous and evergreen temperate tree species. Functional Ecology, 21, 211-218.
DOI URL |
| [5] |
Bader MKF, Leuzinger S, Keel SG, Siegwolf RTW, Hagedorn F, Schleppi P, Körner C ( 2013). Central European hardwood trees in a high-CO2 future: Synthesis of an 8-year forest canopy CO2 enrichment project. Journal of Ecology, 101, 1509-1519.
DOI URL |
| [6] |
Bader MKF, Siegwolf R, Körner C ( 2010). Sustained enhancement of photosynthesis in mature deciduous forest trees after 8 years of free air CO2 enrichment. Planta, 232, 1115-1125.
DOI URL PMID |
| [7] |
Barry KM, Pinkard EA ( 2013). Growth and photosynthetic responses following defoliation and bud removal in eucalypts. Forest Ecology and Management, 293, 9-16.
DOI URL |
| [8] |
Bauerle WL, Hinckley TM, Cermak J, Kucera J, Bible K ( 1999). The canopy water relations of old-growth Douglas-fir trees. Trees, 13, 211-217.
DOI URL PMID |
| [9] |
Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Rodenbeck C, Arain MA, Baldocchi D, Bonan GB, Bondeau A, Cescatti A, Lasslop G, Lindroth A, Lomas M, Luyssaert S, Margolis H, Oleson KW, Roupsard O, Veenendaal E, Viovy N, Williams C, Woodward FI, Papale D ( 2010). Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science, 329, 834-838.
DOI URL PMID |
| [10] |
Berdanier AB, Clark JS ( 2016). Multi-year drought-induced morbidity preceding tree death in Southeastern US forests. Ecological Applications, 26, 17-23.
DOI URL PMID |
| [11] |
Bond BJ, Czarnomski NM, Cooper C, Day ME, Greenwood MS ( 2007). Developmental decline in height growth in Douglas-fir. Tree Physiology, 27, 441-453.
DOI URL PMID |
| [12] |
Bréda N, Huc R, Granier A, Dreyer E ( 2006). Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science, 63, 625-644.
DOI URL |
| [13] |
Burnett AC, Rogers A, Rees M, Osborne CP ( 2016). Carbon source-sink limitations differ between two species with contrasting growth strategies. Plant, Cell & Environment, 39, 2460-2472.
DOI URL PMID |
| [14] |
Cavieres LA, Rada F, Azócar A, García-Núñez C, Cabrera HM ( 2000). Gas exchange and low temperature resistance in two tropical high mountain tree species from the Venezuelan Andes. Acta Oecologica, 21, 203-211.
DOI URL PMID |
| [15] |
Chapin III FS, Schulze E, Mooney HA ( 1990). The ecology and economics of storage in plants. Annual Review of Ecology and Systematics, 21, 423-447.
DOI URL |
| [16] |
Chapin III FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Díaz S ( 2000). Consequences of changing biodiversity. Nature, 405, 234-242.
DOI URL PMID |
| [17] |
Chaves MM, Flexas J, Pinheiro C ( 2009). Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551-560.
DOI URL PMID |
| [18] |
Collett NG, Neumann FG ( 2002). Effects of simulated chronic defoliation in summer on growth and survival of blue gum (Eucalyptus globulus Labill.) within young plantations in northern Victoria. Australian Forestry, 65, 99-106.
DOI URL |
| [19] |
Dannoura M, Epron D, Desalme D, Massonnet C, Tsuji S, Plain C, Priault P, Gérant D ( 2019). The impact of prolonged drought on phloem anatomy and phloem transport in young beech trees. Tree Physiology, 39, 201-210.
DOI URL PMID |
| [20] |
Dawes MA, Hagedorn F, Handa IT, Streit K, Ekblad A, Rixen C, Körner C, Hättenschwiler S ( 2013). An alpine treeline in a carbon dioxide-rich world: Synthesis of a nine-year free-air carbon dioxide enrichment study. Oecologia, 171, 623-637.
DOI URL PMID |
| [21] |
Dawes MA, Hättenschwiler S, Bebi P, Hagedorn F, Handa IT, Körner C, Rixen C ( 2011). Species-specific tree growth responses to 9 years of CO2 enrichment at the alpine treeline. Journal of Ecology, 99, 383-394.
DOI URL |
| [22] | Deppong DO, Cline MG ( 2000). Do leaves control episodic shoot growth in woodyplants? The Ohio Journal of Science, 100, 19-23. |
| [23] |
Deslauriers A, Huang JG, Balducci L, Beaulieu M, Rossi S ( 2016). The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiology, 170, 2072-2084.
DOI URL PMID |
| [24] |
Dietze MC, Sala AN, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R ( 2014). Nonstructural carbon in woody plants. Annual Review of Plant Biology, 65, 667-687.
DOI URL PMID |
| [25] |
Dolezal J, Kopecky M, Dvorsky M, Macek M, Rehakova K, Capkova K, Borovec J, Schweingruber F, Liancourt P, Altman J ( 2019). Sink limitation of plant growth determines tree line in the arid Himalayas. Functional Ecology, 33, 553-565.
DOI URL |
| [26] |
Duan H, Amthor JS, Duursma RA, O’Grady AP, Choat B, Tissue DT ( 2013). Carbon dynamics of eucalypt seedlings exposed to progressive drought in elevated [CO2] and elevated temperature. Tree Physiology, 33, 779-792.
DOI URL PMID |
| [27] |
Dymond CC, Beukema S, Nitschke CR, Coates KD, Scheller RM ( 2016). Carbon sequestration in managed temperate coniferous forests under climate change. Biogeosciences, 13, 1933-1947.
DOI URL |
| [28] |
Epron D, Cabral OMR, Laclau JP, Dannoura M, Packer AP, Plain C, Battie-Laclau P, Moreira MZ, Trivelin PCO, Bouillet JP, Gérant D, Nouvellon Y ( 2016). In situ 13CO2 pulse labelling of field-grown eucalypt trees revealed the effects of potassium nutrition and throughfall exclusion on phloem transport of photosynthetic carbon. Tree Physiology, 36, 6-21.
DOI URL PMID |
| [29] | Evans CG ( 1972). The Quantitative Analysis of Plant Growth. Blackwell Scientific, Oxford. |
| [30] |
Fajardo A, Piper FI ( 2014). An experimental approach to explain the southern Andes elevational treeline. American Journal of Botany, 101, 788-795.
DOI URL PMID |
| [31] |
Fajardo A, Piper FI ( 2017). An assessment of carbon and nutrient limitations in the formation of the southern Andes tree line. Journal of Ecology, 105, 517-527.
DOI URL |
| [32] |
Farquhar GD, von Caemmerer S, Berry JA ( 1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
DOI URL PMID |
| [33] |
Fatichi S, Leuzinger S, Körner C ( 2014). Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytologist, 201, 1086-1095.
DOI URL PMID |
| [34] |
Fatichi S, Pappas C, Zscheischler J, Leuzinger S ( 2019). Modelling carbon sources and sinks in terrestrial vegetation. New Phytologist, 221, 652-668.
DOI URL PMID |
| [35] |
Feild TS, Brodribb T ( 2001). Stem water transport and freeze-thaw xylem embolism in conifers and angiosperms in a Tasmanian treeline heath. Oecologia, 127, 314-320.
DOI URL PMID |
| [36] |
Friend AD, Eckes-Shephard AH, Fonti P, Rademacher TT, Rathgeber CBK, Richardson AD, Turton RH ( 2019). On the need to consider wood formation processes in global vegetation models and a suggested approach. Annals of Forest Science, 76, 49.
DOI URL |
| [37] |
Galiano L, Martínez-Vilalta J, Lloret F ( 2011). Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytologist, 190, 750-759.
DOI URL PMID |
| [38] |
Galiano L, Timofeeva G, Saurer M, Siegwolf R, Martínez-Vilalta J, Hommel R, Gessler A ( 2017). The fate of recently fixed carbon after drought release: Towards unravelling C storage regulation in Tilia platyphyllos and Pinus sylvestris. Plant, Cell & Environment, 40, 1711-1724.
DOI URL PMID |
| [39] |
Gibon Y, Pyl ET, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M ( 2009). Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant, Cell & Environment, 32, 859-874.
DOI URL PMID |
| [40] |
Greenwood MS, Ward MH, Day ME, Adams SL, Bond BJ ( 2008). Age-related trends in red spruce foliar plasticity in relation to declining productivity. Tree Physiology, 28, 225-232.
DOI URL PMID |
| [41] |
Gričar J, Zavadlav S, Jyske T, Lavrič M, Laakso T, Hafner P, Eler K, Vodnik D ( 2019). Effect of soil water availability on intra-annual xylem and phloem formation and non-structural carbohydrate pools in stem of Quercus pubescens. Tree Physiology, 39, 222-233.
DOI URL PMID |
| [42] |
Hagedorn F, Joseph J, Peter M, Luster J, Pritsch K, Geppert U, Kerner R, Molinier V, Egli S, Schaub M, Liu JF, Li MH, Sever K, Weiler M, Siegwolf RTW, Gessler A, Arend M ( 2016). Recovery of trees from drought depends on belowground sink control. Nature Plants, 2, 1-5.
DOI URL PMID |
| [43] |
Handa IT, Körner C, Hättenschwiler S ( 2005). A test of the treeline carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology, 86, 1288-1300.
DOI URL |
| [44] |
Hararuk O, Campbell EM, Antos JA, Parish R ( 2019). Tree rings provide no evidence of a CO2 fertilization effect in old-growth subalpine forests of western Canada. Global Change Biology, 25, 1222-1234.
DOI URL |
| [45] |
Hartmann H, Adams HD, Hammond WM, Hoch G, Landhäusser SM, Wiley E, Zaehle S ( 2018). Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environmental and Experimental Botany, 152, 7-18.
DOI URL |
| [46] |
Hartmann H, McDowell NG, Trumbore S ( 2015). Allocation to carbon storage pools in Norway spruce saplings under drought and low CO2. Tree Physiology, 35, 243-252.
DOI URL PMID |
| [47] |
Hartmann H, Trumbore S ( 2016). Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytologist, 211, 386-403.
DOI URL PMID |
| [48] |
Hesse BD, Goisser M, Hartmann H, Grams TEE ( 2019). Repeated summer drought delays sugar export from the leaf and impairs phloem transport in mature beech. Tree Physiology, 39, 192-200.
DOI URL PMID |
| [49] |
Hillabrand RM, Hacke UG, Lieffers VJ ( 2019). Defoliation constrains xylem and phloem functionality. Tree Physiology, 39, 1099-1108.
DOI URL PMID |
| [50] |
Hoch G, Körner C ( 2009). Growth and carbon relations of tree line forming conifers at constant vs. variable low temperatures. Journal of Ecology, 97, 57-66.
DOI URL |
| [51] |
Hoch G, Popp M, Körner C ( 2002). Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos, 98, 361-374.
DOI URL |
| [52] |
Hoch G, Richter A, Körner C ( 2003). Non-structural carbon compounds in temperate forest trees. Plant, Cell and Environment, 26, 1067-1081.
DOI URL PMID |
| [53] | Hsiao TC, Acevedo E, Fereres E, Henderson DW ( 1976). Water stress, growth, and osmotic adjustment. Philosophical Transactions of the Royal Society B: Biological Sciences, 273, 479-500. |
| [54] |
Huang JB, Hammerbacher A, Weinhold A, Reichelt M, Gleixner G, Behrendt T, Dam NM, Sala AN, Gershenzon J, Trumbore S, Hartmann H ( 2019). Eyes on the future—Evidence for trade-offs between growth, storage and defense in Norway spruce. New Phytologist, 222, 144-158.
DOI URL PMID |
| [55] |
Huang JG, Guo XL, Rossi S, Zhai LH, Yu BY, Zhang SK, Zhang MF ( 2018). Intra-annual wood formation of subtropical Chinese red pine shows better growth in dry season than wet season. Tree Physiology, 38, 1225-1236.
DOI URL PMID |
| [56] |
Ishii HT, Jennings GM, Sillett SC, Koch GW ( 2008). Hydrostatic constraints on morphological exploitation of light in tall Sequoia sempervirens trees. Oecologia, 156, 751-763.
DOI URL PMID |
| [57] |
Jacquet JS, Bosc A, O’Grady A, Jactel H ( 2014). Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Tree Physiology, 34, 367-376.
DOI URL PMID |
| [58] |
Jensen KH, Rio E, Hansen R, Clanet C, Bohr T ( 2009). Osmotically driven pipe flows and their relation to sugar transport in plants. Journal of Fluid Mechanics, 636, 371-396.
DOI URL |
| [59] |
Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC ( 2012). Hydraulic safety margins and embolism reversal in stems and leaves: Why are conifers and angiosperms so different? Plant Science, 195, 48-53.
DOI URL |
| [60] |
Kiorapostolou N, Petit G ( 2019). Similarities and differences in the balances between leaf, xylem and phloem structures in Fraxinus ornus along an environmental gradient. Tree Physiology, 39, 234-242.
DOI URL PMID |
| [61] |
Kirschbaum MUF ( 2011). Does enhanced photosynthesis enhance growth? lessons learned from CO2 enrichment studies. Plant Physiology, 155, 117-124.
DOI URL PMID |
| [62] |
Klein T, Bader MKF, Leuzinger S, Mildner M, Schleppi P, Siegwolf RTW, Körner C ( 2016). Growth and carbon relations of mature Picea abies trees under 5 years of free-air CO2 enrichment. Journal of Ecology, 104, 1720-1733.
DOI URL |
| [63] |
Körner C ( 2003). Carbon limitation in trees. Journal of Ecology, 91, 4-17.
DOI URL |
| [64] |
Körner C ( 2006). Plant CO2 responses: An issue of definition, time and resource supply. New Phytologist, 172, 393-411.
DOI URL PMID |
| [65] | Körner C ( 2012). Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits. Springer, Berlin. |
| [66] |
Körner C ( 2015). Paradigm shift in plant growth control. Current Opinion in Plant Biology, 25, 107-114.
DOI URL PMID |
| [67] |
Landhäusser SM, Lieffers VJ ( 2012). Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees, 26, 653-661.
DOI URL |
| [68] |
Li MH, Jiang Y, Wang A, Li XB, Zhu WZ, Yan CF, Du Z, Shi Z, Lei JP, Schönbeck L, He P, Yu FH, Wang X ( 2018). Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiology, 38, 1345-1355.
DOI URL PMID |
| [69] |
Li MH, Xiao WF, Wang SG, Cheng GW, Cherubini P, Cai XH, Liu XL, Wang XD, Zhu WZ ( 2008). Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiology, 28, 1287-1296.
DOI URL PMID |
| [70] |
Li MH, Yang J ( 2004). Effects of microsite on growth of Pinus cembra in the subalpine zone of the Austrian Alps. Annals of Forest Science, 61, 319-325.
DOI URL |
| [71] | Litton CM, Giardina CP ( 2008). Below-ground carbon flux and partitioning: Global patterns and response to temperature. Functional Ecology, 22, 941-954. |
| [72] | Litton CM, Raich JW, Ryan MG ( 2007). Carbon allocation in forest ecosystems. Global Change Biology, 13, 2089-2109. |
| [73] | Liu YY, Wang AY, An YN, Lian PY, Wu DD, Zhu JJ, Meinzer FC, Hao GY ( 2018). Hydraulics play an important role in causing low growth rate and dieback of aging Pinus sylvestris var. mongolica trees in plantations of Northeast China. Plant, Cell & Environment, 41, 1500-1511. |
| [74] | Luo YQ, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, Mc Murtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB ( 2004). Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience, 54, 731. |
| [75] | MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ ( 2017). Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. Journal of Experimental Botany, 68, 4433-4453. |
| [76] | Maherali H, Pockman WT, Jackson RB ( 2004). Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology, 85, 2184-2199. |
| [77] | Martínez-Vilalta J, Sala AN, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F ( 2016). Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecological Monographs, 86, 495-516. |
| [78] | McCarthy HR, Oren R, Johnsen KH, Gallet-Budynek A, Pritchard SG, Cook CW, LaDeau SL, Jackson RB, Finzi AC ( 2010). Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: Interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytologist, 185, 514-528. |
| [79] | Meinzer FC, Bond BJ, Karanian JA ( 2008). Biophysical constraints on leaf expansion in a tall conifer. Tree Physiology, 28, 197-206. |
| [80] | Michelot A, Simard S, Rathgeber C, Dufrêne E, Damesin C ( 2012). Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiology, 32, 1033-1045. |
| [81] | Millard P, Sommerkorn M, Grelet GA ( 2007). Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytologist, 175, 11-28. |
| [82] | Minchin PEH (2007). Mechanistic modelling of carbon partitioning. In: Vos J, Marcelis LFM, de Visser PHB, Struik PC, Evers JB eds. Functional-Structural Plant Modelling in Crop Production. Springer, Dordrecht. 113-122. |
| [83] | Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques M, Gibon Y ( 2011). Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany, 62, 1715-1729. |
| [84] | Nardini A, Lo Gullo MA, Salleo S ( 2011). Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Science, 180, 604-611. |
| [85] | Nikinmaa E, Hölttä T, Hari P, Kolari P, Mäkelä A, Sevanto S, Vesala T ( 2013). Assimilate transport in phloem sets conditions for leaf gas exchange. Plant, Cell & Environment, 36, 655-669. |
| [86] | Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE ( 2010). CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proceedings of the National Academy of Sciences of the United States of America, 107, 19368-19373. |
| [87] | Palacio S, Camarero JJ, Maestro M, Alla AQ, Lahoz E, Monserrat-Martí G ( 2018). Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees, 32, 777-790. |
| [88] | Palacio S, Hoch G, Sala AN, Körner C, Millard P ( 2014). Does carbon storage limit tree growth? New Phytologist, 201, 1096-1100. |
| [89] | Paul MJ, Foyer CH ( 2001). Sink regulation of photosynthesis. Journal of Experimental Botany, 52, 1383-1400. |
| [90] | Pinkard EA, Eyles A, O’Grady AP ( 2011). Are gas exchange responses to resource limitation and defoliation linked to source:sink relationships? Plant, Cell & Environment, 34, 1652-1665. |
| [91] | Piper FI, Fajardo A ( 2011). No evidence of carbon limitation with tree age and height in Nothofagus pumilio under Mediterranean and temperate climate conditions. Annals of Botany, 108, 907-917. |
| [92] | Piper FI, Fajardo A ( 2014). Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. Journal of Ecology, 102, 1101-1111. |
| [93] | Piper FI, Fajardo A, Hoch G ( 2017). Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiology, 37, 1001-1010. |
| [94] | Power SA ( 1994). Temporal trends in twig growth of Fagus sylvatica L. and their relationships with environmental factors. Forestry, 67, 13-30. |
| [95] | Poyatos R, Aguadé D, Galiano L, Mencuccini M, Martínez-Vilalta J ( 2013). Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytologist, 200, 388-401. |
| [96] | Puri E, Hoch G, Körner C ( 2015). Defoliation reduces growth but not carbon reserves in Mediterranean Pinus pinaster trees. Trees, 29, 1187-1196. |
| [97] | Quentin AG, O’Grady AP, Beadle CL, Mohammed C, Pinkard EA ( 2012). Interactive effects of water supply and defoliation on photosynthesis, plant water status and growth of Eucalyptus globulus Labill. Tree Physiology, 32, 958-967. |
| [98] | Rosati A, Paoletti A, Al Hariri R, Morelli A, Famiani F ( 2018). Resource investments in reproductive growth proportionately limit investments in whole-tree vegetative growth in young olive trees with varying crop loads. Tree Physiology, 38, 1267-1277. |
| [99] | Rossi S, Deslauriers A, Anfodillo T, Carraro V ( 2007). Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia, 152, 1-12. |
| [100] | Rossi S, Deslauriers A, Gričar J, Seo JW, Rathgeber CB, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R ( 2008). Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography, 17, 696-707. |
| [101] | Ryan MG, Oren R, Waring RH ( 2018). Fruiting and sink competition. Tree Physiology, 38, 1261-1266. |
| [102] | Ryan MG, Phillips N, Bond BJ ( 2006). The hydraulic limitation hypothesis revisited. Plant, Cell & Environment, 29, 367-381. |
| [103] | Ryan MG, Yoder BJ ( 1997). Hydraulic limits to tree height and tree growth. BioScience, 47, 235-242. |
| [104] | Sala AN, Hoch G ( 2009). Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant, Cell & Environment, 32, 22-30. |
| [105] | Sala AN, Piper F, Hoch G ( 2010). Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist, 186, 274-281. |
| [106] | Sala AN, Woodruff DR, Meinzer FC ( 2012). Carbon dynamics in trees: Feast or famine? Tree Physiology, 32, 764-775. |
| [107] | Salmon Y, Dietrich L, Sevanto S, Hölttä T, Dannoura M, Epron D ( 2019). Drought impacts on tree phloem: From cell-level responses to ecological significance. Tree Physiology, 39, 173-191. |
| [108] | Schmid S, Palacio S, Hoch G ( 2017). Growth reduction after defoliation is independent of CO2 supply in deciduous and evergreen young oaks. New Phytologist, 214, 1479-1490. |
| [109] | Sevanto S ( 2014). Phloem transport and drought. Journal of Experimental Botany, 65, 1751-1759. |
| [110] | Sigurdsson BD, Medhurst JL, Wallin G, Eggertsson O, Linder S ( 2013). Growth of mature boreal Norway spruce was not affected by elevated [CO2] and/or air temperature unless nutrient availability was improved. Tree Physiology, 33, 1192-1205. |
| [111] | Simard S, Giovannelli A, Treydte K, Traversi ML, King GM, Frank D, Fonti P ( 2013). Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiology, 33, 913-923. |
| [112] | Smith AM, Stitt M ( 2007). Coordination of carbon supply and plant growth. Plant, Cell & Environment, 30, 1126-1149. |
| [113] | Steppe K, Sterck F, Deslauriers A ( 2015). Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends in Plant Science, 20, 335-343. |
| [114] | Stevens GC, Fox JF ( 1991). The causes of treeline. Annual Review of Ecology and Systematics, 22, 177-191. |
| [115] | Stribley GH, Ashmore MR ( 2002). Quantitative changes in twig growth pattern of young woodland beech (Fagus sylvatica L.) in relation to climate and ozone pollution over 10 years. Forest Ecology and Management, 157, 191-204. |
| [116] | Susiluoto S, Hilasvuori E, Berninger F ( 2010). Testing the growth limitation hypothesis for subarctic Scots pine. Journal of Ecology, 98, 1186-1195. |
| [117] | Tardieu F, Granier C, Muller B ( 2011). Water deficit and growth. Co-ordinating processes without an orchestrator? Current Opinion in Plant Biology, 14, 283-289. |
| [118] | Trugman AT, Detto M, Bartlett MK, Medvigy D, Anderegg WRL, Schwalm C, Schaffer B, Pacala SW ( 2018). Tree carbon allocation explains forest drought-kill and recovery patterns. Ecology Letters, 21, 1552-1560. |
| [119] | van der Sleen P, Groenendijk P, Vlam M, Anten NPR, Boom A, Bongers F, Pons TL, Terburg G, Zuidema PA ( 2015). No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nature Geoscience, 8, 24-28. |
| [120] | von Arx G, Arzac A, Fonti P, Frank D, Zweifel R, Rigling A, Galiano L, Gessler A, Olano JM ( 2017). Responses of sapwood ray parenchyma and non-structural carbohydrates of Pinus sylvestris to drought and long-term irrigation. Functional Ecology, 31, 1371-1382. |
| [121] | Wang H, Prentice IC, Davis TW, Keenan TF, Wright IJ, Peng CH ( 2017). Photosynthetic responses to altitude: An explanation based on optimality principles. New Phytologist, 213, 976-982. |
| [122] | Wardlaw IF ( 1990). The control of carbon partitioning in plants. New Phytologist, 116, 341-381. |
| [123] | Weber R, Gessler A, Hoch G ( 2019). High carbon storage in carbon-limited trees. New Phytologist, 222, 171-182. |
| [124] | White AC, Rogers A, Rees M, Osborne CP ( 2016). How can we make plants grow faster? A source-sink perspective on growth rate. Journal of Experimental Botany, 67, 31-45. |
| [125] | Wiley E, Casper BB, Helliker BR ( 2017). Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. Journal of Ecology, 105, 412-424. |
| [126] | Wiley E, Helliker B ( 2012). A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist, 195, 285-289. |
| [127] | Yan JH, Zhang YP, Yu GR, Zhou GY, Zhang LM, Li K, Tan ZH, Sha LQ ( 2013). Seasonal and inter-annual variations in net ecosystem exchange of two old-growth forests in southern China. Agricultural and Forest Meteorology, 182- 183, 257-265. |
| [128] | Zimmermann MH, Brown CL ( 1974). Trees, Structure and Function. Springer, New York. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn