

Chin J Plant Ecol ›› 2023, Vol. 47 ›› Issue (10): 1441-1452.DOI: 10.17521/cjpe.2022.0227
• Research Articles • Previous Articles Next Articles
SHI Sheng-Bo1,4,*( ), SHI Rui2, ZHOU Dang-Wei1,3, ZHANG Wen4
), SHI Rui2, ZHOU Dang-Wei1,3, ZHANG Wen4
Received:2022-06-06
Accepted:2022-10-10
Online:2023-10-20
Published:2023-11-23
Supported by:SHI Sheng-Bo, SHI Rui, ZHOU Dang-Wei, ZHANG Wen. Effects of low temperature on photochemical and non-photochemical energy dissipation of Kobresia pygmaea leaves[J]. Chin J Plant Ecol, 2023, 47(10): 1441-1452.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2022.0227

Fig. 1 Kobresia pygmaea meadow and plant configuration. A, Natural landscape of K. pygmaea-forb meadow. B, Cushion aggregation growth pattern of K. pygmaea. C, Developed root system is mainly distributed within a depth of 10 cm, forming a dense layer with the soil. D, Plants with stubby rhizomes and dense brown persistent leaf sheaths.
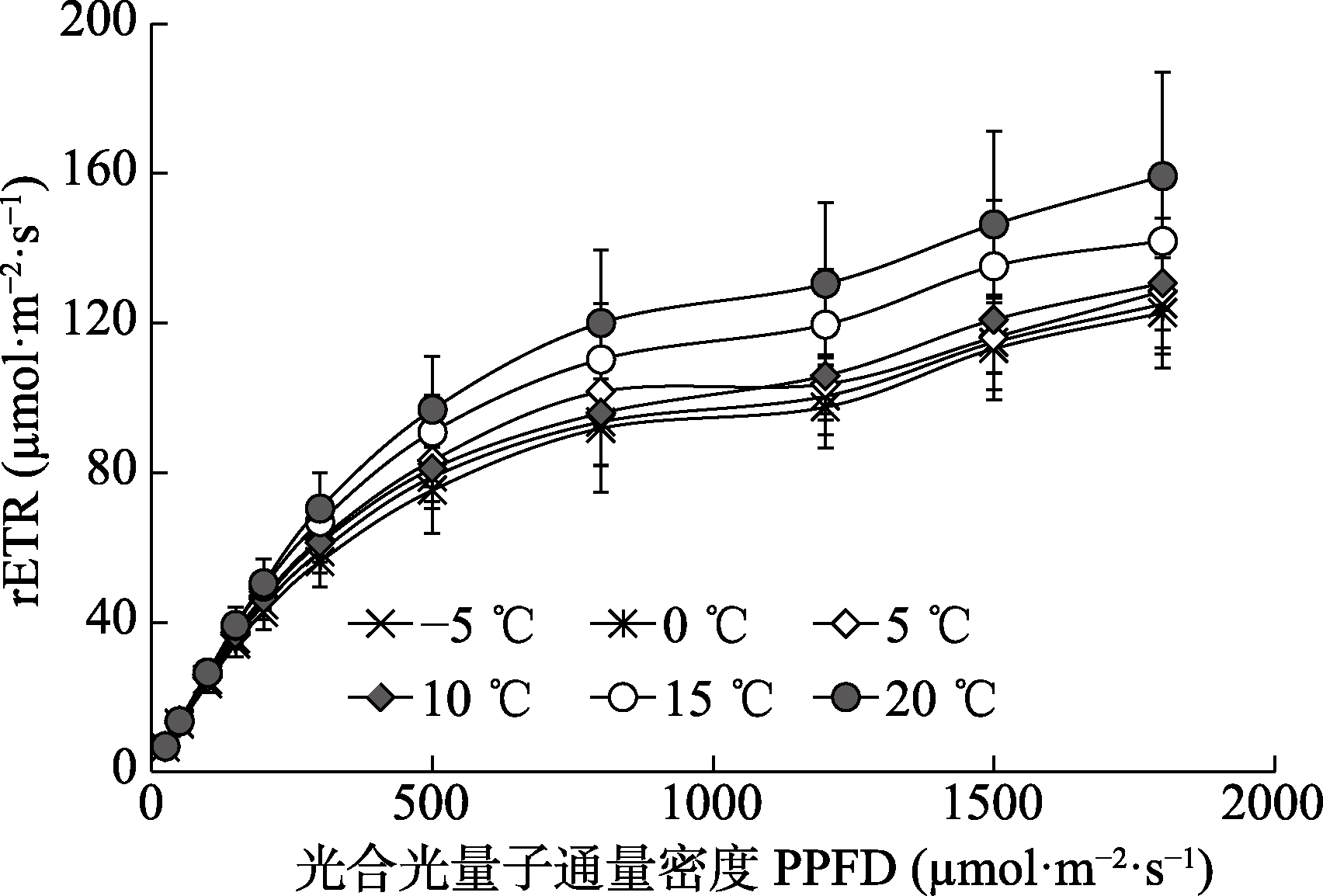
Fig. 2 Effects of measurement temperature on rapid light- response curves of the relative electron transfer rate through photosystem II (rETR) of Kobresia pygmaea leaves (mean ± SD, n = 60). PPFD, photosynthetical active photon flux density.
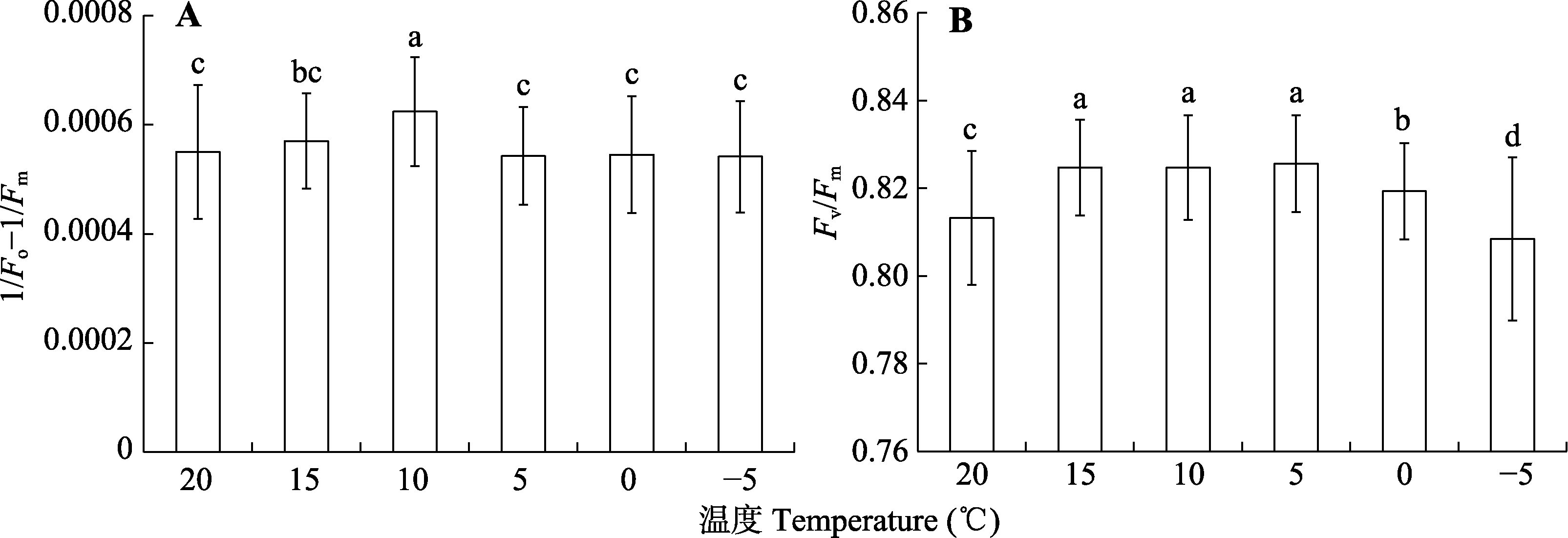
Fig. 3 Response of the maximum quantum efficiency of photosystem II photochemistry (Fv/Fm and 1/Fo - 1/Fm) of Kobresia pygmaea leaves to temperature. Different lowercase letters indicate significant differences of Fv/Fm and 1/Fo - 1/Fm among different measurement temperature degree, respectively (α = 0.05; mean ± SD, n = 60).
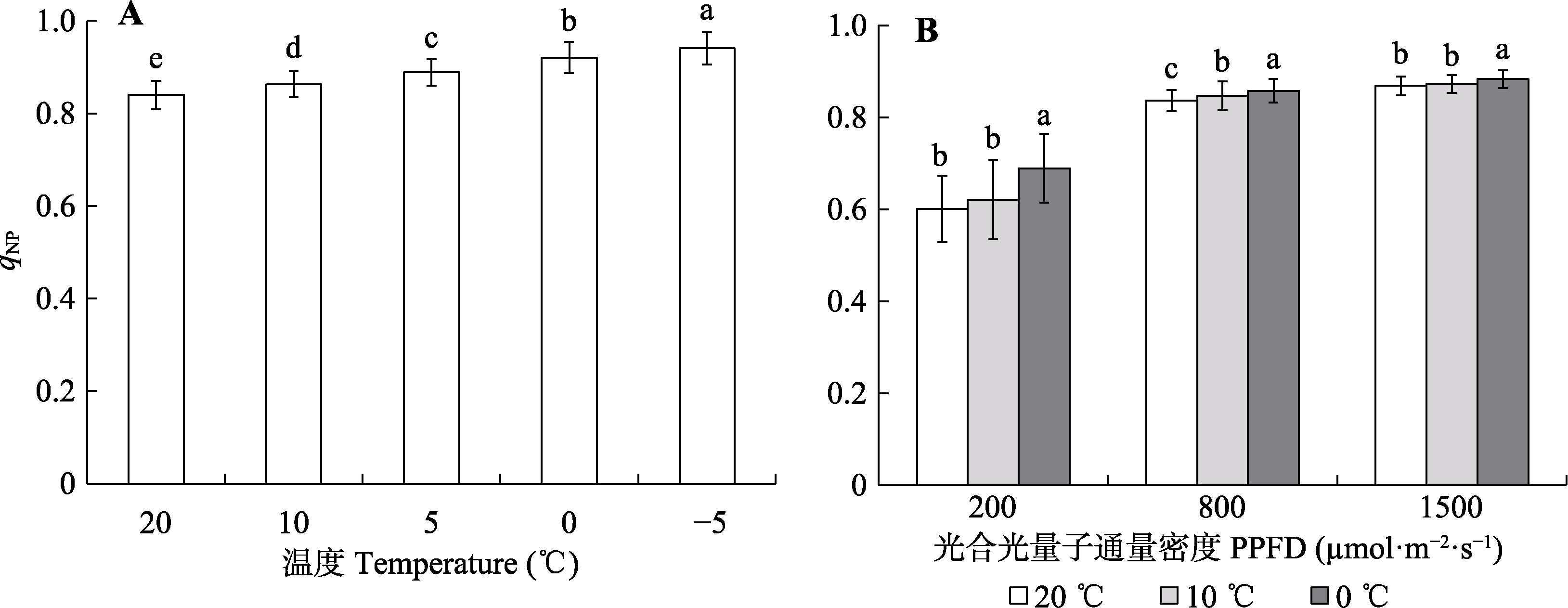
Fig. 4 Response of the photosystem II non-photochemical quenching coefficient (qNP) in Kobresia pygmaea leaves to temperature and its variation to steady-state light intensities. Different lowercase letters in A indicate significant differences among different measurement temperature under 1 000 μmol·m-2·s-1 steady-state light intensity (α = 0.05; mean ± SD, n = 55); different lowercase letters in B indicate significant differences among different temperature under the same steady-state light intensity (α = 0.05; mean ± SD, n = 80). PPFD, photosynthetical active photon flux density.
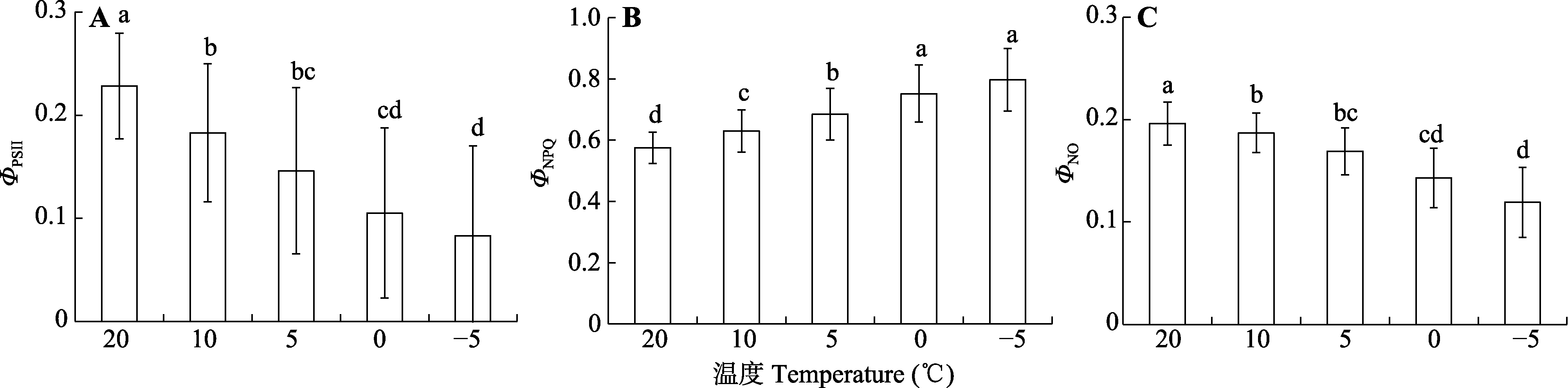
Fig. 5 Effects of temperature on the photosystem II actual photochemical efficiency (ΦPSII) (A), the quantum yield of regulated energy dissipation (ΦNPQ) (B) and non-regulated energy dissipation (ΦNO) (C) in Kobresia pygmaea leaves. Different lowercase letters indicate significant differences among different measurement temperature under 1 000 μmol·m-2·s-1 steady-state light intensity (α = 0.05; mean ± SD, n = 55).
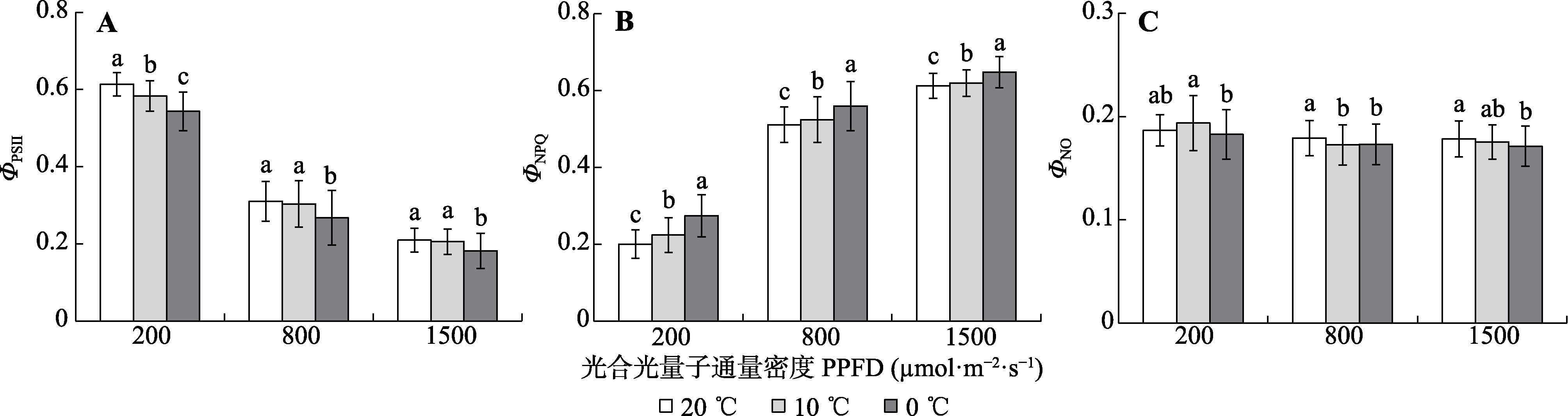
Fig. 6 Photosystem II actual photochemical efficiency (ΦPSII) (A), the quantum yield of regulated energy dissipation (ΦNPQ) (B), and non-regulated energy dissipation (ΦNO) (C) in Kobresia pygmaea leaves under different temperature degrees. Different lowercase letters indicate significant differences among measurement temperature degrees at the same light intensity (α = 0.05; mean ± SD, n = 80). PPFD, photosynthetical active photon flux density.
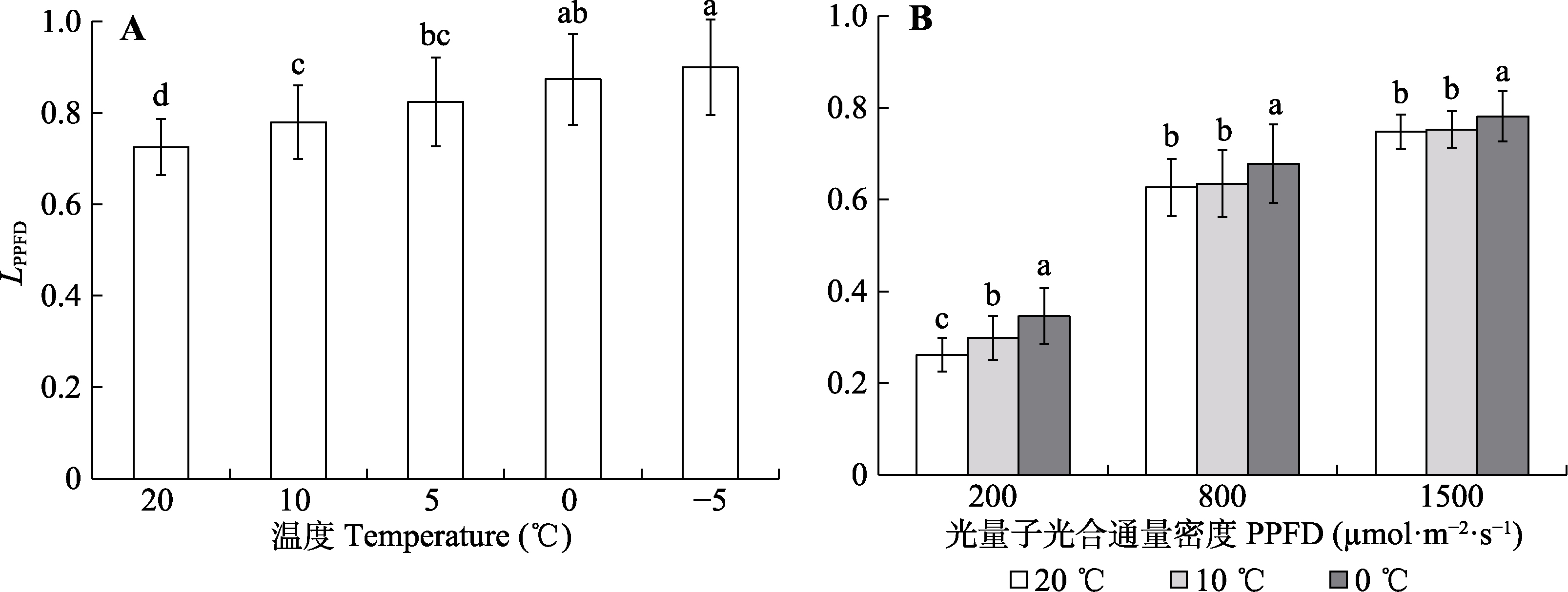
Fig. 7 Response of the relative limitation of photosystem II photochemical efficiency (LPPFD) in Kobresia pygmaea leaves to measurement temperature and their variation with steady-state light intensities. Different lowercase letters in A indicate significant differences among different temperature under 1 000 μmol·m-2·s-1 steady-state light intensity (mean ± SD, n = 55); different lowercase letters in B indicate significant differences among different measurement temperature under the same steady-state light intensity (α = 0.05; mean ± SD, n = 80). PPFD, photosynthetical active photon flux density.
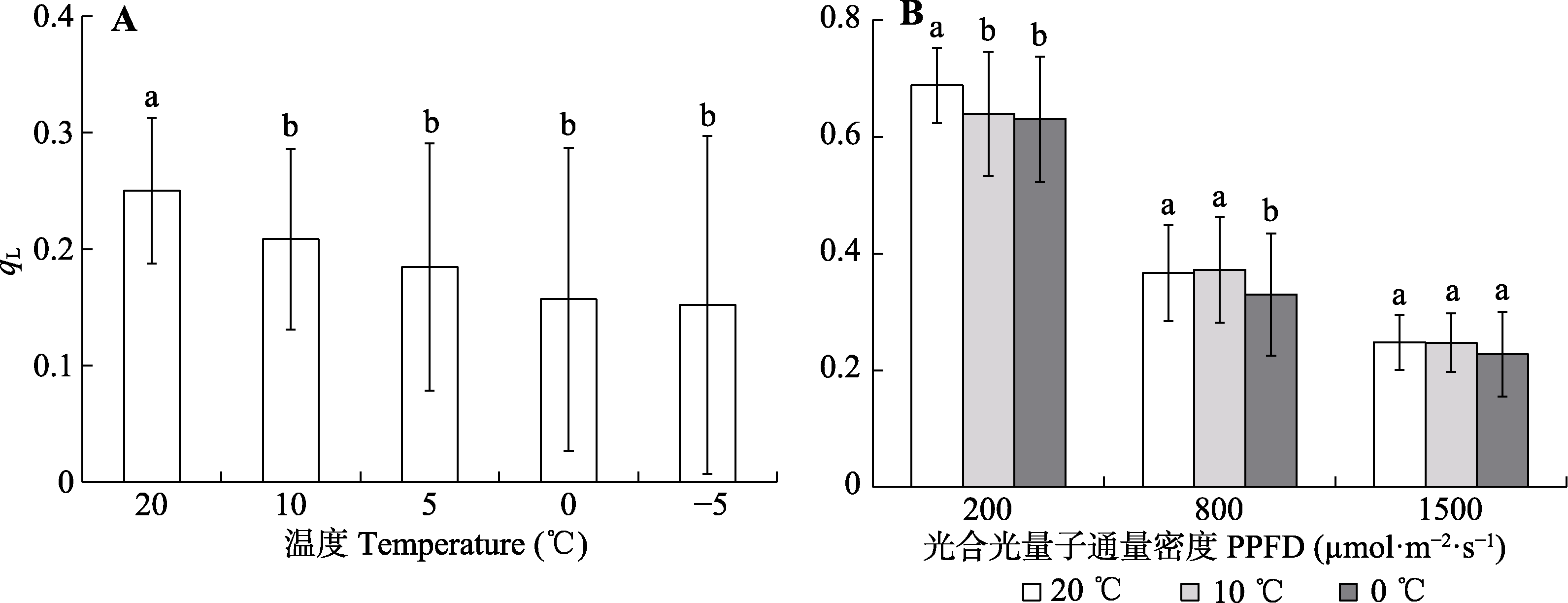
Fig. 8 Response of the fraction of open photosystem II centers (qL) in Kobresia pygmaea leaves to measurement temperature and their variation to steady-state light intensities. Different lowercase letters in A indicate significant differences among different measurement temperature under 1 000 μmol·m-2·s-1 steady-state light intensity (α = 0.05; mean ± SD, n = 55); different lowercase letters in B indicate significant differences among different measurement temperature under the same steady-state light intensity (α = 0.05; mean ± SD, n = 80). PPFD, photosynthetical active photon flux density.
| PPFD | 低温 Low temperature | PPFD ×低温 PPFD × Low temperature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | η2 | F | p | η2 | F | p | η2 | |
| qL | 264.712 | 0 | 0.358 | 7.927 | 0 | 0.032 | 0.927 | 0.397 | 0.004 |
| qNP | 163.739 | 0 | 0.257 | 23.102 | 0 | 0.089 | 0.848 | 0.429 | 0.004 |
| ΦPSII | 417.730 | 0 | 0.468 | 22.572 | 0 | 0.087 | 0.948 | 0.388 | 0.004 |
| ΦNO | 0 | 0.991 | 0 | 5.569 | 0.004 | 0.023 | 0.707 | 0.493 | 0.003 |
| ΦNPQ | 476.751 | 0 | 0.501 | 33.935 | 0 | 0.125 | 0.788 | 0.455 | 0.003 |
| rETR | 174.423 | 0 | 0.269 | 24.480 | 0 | 0.094 | 0.276 | 0.759 | 0.001 |
| LPPFD | 417.730 | 0 | 0.468 | 22.572 | 0 | 0.087 | 0.948 | 0.388 | 0.004 |
Table 1 Interaction effects analysis of high light intensity and low temperature on chlorophyll fluorescence parameters of kobresia pygmaea
| PPFD | 低温 Low temperature | PPFD ×低温 PPFD × Low temperature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | η2 | F | p | η2 | F | p | η2 | |
| qL | 264.712 | 0 | 0.358 | 7.927 | 0 | 0.032 | 0.927 | 0.397 | 0.004 |
| qNP | 163.739 | 0 | 0.257 | 23.102 | 0 | 0.089 | 0.848 | 0.429 | 0.004 |
| ΦPSII | 417.730 | 0 | 0.468 | 22.572 | 0 | 0.087 | 0.948 | 0.388 | 0.004 |
| ΦNO | 0 | 0.991 | 0 | 5.569 | 0.004 | 0.023 | 0.707 | 0.493 | 0.003 |
| ΦNPQ | 476.751 | 0 | 0.501 | 33.935 | 0 | 0.125 | 0.788 | 0.455 | 0.003 |
| rETR | 174.423 | 0 | 0.269 | 24.480 | 0 | 0.094 | 0.276 | 0.759 | 0.001 |
| LPPFD | 417.730 | 0 | 0.468 | 22.572 | 0 | 0.087 | 0.948 | 0.388 | 0.004 |
| [1] |
Baker NR (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89-113.
DOI PMID |
| [2] |
Baker NR, Rosenqvist E (2004). Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. Journal of Experimental Botany, 55, 1607-1621.
DOI PMID |
| [3] |
Bilger W, Björkman O (1990). Role of the xanthophyll cycle in photoprotection elucidated by measurements of light- induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research, 25, 173-185.
DOI PMID |
| [4] |
Costa-Broseta Á, Perea-Resa C, Castillo MC, Ruíz MF, Salinas J, León J (2019). Nitric oxide deficiency decreases C-repeat binding factor-dependent and -independent induction of cold acclimation. Journal of Experimental Botany, 70, 3283-3296.
DOI PMID |
| [5] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [6] |
Govindjee (2002). A role for a light-harvesting antenna complex of photosystem II in photoprotection. The Plant Cell, 14, 1663-1668.
DOI URL |
| [7] |
Guarini JM, Morita C (2009). Modelling the dynamics of the electron transport rate measured by PAM fluorimetry during rapid light curve experiments. Photosynthetica, 47, 206-214.
DOI URL |
| [8] |
Hendrickson L, Furbank RT, Chow WS (2004). A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynthesis Research, 82, 73-81.
DOI PMID |
| [9] | Ke YY, Chen X, Ni QQ, Zhang LL, Liu LZ, Xu H, Wei FZ, Li JC (2021). Research progress of the metabolism of reactive oxygen species and its regulation mechanisms in wheat under low temperature stress. Barley and Cereal Sciences, 38(1), 1-6. |
| [柯媛媛, 陈翔, 倪芊芊, 张乐乐, 刘绿洲, 许辉, 魏凤珍, 李金才 (2021). 低温逆境胁迫下小麦ROS代谢及调控研究进展. 大麦与谷类科学, 38(1), 1-6.] | |
| [10] |
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research, 79, 209-218.
DOI PMID |
| [11] | Larcher W (1980). Physiological Plant Ecology. 2nd ed. Spring-Verlag, New York. 5-60. |
| [12] | Li XJ, Cui HJ (2018). Research progress on the physiological response of plants to environmental stress. Shandong Forestry Science and Technology, 48(6), 90-94. |
| [李晓靖, 崔海军 (2018). 低温胁迫下植物光合生理研究进展. 山东林业科技, 48(6), 90-94.] | |
| [13] | Li YK, Lin L, Zhang FW, Liang DY, Wang X, Cao GM (2010). Kobresia pygmaea community—Disclimax of alpine meadow zonal vegetation in the pressure of grazing. Journal of Mountain Science, 28, 257-265. |
| [李以康, 林丽, 张法伟, 梁东营, 王溪, 曹广民 (2010). 小嵩草群落——高寒草甸地带性植被放牧压力下的偏途顶极群落. 山地学报, 28, 257-265.] | |
| [14] |
Lima Neto MC, Lobo AKM, Martins MO, Fontenele AV, Silveira JAG (2014). Dissipation of excess photosynthetic energy contributes to salinity tolerance: a comparative study of salt-tolerant Ricinus communis and salt-sensitive Jatropha curcas. Journal of Plant Physiology, 171, 23-30.
DOI URL |
| [15] |
Liu B, Wang XY, Cao Y, Arora R, Zhou H, Xia YP (2020). Factors affecting freezing tolerance: a comparative transcriptomics study between field and artificial cold acclimations in overwintering evergreens. The Plant Journal, 103, 2279-2300.
DOI URL |
| [16] | Lu CF, Jian LC, Ben GY (2000). Photosynthesis in alpine plant Lagotis brevituba and its response to freezing stress. Chinese Bulletin of Botany, 17, 559-564. |
| [卢存福, 简令成, 贲桂英 (2000). 高山植物短管兔儿草光合作用特性及其对冰冻胁迫的反应. 植物学通报, 17, 559-564.] | |
| [17] |
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence—A practical guide. Journal of Experimental Botany, 51, 659-668.
DOI PMID |
| [18] | Miehe G, Miehe S, Kaiser K, Liu JQ, Zhao XQ (2008). Status and dynamics of the Kobresia pygmaea ecosystem on the Tibetan Plateau. Ambio, 37, 258-265. |
| [19] |
Miehe G, Schleuss PM, Seeber E, Babel W, Biermann T, Braendle M, Chen FH, Coners H, Foken T, Gerken T, Graf HF, Guggenberger G, Hafner S, Holzapfel M, Ingrisch J, et al. (2019). The Kobresia pygmaea ecosystem of the Tibetan highlands—Origin, functioning and degradation of the world’s largest pastoral alpine ecosystem Kobresia pastures of Tibet. Science of the Total Environment, 648, 754-771.
DOI URL |
| [20] |
Murchie EH, Niyogi KK (2011). Manipulation of photoprotection to improve plant photosynthesis. Plant Physiology, 155, 86-92.
DOI PMID |
| [21] |
Niyogi KK, Truong TB (2013). Evolution of flexible non- photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Current Opinion in Plant Biology, 16, 307-314.
DOI URL |
| [22] |
Oxborough K, Baker NR (1997). Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components— Calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynthesis Research, 54, 135-142.
DOI URL |
| [23] |
Peng SM, Du QY, Lin AW, Hu B, Xiao K, Xi YL (2015). Observation and estimation of photosynthetically active radiation in Lhasa (Tibetan Plateau). Advances in Space Research, 55, 1604-1612.
DOI URL |
| [24] | Sáez PL, Bravo LA, Latsague MI, Toneatti MJ, Sánchez-Olate M, Ríos DG (2013). Light energy management in micropropagated plants of Castanea sativa, effects of photoinhibition. Plant Science, 201- 202, 12-24. |
| [25] | Schreiber U, Bilger W, Neubauer C (1995). Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis//Schulze ED, Caldwell MM. Ecophysiology of Photosynthesis. Springer-Verlag, Berlin, Heideberg. 49-70. |
| [26] | Shi SB, Li TC, Li M, Liu SZ, Li AD, Ma JP (2015). Interaction effect analysis of soil drought and strong light on PSII non-photochemical quenching in Kobresia pygmaea leaves. Plant Physiology Journal, 51, 1678-1689. |
| [师生波, 李天才, 李妙, 刘世增, 李爱德, 马剑平 (2015). 土壤干旱和强光对高山嵩草叶片PSII反应中心非光化学猝灭的交互影响分析. 植物生理学报, 51, 1678-1689.] | |
| [27] |
Shi SB, Zhou DW, Li TC, De KJ, Gao XZ, Ma JL, Sun T, Wang FL (2023). Responses of photosynthetic function of Kobresia pygmaea to overnight low temperature on the Qingzang Plateau. Chinese Journal of Plant Ecology, 47, 361-373.
DOI URL |
|
[师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳 (2023). 青藏高原高山嵩草光合功能对模拟夜间低温的响应. 植物生态学报, 47, 361-373.]
DOI |
|
| [28] | Sun BG, Long RJ, Wang CT (2007). A study on the plant population phenology in Qinghai-Tibet Plateau Kobrecia pygmaea meadow. Pratacultural Science, 24, 16-20. |
| [孙步功, 龙瑞军, 王长庭 (2007). 青藏高原冷龙岭南麓高寒小嵩草草甸植物种群物候学研究. 草业科学, 24, 16-20.] | |
| [29] |
Tikkanen M, Mekala NR, Aro EM (2014). Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochimica et Biophysica Acta, 1837, 210-215.
DOI PMID |
| [30] |
Valizadeh-Kamran R, Toorchi M, Mogadam M, Mohammadi H, Pessarakli M (2018). Effects of freeze and cold stress on certain physiological and biochemical traits in sensitive and tolerant barley (Hordeum vulgare) genotypes. Journal of Plant Nutrition, 41, 102-111.
DOI URL |
| [31] | Wang CT, Long RJ, Ding LM (2004). Study of alpine meadow of basic characteristic in Qinghai Tibet Plateau. Pratacultural Science, 21, 16-19. |
| [王长庭, 龙瑞军, 丁路明 (2004). 青藏高原高寒嵩草草甸基本特征的研究. 草业科学, 21, 16-19.] | |
| [32] | Wang WY, Wang QJ, Deng ZF (1998). Communities structural characteristic and plant distribution pattern in alpine Kobresia pygmaea meadow, Haibei region of Qinghai Province. Acta Phytoecologica Sinica, 22, 336-343. |
| [王文颖, 王启基, 邓自发 (1998). 青海海北地区高山嵩草草甸植物群落的结构特征及其分布格局. 植物生态学报, 22, 336-343.] | |
| [33] | Wang YJ, Wei XH, Yang P (2005). Effects of over-grazing on vegetation degradation of Kobresia pygmaea meadow in Nagqu, Tibet. Journal of Lanzhou University (Natural Sciences), 41, 32-38. |
| [王亚军, 魏兴琥, 杨萍 (2005). 超载放牧对那曲地区高山嵩草草甸植被退化的影响. 兰州大学学报(自然科学版), 41, 32-38.] | |
| [34] | Wu FZ, Wang HX, Xu GH, Zhang ZC (2015). Research progress on the physiological and molecular mechanisms of woody plants under low temperature stress. Scientia Silvae Sinicae, 51(7), 116-128. |
| [乌凤章, 王贺新, 徐国辉, 张自川 (2015). 木本植物低温胁迫生理及分子机制研究进展. 林业科学, 51(7), 116-128.] | |
| [35] | Xiang HT, Zheng DF, He N, Li W, Wang ML, Wang SY (2021). Research progress on the physiological response of plants to low temperature and the amelioration effectiveness of exogenous ABA. Acta Prataculturae Sinica, 30, 208-219. |
|
[项洪涛, 郑殿峰, 何宁, 李琬, 王曼力, 王诗雅 (2021). 植物对低温胁迫的生理响应及外源脱落酸缓解胁迫效应的研究进展. 草业学报, 30, 208-219.]
DOI |
|
| [36] | Xu DQ (2002). Photosynthetic Efficiency. Shanghai Scientific and Technical Press, Shanghai. |
| [许大全 (2002). 光合作用效率. 上海科学技术出版社, 上海.] | |
| [37] |
Yamori W, Hikosaka K, Way DA (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research, 119, 101-117.
DOI PMID |
| [38] |
Yu BH, Lu CH (2011). Assessment of ecological vulnerability on the Tibetan Plateau. Geographical Research, 30, 2289-2295.
DOI |
| [于伯华, 吕昌河 (2011). 青藏高原高寒区生态脆弱性评价. 地理研究, 30, 2289-2295.] | |
| [39] | Zhang XS (1978). The plateau zonality of vegetation in Xizang. Acta Botanica Sinica, 20, 140-149. |
| [张新时 (1978). 西藏高原植被的高原地带性. 植物学报, 20, 140-149.] | |
| [40] | Zhou J, Zeng XY, He W, Li JY, Zhang W, Li XQ (2016). Mechanism of chilling injury in Jacaranda acutifolia Humb. et Bonpl. under chilling stress. Southwest China Journal of Agricultural Sciences, 29, 74-80. |
| [周静, 曾学英, 贺维, 李佳泳, 张炜, 李晓清 (2016). 低温胁迫下蓝花楹的耐寒生理机制分析. 西南农业学报, 29, 74-80.] | |
| [41] | Zhou XM (2001). Chinese Kobresia Meadow. Science Press, Beijing. |
| [周兴民 (2001). 中国嵩草草甸. 科学出版社, 北京.] |
| [1] | ZHAO Yan-Chao, CHEN Li-Tong. Soil nutrients modulate response of aboveground biomass to warming in alpine grassland on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(8): 1071-1081. |
| [2] | REN Pei-Xin, LI Peng, PENG Chang-Hui, ZHOU Xiao-Lu, YANG Ming-Xia. Temporal and spatial variation of vegetation photosynthetic phenology in Dongting Lake basin and its response to climate change [J]. Chin J Plant Ecol, 2023, 47(3): 319-330. |
| [3] | SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(3): 361-373. |
| [4] | LIN Ma-Zhen, HUANG Yong, LI Yang, SUN Jian. Geographical distribution characteristics and influencing factors of plant survival strategies in an alpine grassland [J]. Chin J Plant Ecol, 2023, 47(1): 41-50. |
| [5] | ZHU Yu-Ying, ZHANG Hua-Min, DING Ming-Jun, YU Zi-Ping. Changes of vegetation greenness and its response to drought-wet variation on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(1): 51-64. |
| [6] | WEI Yao, MA Zhi-Yuan, ZHOU Jia-Ying, ZHANG Zhen-Hua. Experimental warming changed reproductive phenology and height of alpine plants on the Qingzang Plateau [J]. Chin J Plant Ecol, 2022, 46(9): 995-1004. |
| [7] | JIN Yi-Li, WANG Hao-Yan, WEI Lin-Feng, HOU Ying, HU Jing, WU Kai, XIA Hao-Jun, XIA Jie, ZHOU Bo-Rui, LI Kai, NI Jian. A plot-based dataset of plant community on the Qingzang Plateau [J]. Chin J Plant Ecol, 2022, 46(7): 846-854. |
| [8] | LU Jing, MA Zong-Qi, GAO Peng-Fei, FAN Bao-Li, SUN Kun. Changes in the Hippophae tibetana population structure and dynamics, a pioneer species of succession, to altitudinal gradients in the Qilian Mountains, China [J]. Chin J Plant Ecol, 2022, 46(5): 569-579. |
| [9] | HU Xiao-Fei, WEI Lin-Feng, CHENG Qi, WU Xing-Qi, NI Jian. A climate diagram atlas of Qingzang Plateau [J]. Chin J Plant Ecol, 2022, 46(4): 484-492. |
| [10] | WU Zan, PENG Yun-Feng, YANG Gui-Biao, LI Qin-Lu, LIU Yang, MA Li-Hua, YANG Yuan-He, JIANG Xian-Jun. Effects of land degradation on soil and microbial stoichiometry in Qingzang Plateau alpine grasslands [J]. Chin J Plant Ecol, 2022, 46(4): 461-472. |
| [11] | ZHENG Zhou-Tao, ZHANG Yang-Jian. Variation in ecosystem water use efficiency and its attribution analysis during 1982-2018 in Qingzang Plateau [J]. Chin J Plant Ecol, 2022, 46(12): 1486-1496. |
| [12] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [13] | XUE Jin-Ru, LÜ Xiao-Liang. Assessment of vegetation productivity under the implementation of ecological programs in the Loess Plateau based on solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2022, 46(10): 1289-1304. |
| [14] | Ning LIU, Shou-Zhang PENG, Yun-Ming CHEN. Temporal effects of climate factors on vegetation growth on the Qingzang Plateau, China [J]. Chin J Plant Ecol, 2022, 46(1): 18-26. |
| [15] | NIE Xiu-Qing, WANG Dong, ZHOU Guo-Ying, XIONG Feng, DU Yan-Gong. Soil microbial biomass carbon, nitrogen, phosphorus and their stoichiometric characteristics in alpine wetlands in the Three Rivers Sources Region [J]. Chin J Plant Ecol, 2021, 45(9): 996-1005. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn