

植物生态学报 ›› 2016, Vol. 40 ›› Issue (9): 942-951.DOI: 10.17521/cjpe.2016.0001
收稿日期:2015-01-03
接受日期:2016-07-19
出版日期:2016-09-10
发布日期:2016-09-29
通讯作者:
樊大勇
基金资助:
Da-Yong FAN*( ), Zeng-Juan FU, Zong-Qiang XIE, Rong-Gui LI, Shu-Min ZHANG
), Zeng-Juan FU, Zong-Qiang XIE, Rong-Gui LI, Shu-Min ZHANG
Received:2015-01-03
Accepted:2016-07-19
Online:2016-09-10
Published:2016-09-29
Contact:
Da-Yong FAN
摘要:
研究叶片内部光合能力及其异质性, 是光合作用生化模型、光抑制机理、光保护机理等光合生理生态热点问题的重要前提, 但目前缺少可以进行活体测定的装置。该文作者对Vogelmann和Evans (2002)的装置进行了改进, 获得了叶片内部光系统II (PSII)最大光化学量子效率(Fv/Fm)及其异质性影像, 并采用基于Matlab软件自编的图像处理程序对其进行了分析, 研究了不同时间光抑制处理导致的叶内Fv/Fm及其异质性的变化。研究发现: 叶片内部不同层叶肉组织Fv/Fm存在异质性。强光照射导致叶片内部不同层叶肉组织Fv/Fm值下降, 但靠近上表皮的栅栏组织具有较强的抗光抑制能力。光抑制导致叶片内部Fv/Fm异质性的变化, 短期光抑制导致Fv/Fm异质性变大, 这可能与部分叶绿体避光运动相关, 长期光抑制导致Fv/Fm异质性变小, 说明叶绿体避光运动这种光保护机制已经失效。相比于其他类型叶绿素荧光仪, 此装置可以完整获得活体叶片内部Fv/Fm及其异质性影像数据, 这对于系统研究叶片内部光合能力及其异质性机制, 以及为进一步研究一些光合生理生态热点问题提供了有力工具, 具有进一步研发和应用的前景。
樊大勇, 付增娟, 谢宗强, 李荣贵, 张淑敏. 调制式荧光影像新技术: 叶片内部最大光化学量子效率及其异质性的活体测定. 植物生态学报, 2016, 40(9): 942-951. DOI: 10.17521/cjpe.2016.0001
Da-Yong FAN, Zeng-Juan FU, Zong-Qiang XIE, Rong-Gui LI, Shu-Min ZHANG. A new technology of modulated Chl a fluorescence image: In vivo measurement of the PSII maximum photochemical efficiency and its heterogeneity within leaves. Chinese Journal of Plant Ecology, 2016, 40(9): 942-951. DOI: 10.17521/cjpe.2016.0001
| 参数 Index | 型号 Type | |||
|---|---|---|---|---|
| PAM101-102-1031) | IMAGING-PAM | PEA | 本仪器 This set-up | |
| 信号采集方式 Signal acquisition mode | 调制-锁相放大 Lock-in amplifier | CCD连续采集 CCD continuous collection | 光电连续采集 Photoelectric continuous collection | CCD连续采集 CCD continuous collection |
| 荧光数据形式 Fluorescence data format | 点式 Point | 图像 Image | 点式 Point | 图像 Image |
| 测量光光强 The measuring light intensity | 20-302) | 10-1 000 | 3 000 | 1 0003) |
| 测量光脉冲宽度 The measuring light pulse width | 2 μs4) | 1.8 ms | 2 s | 160 μs |
| 测量光周期 The measuring cycle duration | 600 μs5) | 125-1 000 ms | NA | 3.3 ms |
| 测量光脉冲宽度在OJIP的位置 The position of the measuring light pulse in OJIP curve | O段 At O stage | 接近J段 Close to J stage | NA | 接近O段 Close to O stage |
| 测量光占空比 The measuring light duty cycle | 1/300 | 1.8/(125-1000) | NA | 1/30 |
| 饱和光光强 Saturating light intensity (μmol·m-2·s-1) | >3 000 | 800-1 200 | 3 000 | 1 000 |
| 测量光光强是否与饱和光光强一致 Is the measuring light intensity consistent with the saturating light intensity? | 否 No | 否6) No6) | 是 Yes | 是 Yes |
| 可否得到叶水平面荧光异质性 Can the fluorescence image on the leaf surface be obtained? | 否 No | 可以 Yes | 否 No | 可以7) Yes7) |
| 可否得到叶横截面荧光异质性 Can the fluorescence across leaf section be obtained? | 否 No | 否8) No8) | 否 No | 可以9) Yes9) |
| 测量光和饱和光的均匀性 The uniformity of measuring and saturating light source | 均匀 Uniform | 一定范围内均匀 Uniform within a specific range | 均匀 Uniform | 均匀10) Uniform10) |
表1 目前常见进行叶绿素荧光最大光化学量子效率(Fv/Fm)测定的荧光仪性能对比
Table 1 The comparison of current fluorometers which can measure maximum photochemical efficiency (Fv/Fm)
| 参数 Index | 型号 Type | |||
|---|---|---|---|---|
| PAM101-102-1031) | IMAGING-PAM | PEA | 本仪器 This set-up | |
| 信号采集方式 Signal acquisition mode | 调制-锁相放大 Lock-in amplifier | CCD连续采集 CCD continuous collection | 光电连续采集 Photoelectric continuous collection | CCD连续采集 CCD continuous collection |
| 荧光数据形式 Fluorescence data format | 点式 Point | 图像 Image | 点式 Point | 图像 Image |
| 测量光光强 The measuring light intensity | 20-302) | 10-1 000 | 3 000 | 1 0003) |
| 测量光脉冲宽度 The measuring light pulse width | 2 μs4) | 1.8 ms | 2 s | 160 μs |
| 测量光周期 The measuring cycle duration | 600 μs5) | 125-1 000 ms | NA | 3.3 ms |
| 测量光脉冲宽度在OJIP的位置 The position of the measuring light pulse in OJIP curve | O段 At O stage | 接近J段 Close to J stage | NA | 接近O段 Close to O stage |
| 测量光占空比 The measuring light duty cycle | 1/300 | 1.8/(125-1000) | NA | 1/30 |
| 饱和光光强 Saturating light intensity (μmol·m-2·s-1) | >3 000 | 800-1 200 | 3 000 | 1 000 |
| 测量光光强是否与饱和光光强一致 Is the measuring light intensity consistent with the saturating light intensity? | 否 No | 否6) No6) | 是 Yes | 是 Yes |
| 可否得到叶水平面荧光异质性 Can the fluorescence image on the leaf surface be obtained? | 否 No | 可以 Yes | 否 No | 可以7) Yes7) |
| 可否得到叶横截面荧光异质性 Can the fluorescence across leaf section be obtained? | 否 No | 否8) No8) | 否 No | 可以9) Yes9) |
| 测量光和饱和光的均匀性 The uniformity of measuring and saturating light source | 均匀 Uniform | 一定范围内均匀 Uniform within a specific range | 均匀 Uniform | 均匀10) Uniform10) |
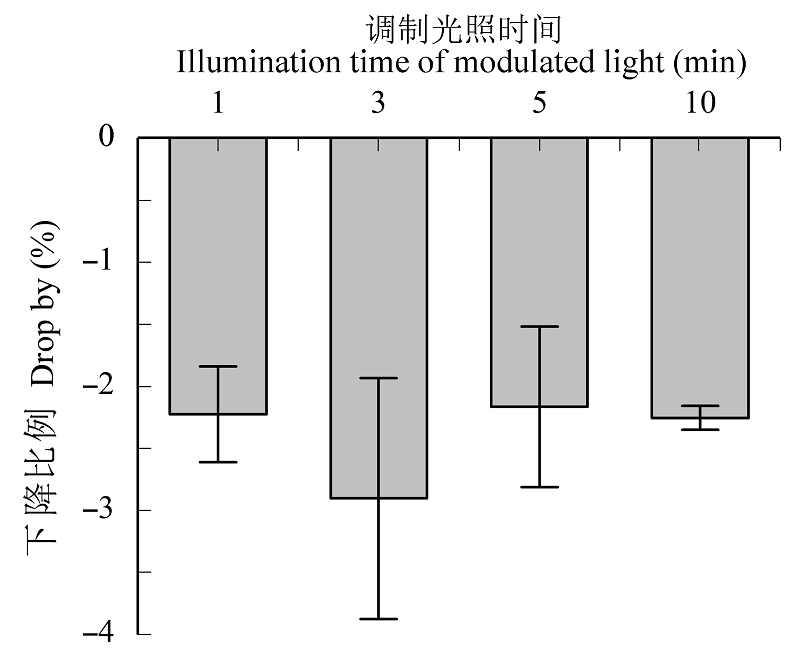
图3 本仪器设置的调制光对叶绿素荧光最大光化学量子效率(Fv/Fm)测定值的影响(平均值±标准偏差)。
Fig. 3 Effect of the current set-up of modulated measuring light on maximum photochemical efficiency (Fv/Fm) values (mean ± SD).
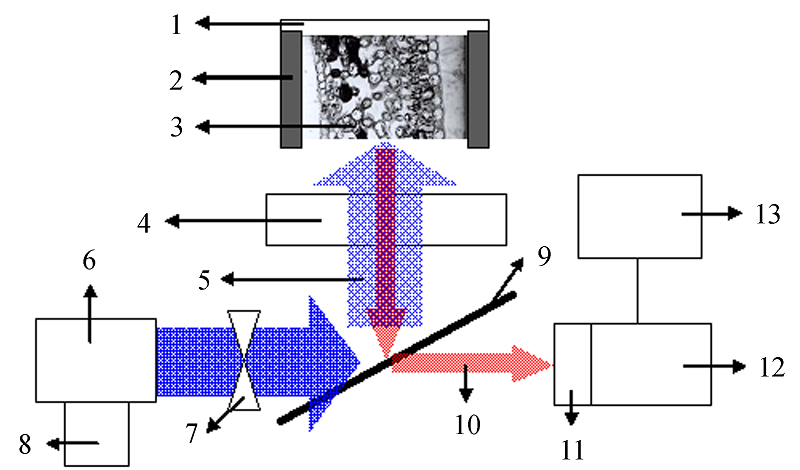
图1 仪器装置图。1 叶夹; 2 垫片; 3 叶片; 4 显微镜物镜; 5 入射调制测量光(用于测定Fo)及饱和强光(用于测定Fm); 6 蓝色激光LED; 7 斩波器(在测定Fo时打开, 产生调制测量光, 在测定Fm时关闭, 使得饱和强光通过); 8 恒流源; 9 显微镜二向分光镜; 10 叶绿素荧光; 11 短波截止滤光片(RG9); 12 显微镜自带CCD; 13 计算机。Fo, 暗适应状态下的最小荧光产量; Fm, 暗适应状态下的最大荧光产量。
Fig. 1 Block diagram of the experimental set-up of measurement of chlorophyll fluorescence yield within leaves. 1, leaf clip; 2, leaf pad; 3, leaf disk; 4, microscope objective; 5, incident modulated measuring light (for the determination of Fo) and saturate light (for the determination of Fm); 6, blue laser LED; 7, chopper (when turned on, modulated measuring light is created, for the determination of Fo; when turned off, continuous strong light is created, for the determination of Fm); 8, constant current source; 9, beam splitter; 10, chl a fluorescence; 11, short-wave cut-off filter (RG9); 12, charge coupled device (CCD); 13, computer. Fo, minimum fluorescence yield in dark-adapted state; Fm, maximum fluorescence yield in dark- adapted state
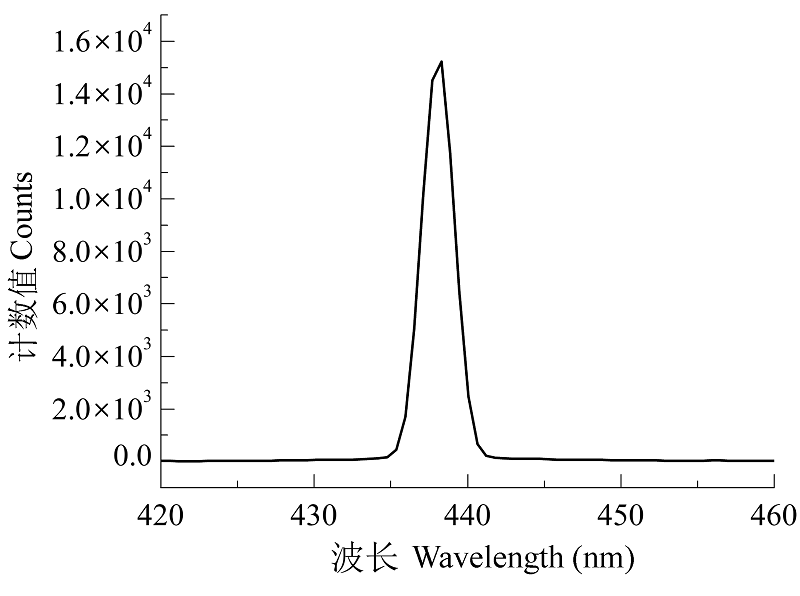
图2 1 W大功率蓝色激光二极管的光谱。用AvaSpec- ULS2048×64光纤光谱仪(Avantes, Apeldoorn, the Netherlands)测定。
Fig. 2 Spectrum of 1 W high power blue laser diode. Measured by a fiber optic spectrometer AvaSpec-ULS2048×64 (Avantes, Apeldoorn, the Netherlands).
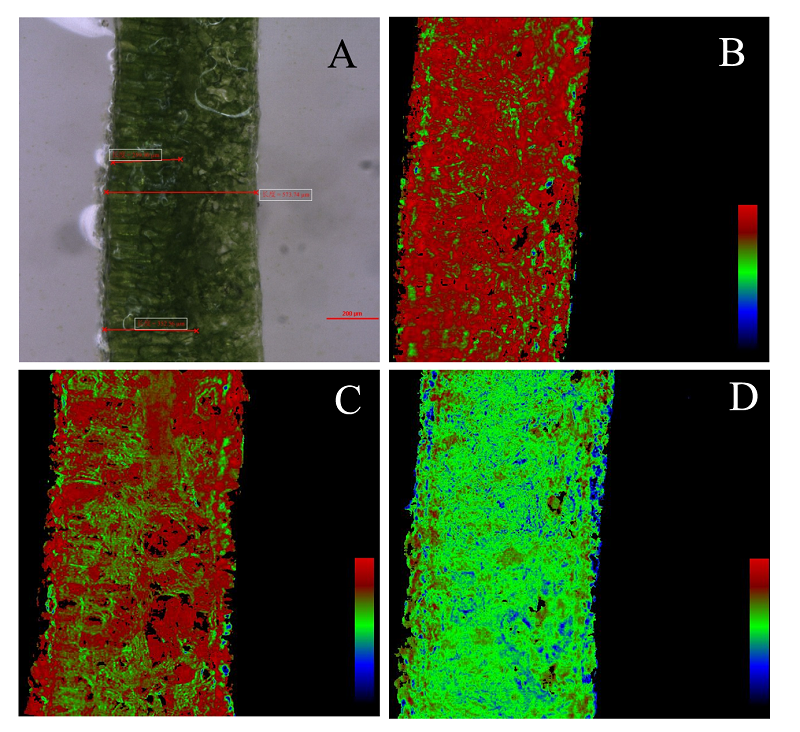
图4 对照叶片和光抑制处理后沿叶横截面的最大光化学量子效率(Fv/Fm)异质性假彩色影像。A, 徒手切片。B, 0小时。C, 1小时。D, 3小时。
Fig. 4 The false color images of maximum photochemical efficiency (Fv/Fm) across leaf section after photoinhibition. A, free-hand section showing the thickness of leaf. B, 0 h. C, 1 h. D, 3 h.
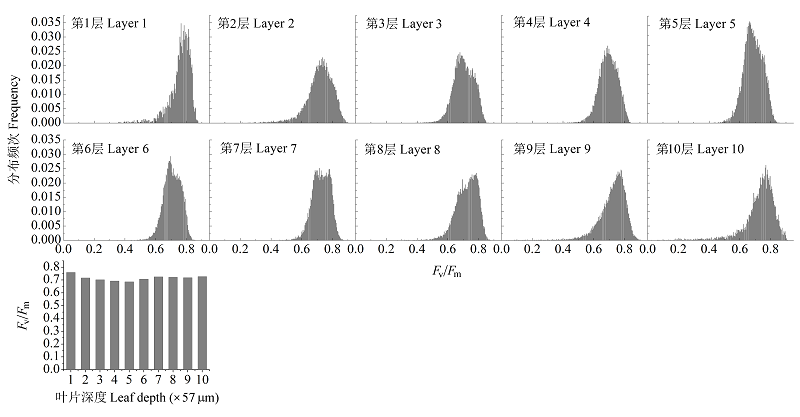
图6 光抑制1小时后叶片沿叶横截面的最大光化学量子效率(Fv/Fm)异质性。
Fig. 6 Maximum photochemical efficiency (Fv/Fm) heterogeneity across leaf section of leaf with 1 hour photoinhibition.
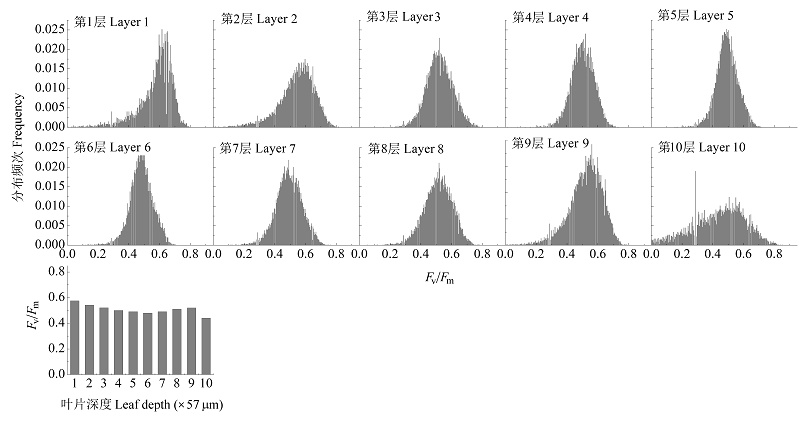
图7 光抑制3 h后沿叶横截面的最大光化学量子效率(Fv/Fm)异质性。
Fig. 7 Maximum photochemical efficiency (Fv/Fm) heterogeneity across leaf section of leaf with 3 h photoinhibition.
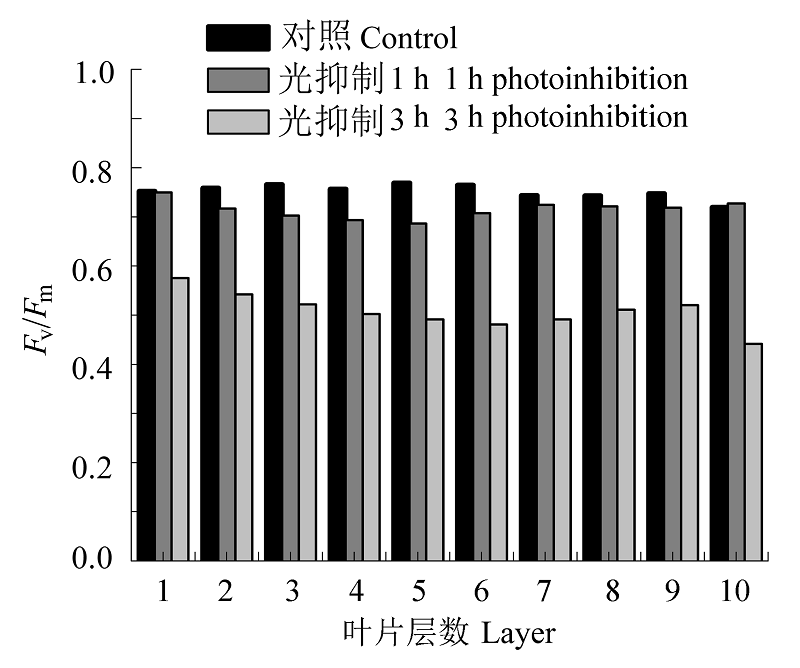
图8 对照和光抑制处理后沿叶横截面的最大光化学量子效率(Fv/Fm)差异。
Fig. 8 Maximum photochemical efficiency (Fv/Fm) curves across leaf section of the control and photoinhibited leaves.
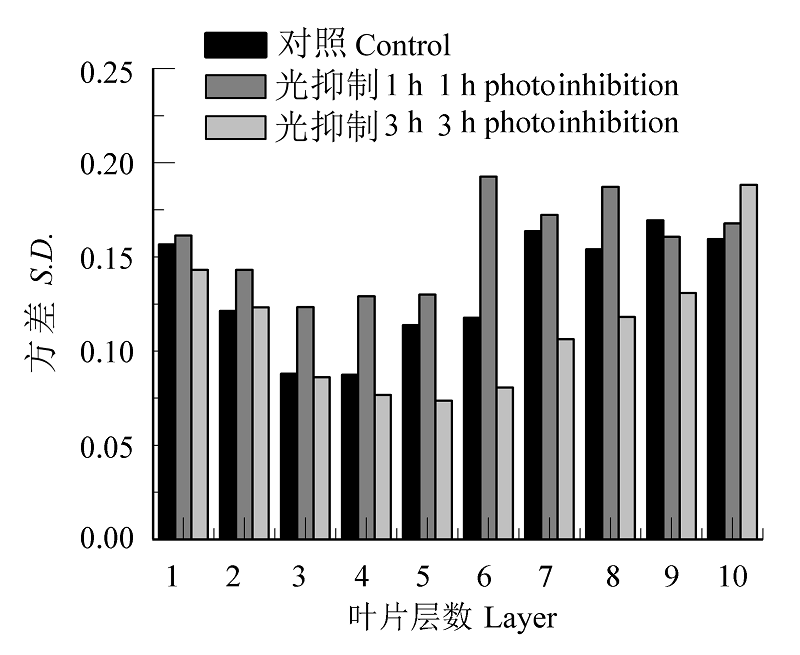
图9 对照和光抑制处理后沿叶横截面的最大光化学量子效率(Fv/Fm)变异性(S.D.)。
Fig. 9 Maximum photochemical efficiency (Fv/Fm) variability (S.D.) along the cross section of control and photoinhibited leaves.
| 1 | Aro EM, McMaffery S, Anderson JM (1993). Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances.Plant Physiology, 103, 835-843. |
| 2 | Baker NR (2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo.Annual Review of Plant Biology, 59, 89-113. |
| 3 | Cao J, Govindjee (1990). Chlorophyll a fluorescence transient as an indicator of active and inactive photosystem II in thylakoid membranes.Biochimica et Biophysica Acta- Bioenergetics, 1015, 180-188. |
| 4 | Croxdale JG, Omasa K (1990). Chlorophyll-a fluorescence and carbon assimilation in developing leaves of light-grown cucumber.Plant Physiology, 93, 1078-1082. |
| 5 | Danielsson R, Albertsson PA, Mamedov F, Styring S (2004). Quantification, of photosystem I and II in different parts of the thylakoid membrane from spinach.Biochimica et Biophysica Acta-Bioenergetics, 1608, 53-61. |
| 6 | Farquhar GD, Caemmerer SV, Berry JA (1980). A biochemical- model of photosynthetic CO2 assimilation in leaves of C3 species.Planta, 149, 78-90. |
| 7 | Fan DY, Ye ZP, Wang SC, Chow WS (2015). Multiple roles of oxygen in the photoinactivation and dynamic repair of photosystem II in spinach leaves.Photosynthesis Research. doi: 10.1007/s11120-015-0185-y. |
| 8 | Hakala M, Tuominen I, Keranen M, Tyystjarvi T, Tyystjarvi E (2005). Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II.Biochimica et Biophysica Acta-Bioenergetics, 1706, 68-80. |
| 9 | Hu YY, Fan DY, Losciale P, Chow W, Zhang WF (2013). Whole-tissue determination of the rate coefficients of photoinactivation and repair of photosystem II in cotton leaf discs based on flash-induced P700 redox kinetics.Photosynthesis Research, 117, 517-528. |
| 10 | Kagawa T, Wada M (1999). Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation.Plant Physiology, 119, 917-923. |
| 11 | Melis A (1991). Dynamics of photosynthetic membrane- composition and function.Biochimica et Biophysica Acta, 1058, 87-106. |
| 12 | Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011). Intra-leaf gradients of photoinhibition induced by different color lights: Implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers.New Phytologist, 191, 146-159. |
| 13 | Osmond CB (1994). What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR eds. Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field. BIOS Scientific Publishing, Oxford, UK. 1-24. |
| 14 | Oxborough K (2004). Using chlorophyll a fluorescence imaging to monitor photosynthetic performance, In: Papageorgiou GC, Govindjee eds. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer Press, Dordrecht, The Netherland. 409-428. |
| 15 | Schreiber U, Kuhl M, Klimant I, Reising H (1996). Measurement of chlorophyll fluorescence within leaves using a modified PAM fluorometer with a fiber-optic microprobe. Photosynthesis Research, 47, 103-109. |
| 16 | Strasser RJ, Srivastava A, Govindjee (1995). Polyphasic chlorophyll-alpha fluorescence transient in plants and cyanobacteria.Photochemistry and Photobiology, 61, 32-42. |
| 17 | Strasser RJ, Tsimilli-Michael M, Srivastava A (2004). Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee eds. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer Press, Dordrecht, the Netherland. 321-362. |
| 18 | Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R (2009). Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green.Plant and Cell Physiology, 50, 684-697. |
| 19 | Terashima I, Inoue Y (1985). Palisade tissue chloroplasts and spongy tissue chloroplasts in spinach: Biochemical and ultrastructural differences.Plant and Cell Physiology, 26, 63-75. |
| 20 | Tyystjärvi E (2013). Photoinhibition of photosystem II.International Review of Cell and Molecular Biology, 300, 243-303. |
| 21 | Vogelmann TC, Bjorn LO (1984). Measurement of light gradients and spectral regime in plant-tissue with a fiber optic probe.Physiologia Plantarum, 60, 361-368. |
| 22 | Vogelmann TC, Evans JR (2002). Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence.Plant, Cell & Environment, 25, 1313-1323. |
| [1] | 胡文海, 张斯斯, 肖宜安, 闫小红. 两种杜鹃花属植物对长期遮阴后全光照环境的生理响应及其光保护机制[J]. 植物生态学报, 2015, 39(11): 1093-1100. |
| [2] | 李志真, 刘东焕, 赵世伟, 姜闯道, 石雷. 环境强光诱导玉簪叶片光抑制的机制[J]. 植物生态学报, 2014, 38(7): 720-728. |
| [3] | 师生波, 张怀刚, 师瑞, 李妙, 陈文杰, 孙亚男. 青藏高原春小麦叶片光合作用的光抑制及PSII反应中心光化学效率的恢复分析[J]. 植物生态学报, 2014, 38(4): 375-386. |
| [4] | 张超, 占东霞, 张鹏鹏, 张亚黎, 罗宏海, 张旺锋. 棉花苞叶光呼吸和PSII热耗散对土壤水分的响应[J]. 植物生态学报, 2014, 38(4): 387-395. |
| [5] | 刘柿良, 马明东, 潘远智, 魏刘利, 何成相, 杨开茂. 不同光强对两种桤木幼苗光合特性和抗氧化系统的影响[J]. 植物生态学报, 2012, 36(10): 1062-1074. |
| [6] | 宋旭丽, 胡春梅, 孟静静, 侯喜林, 何启伟, 李新国. NaCl胁迫加重强光胁迫下超大甜椒叶片的光系统II和光系统I的光抑制[J]. 植物生态学报, 2011, 35(6): 681-686. |
| [7] | 张亚黎, 罗毅, 姚贺盛, 田景山, 罗宏海, 张旺锋. 田间条件下海岛棉和陆地棉花铃期叶片光保护的机制[J]. 植物生态学报, 2010, 34(10): 1204-1212. |
| [8] | 陈华新, 陈玮, 姜闯道, 高辉远, 邹琦. 光温交叉处理对小麦紫黄质脱环氧化酶活性及其热耗散能力的影响[J]. 植物生态学报, 2008, 32(5): 1015-1022. |
| [9] | 杨兴洪, 邹琦, 赵世杰. 遮荫和全光下生长的棉花光合作用和叶绿素荧光特征[J]. 植物生态学报, 2005, 29(1): 8-15. |
| [10] | 蔡志全, 曹坤芳, 齐欣. 热带雨林冠层树种绒毛番龙眼两种发育阶段叶片的光抑制[J]. 植物生态学报, 2003, 27(2): 210-217. |
| [11] | 张教林, 曹坤芳. 光照对两种热带雨林树种幼苗光合能力、热耗散和抗氧化系统的影响[J]. 植物生态学报, 2002, 26(6): 639-646. |
| [12] | 冯玉龙, 张亚杰, 朱春全. 调控活性氧代谢对渗透胁迫时杨树光合作用光抑制的影响[J]. 植物生态学报, 2001, 25(4): 451-459. |
| [13] | 张守仁, 高荣孚. 光胁迫下杂种杨无性系光合生理生态特性的研究[J]. 植物生态学报, 2000, 24(5): 528-533. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19