

植物生态学报 ›› 2013, Vol. 37 ›› Issue (1): 70-79.DOI: 10.3724/SP.J.1258.2013.00008
刘华伟1,2,*( ), 林晓军1,2, 孙超1,2, 李强1,2, 杨呼1,2, 郭蔼光1,2,**(
), 林晓军1,2, 孙超1,2, 李强1,2, 杨呼1,2, 郭蔼光1,2,**( )
)
收稿日期:2012-09-10
接受日期:2012-11-08
出版日期:2013-09-10
发布日期:2013-01-15
通讯作者:
刘华伟,郭蔼光
作者简介:**(E-mail:guoaiguang@yahoo.com.cn)基金资助:
LIU Hua-Wei1,2,*( ), LIN Xiao-Jun1,2, SUN Chao1,2, LI Qiang1,2, YANG Hu1,2, GUO Ai-Guang1,2,**(
), LIN Xiao-Jun1,2, SUN Chao1,2, LI Qiang1,2, YANG Hu1,2, GUO Ai-Guang1,2,**( )
)
Received:2012-09-10
Accepted:2012-11-08
Online:2013-09-10
Published:2013-01-15
Contact:
LIU Hua-Wei,GUO Ai-Guang
摘要:
苗期是小麦(Triticum aestivum)物质和能量积累的关键时期, 苗期干旱影响小麦的后期群体建成。利用田菁茎瘤固氮根瘤菌(Azorhizobium caulinodans) ‘ORS571’与巴西固氮螺菌(Azospirillum brasilense) ‘Yu62’浸种侵染小麦和聚乙二醇(PEG)模拟渗透胁迫, 研究渗透胁迫下接菌小麦种子的发芽状况; 利用固氮菌涂抹小麦幼苗叶部, 测定PEG模拟渗透胁迫下小麦幼苗根体积、叶片相对含水量、脯氨酸含量及可溶性蛋白含量, 探究固氮菌增强小麦幼苗抗渗透胁迫的能力。结果表明, 接种混合固氮菌后在渗透胁迫下小麦种子的发芽率明显提高; 在渗透胁迫下叶部涂抹固氮菌小麦的根体积、叶片相对含水量、脯氨酸含量及可溶性蛋白含量明显升高, 表明接种固氮菌可提高小麦幼苗的抗渗透胁迫能力, 且混合固氮菌对小麦幼苗叶片的增强效果优于单一固氮菌。
刘华伟, 林晓军, 孙超, 李强, 杨呼, 郭蔼光. 接种两种固氮菌增强小麦幼苗抗渗透胁迫及生长能力. 植物生态学报, 2013, 37(1): 70-79. DOI: 10.3724/SP.J.1258.2013.00008
LIU Hua-Wei, LIN Xiao-Jun, SUN Chao, LI Qiang, YANG Hu, GUO Ai-Guang. Inoculation two azotobacter enhancing osmotic stress resistance and growth in wheat seedling. Chinese Journal of Plant Ecology, 2013, 37(1): 70-79. DOI: 10.3724/SP.J.1258.2013.00008
| 小麦品种 Wheat cultivar | 菌根处理 Mycorrhizal treatment | 渗透胁迫 Osmotic stress (%) | 复水 Rehydration (%) | ||||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | |||
| ‘绵阳19’ ‘Mianyang 19’ | 不接种 No infected | 97.44 ± 0.947a | 89.99 ± 2.326b | 86.99 ± 0.803b | 92.67 ± 1.042b | 96.56 ± 1.581b | |
| 接种 Infected | 98.08 ± 1.782a | 96.87 ± 1.160c | 98.05 ± 0.949e | 98.02 ± 0.179c | 97.00 ± 0.399b | ||
| ‘西农979’ ‘Xinong 979’ | 不接种 No infected | 95.68 ± 0.322a | 92.64 ± 2.056bc | 77.74 ± 1.738a | 94.46 ± 0.249b | 95.08 ± 1.499ab | |
| 接种 Infected | 95.67 ± 0.847a | 94.91 ± 2.992c | 90.79 ± 0.292c | 95.76 ± 0.020bc | 97.62 ± 1.876b | ||
| ‘周麦18’ ‘Zhoumai18’ | 不接种 No infected | 97.28 ± 1.079a | 82.48 ± 1.067a | 74.67 ± 4.456a | 93.48 ± 0.800b | 96.27 ± 1.810b | |
| 接种 Infected | 97.28 ± 0.606a | 95.05 ± 1.959c | 90.22 ± 0.695c | 97.43 ± 2.094c | 98.07 ± 1.480b | ||
| ‘小偃22’ ‘Xiaoyan 22’ | 不接种 No infected | 95.73 ± 0.371a | 84.01 ± 0.773a | 82.25 ± 1.723b | 87.78 ± 1.824a | 93.91 ± 0.292a | |
| 接种 Infected | 95.79 ± 0.713a | 97.86 ± 0.467c | 93.66 ± 0.834d | 97.05 ± 1.228c | 92.06 ± 1.093a | ||
表1 渗透胁迫下小麦叶片的相对含水量(平均值±标准偏差)
Table 1 Relative water content of wheat laminas under osmotic stress (mean ± SD)
| 小麦品种 Wheat cultivar | 菌根处理 Mycorrhizal treatment | 渗透胁迫 Osmotic stress (%) | 复水 Rehydration (%) | ||||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | |||
| ‘绵阳19’ ‘Mianyang 19’ | 不接种 No infected | 97.44 ± 0.947a | 89.99 ± 2.326b | 86.99 ± 0.803b | 92.67 ± 1.042b | 96.56 ± 1.581b | |
| 接种 Infected | 98.08 ± 1.782a | 96.87 ± 1.160c | 98.05 ± 0.949e | 98.02 ± 0.179c | 97.00 ± 0.399b | ||
| ‘西农979’ ‘Xinong 979’ | 不接种 No infected | 95.68 ± 0.322a | 92.64 ± 2.056bc | 77.74 ± 1.738a | 94.46 ± 0.249b | 95.08 ± 1.499ab | |
| 接种 Infected | 95.67 ± 0.847a | 94.91 ± 2.992c | 90.79 ± 0.292c | 95.76 ± 0.020bc | 97.62 ± 1.876b | ||
| ‘周麦18’ ‘Zhoumai18’ | 不接种 No infected | 97.28 ± 1.079a | 82.48 ± 1.067a | 74.67 ± 4.456a | 93.48 ± 0.800b | 96.27 ± 1.810b | |
| 接种 Infected | 97.28 ± 0.606a | 95.05 ± 1.959c | 90.22 ± 0.695c | 97.43 ± 2.094c | 98.07 ± 1.480b | ||
| ‘小偃22’ ‘Xiaoyan 22’ | 不接种 No infected | 95.73 ± 0.371a | 84.01 ± 0.773a | 82.25 ± 1.723b | 87.78 ± 1.824a | 93.91 ± 0.292a | |
| 接种 Infected | 95.79 ± 0.713a | 97.86 ± 0.467c | 93.66 ± 0.834d | 97.05 ± 1.228c | 92.06 ± 1.093a | ||
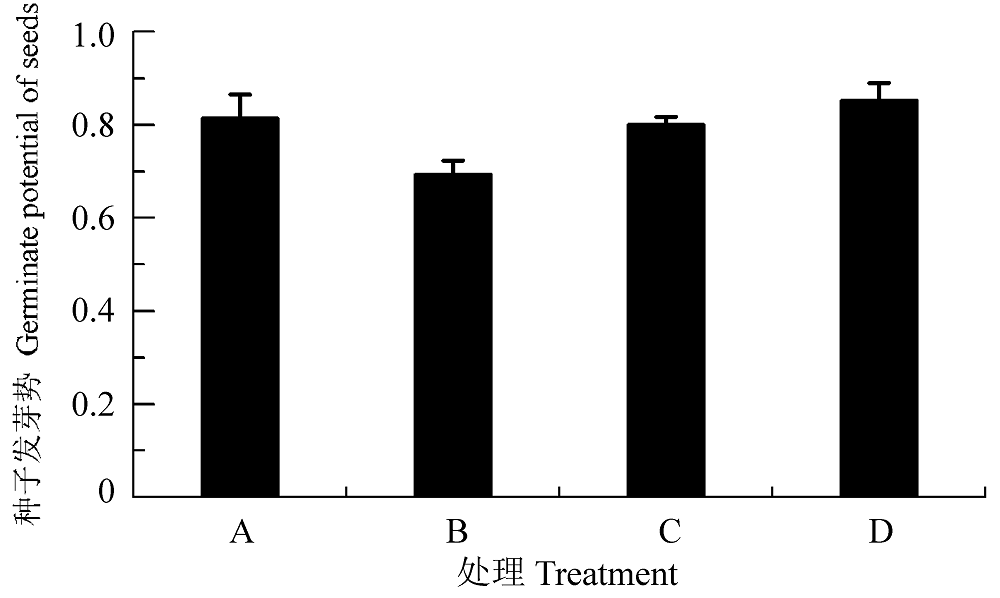
图2 渗透胁迫下小麦种子接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌的发芽势(平均值±标准偏差)。A, PBS正对照; B, 渗透胁迫处理负对照; C, 渗透胁迫下接种田菁茎瘤固氮根瘤菌; D, 渗透胁迫下接种巴西固氮螺菌。
Fig. 2 Germinate potential of wheat seeds infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress (mean ± SD). A, PBS positive control; B, negative control under osmotic stress; C, treatment of infection by Azorhizobium caulinodans under osmotic stress; D, treatment of infection by Azospirillum brasilense under osmotic stress.
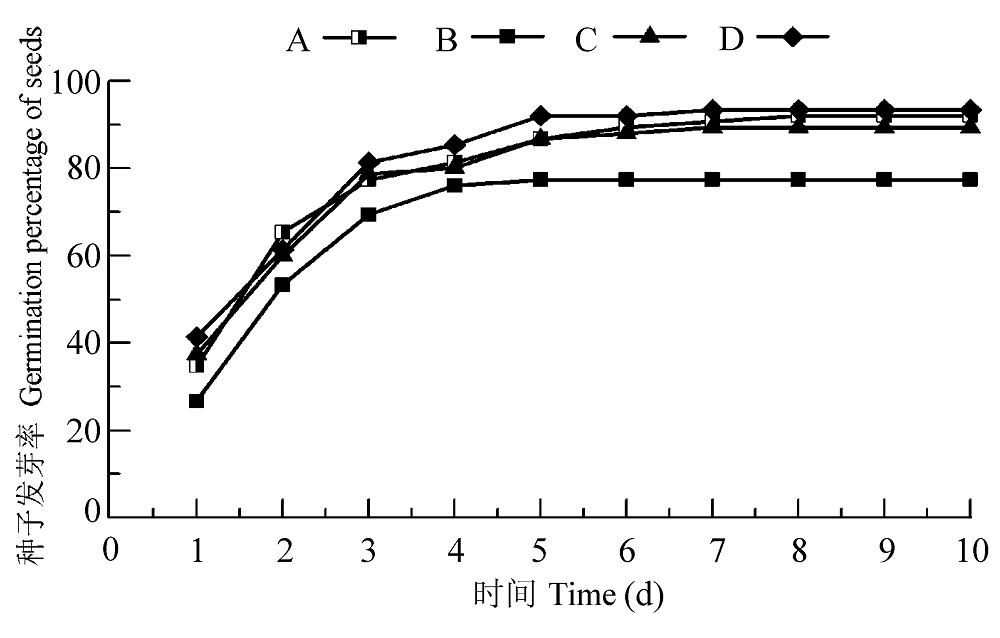
图3 渗透胁迫小麦种子接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌的发芽率。A, PBS正对照; B, 渗透胁迫处理负对照; C, 渗透胁迫下接种田菁茎瘤固氮根瘤菌; D, 渗透胁迫下接种巴西固氮螺菌。
Fig. 3 Germination percentage of wheat seeds infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress. A, PBS positive control; B, negative control under osmotic stress; C, treatment of infection by Azorhizobium caulinodans under osmotic stress; D, treatment of infection by Azospirillum brasilense under osmotic stress.
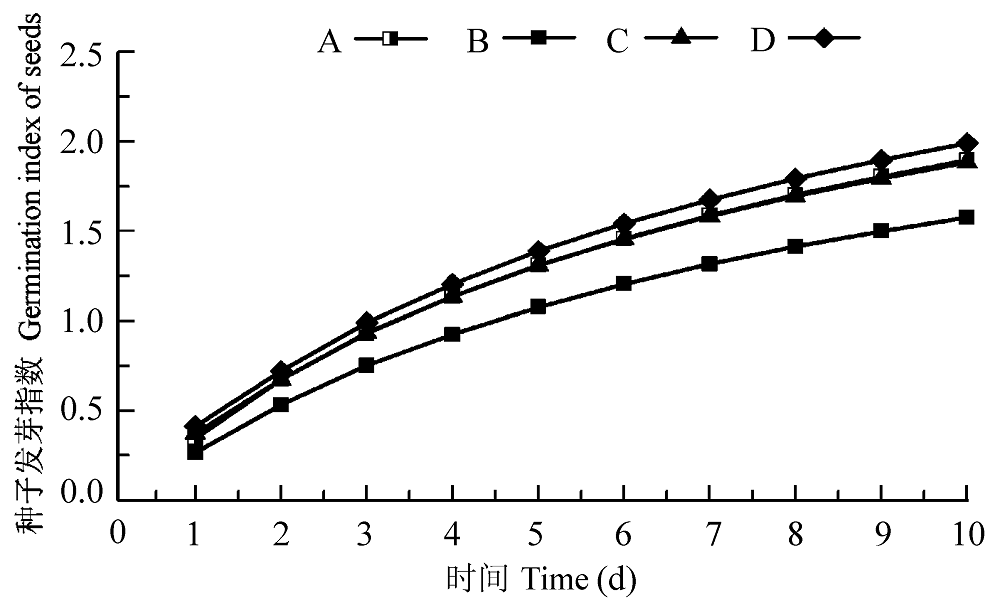
图4 渗透胁迫下小麦种子接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌的发芽指数。A, PBS正对照; B, 渗透胁迫处理负对照; C, 渗透胁迫下接种田菁茎瘤固氮根瘤菌; D, 渗透胁迫下接种巴西固氮螺菌。
Fig. 4 Germination index of wheat seeds infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress. A, PBS positive control; B, negative control under osmotic stress; C, treatment of infection by Azorhizobium caulinodans under osmotic stress; D, treatment of infection by Azospirillum brasilense under osmotic stress.
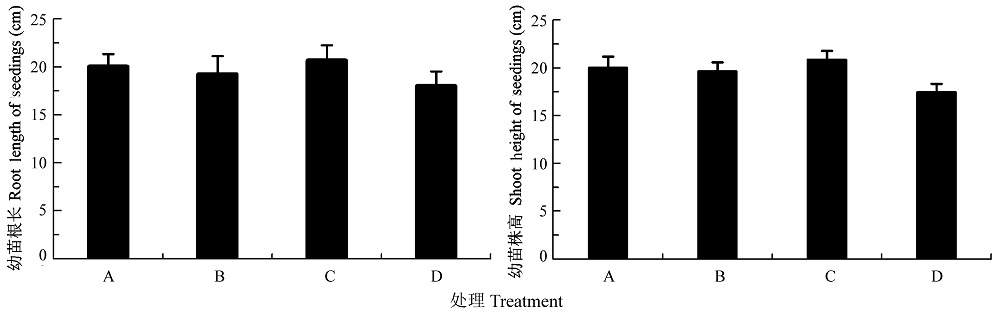
图5 渗透胁迫下接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌对小麦幼苗根长与株高的影响(平均值±标准偏差)。A, 接种田菁茎瘤固氮根瘤菌; B,接种巴西固氮螺菌; C, 接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌; D, 对照。
Fig. 5 Effects of wheat seedling root length and plant height infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress (mean ± SD). A, infected by Azorhizobium caulinodans; B, infected by Azospirillum brasilense; C, infected by Azorhizobium caulinodans and Azospirillum brasilense; D, control.
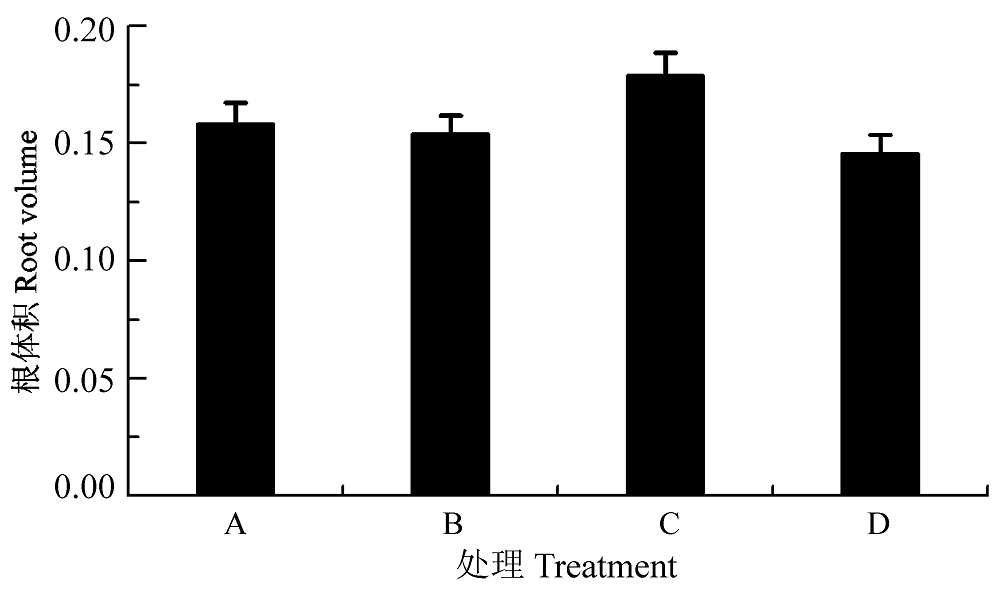
图6 渗透胁迫下叶片接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌小麦幼苗的根体积(平均值±标准偏差)。A, 接种田菁茎瘤固氮根瘤菌; B,接种巴西固氮螺菌; C, 接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌; D, 对照。
Fig. 6 Root volume of wheat seedlings infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress (mean ± SD). A, infected by Azorhizobium caulinodans; B, infected by Azospirillum brasilense; C, infected by Azorhizobium caulinodans and Azospirillum brasilense; D, control.
| 茎瘤固氮根瘤菌 Azorhizobium caulinodans | 巴西固氮螺菌 Azospirillum brasilense | |||||
|---|---|---|---|---|---|---|
| 叶 Leaf (×107) | 14.2 | 11.9 | 12.1 | 16.4 | 15.2 | 14.2 |
| 根 Root (×107) | 2.4 | 1.9 | 2.1 | 1.8 | 2.0 | 1.8 |
表2 接种6天后不同小麦幼苗器官中田菁茎瘤固氮根瘤菌和巴西固氮螺菌定殖数目(3次重复)
Table 2 Colonization number of Azorhizobium caulinodans and Azospirillum brasilense in different wheat seedling organs after infected six days (three repeat)
| 茎瘤固氮根瘤菌 Azorhizobium caulinodans | 巴西固氮螺菌 Azospirillum brasilense | |||||
|---|---|---|---|---|---|---|
| 叶 Leaf (×107) | 14.2 | 11.9 | 12.1 | 16.4 | 15.2 | 14.2 |
| 根 Root (×107) | 2.4 | 1.9 | 2.1 | 1.8 | 2.0 | 1.8 |
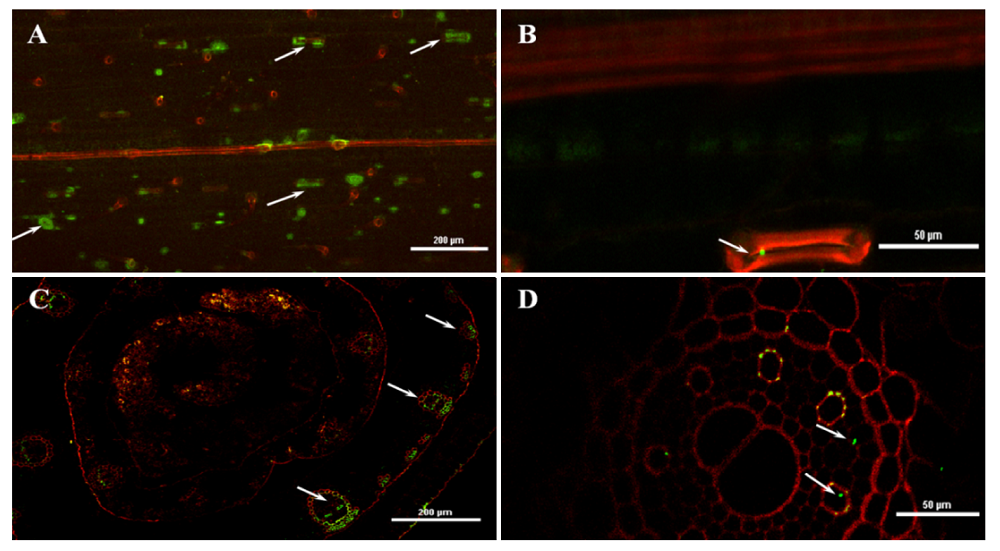
图7 激光共聚焦显微镜检测绿色荧光蛋白(GFP)-田菁茎瘤固氮根瘤菌在小麦体内的定殖。A, GFP-田菁茎瘤固氮根瘤菌吸附在小麦的叶背面, 箭头所示为分布气孔处的GFP-田菁茎瘤固氮根瘤菌。B, GFP-田菁茎瘤固氮根瘤菌定殖在气孔处的局部放大照片。C, GFP-田菁茎瘤固氮根瘤菌定殖在小麦茎的维管组织中。D, GFP-田菁茎瘤固氮根瘤菌定殖在小麦根的维管组织中。
Fig. 7 Colonization of green fluorescent protein (GFP)-Azorhizobium caulinodans in the wheat seeding via confocal laser scanning microscopy. A, Colonization of GFP-A. caulinodans in the wheat blade back, arrow point to stomas. B, Amplifying colonization of GFP-A. caulinodans in the middle of stoma. C, Colonization of GFP-A. caulinodans in the wheat stem vascular tissue. D, Colonization of GFP-A. caulinodans in the wheat root vascular tissue.
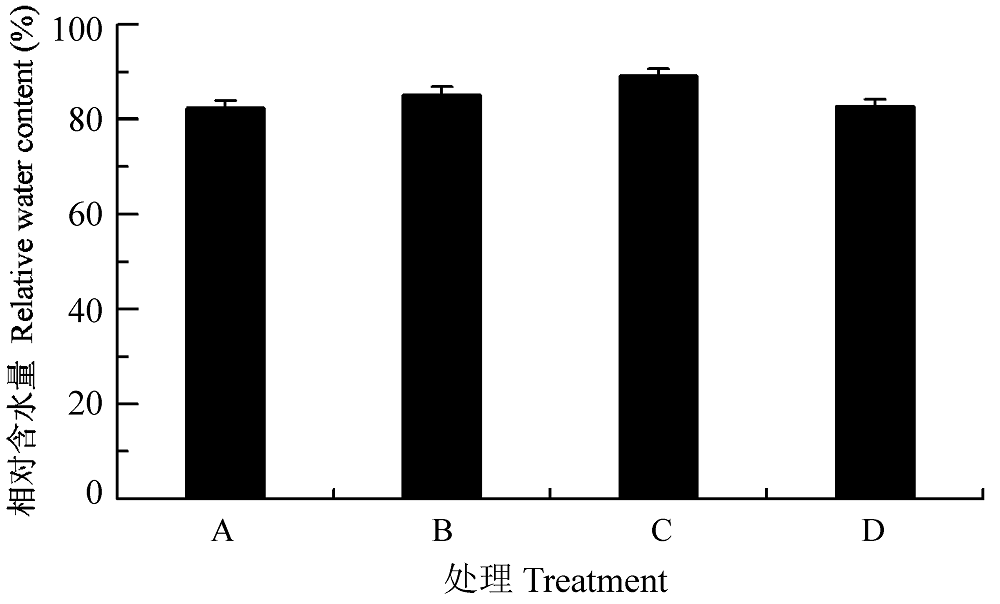
图8 渗透胁迫下叶片接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌小麦幼苗的相对含水量(平均值±标准偏差)。A, 接种田菁茎瘤固氮根瘤菌; B,接种巴西固氮螺菌; C, 接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌; D, 对照。
Fig. 8 Relative water content of wheat seedlings infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress (mean ± SD). A, infected by Azorhizobium caulinodans; B, infected by Azospirillum brasilense; C, infected by Azorhizobium caulinodans and Azospirillum brasilense; D, control.
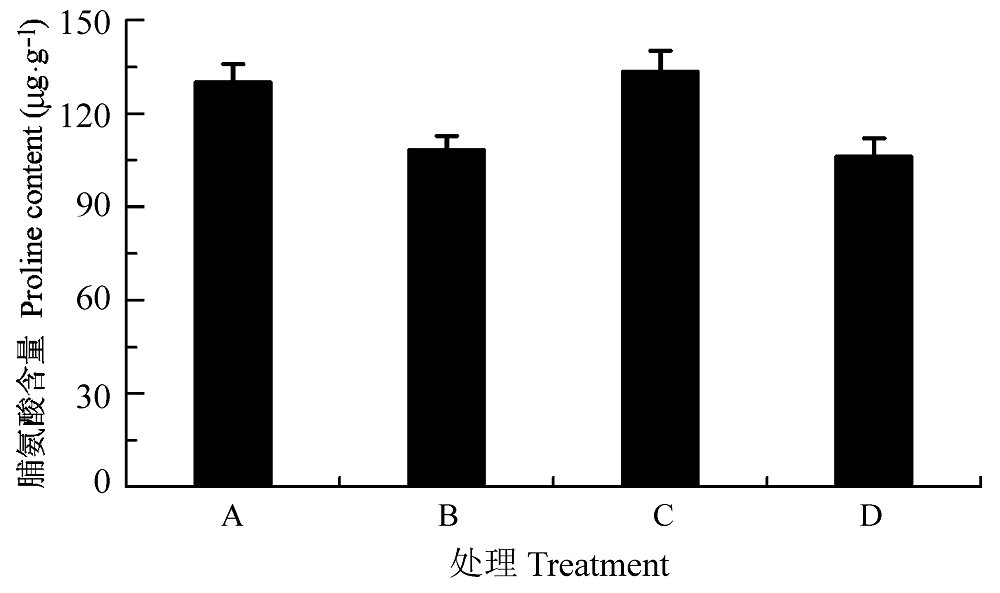
图9 渗透胁迫下叶片接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌小麦幼苗的脯氨酸含量(平均值±标准偏差)。A, 接种田菁茎瘤固氮根瘤菌; B,接种巴西固氮螺菌; C, 接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌; D, 对照。
Fig. 9 Proline content of wheat seedlings infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress (mean ± SD). A, infected by Azorhizobium caulinodans; B, infected by Azospirillum brasilense; C, infected by Azorhizobium caulinodans and Azospirillum brasilense; D, control.
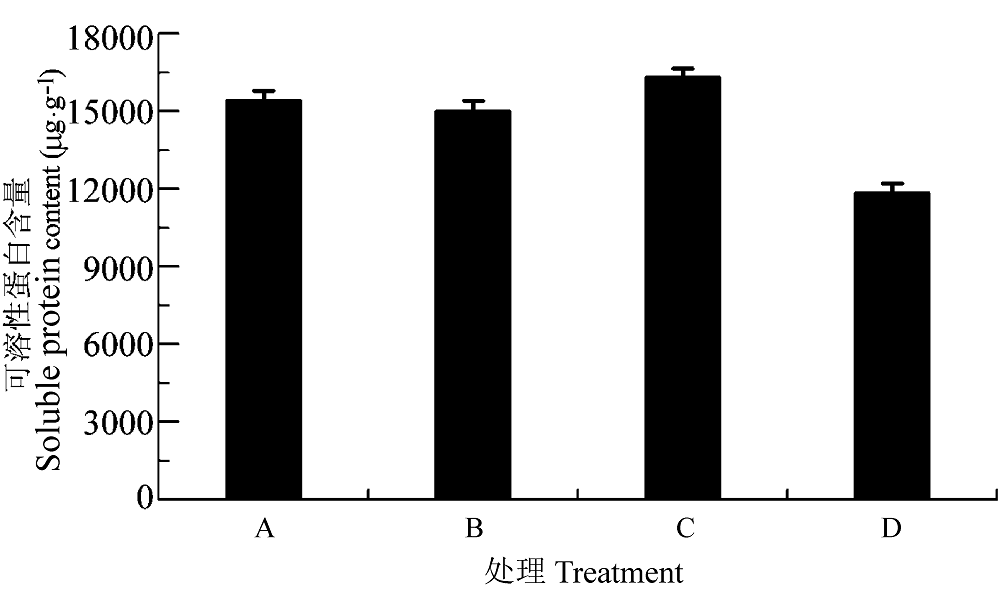
图10 渗透胁迫下叶片接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌小麦幼苗的可溶性蛋白含量(平均值±标准偏差)。A, 接种田菁茎瘤固氮根瘤菌; B,接种巴西固氮螺菌; C, 接种田菁茎瘤固氮根瘤菌和巴西固氮螺菌; D, 对照。
Fig. 10 Soluble protein content of wheat seedlings infected by Azorhizobium caulinodans and Azospirillum brasilense under osmotic stress (mean ± SD). A, infected by Azorhizobium caulinodans; B, infected by Azospirillum brasilense; C, infected by Azorhizobium caulinodans and Azospirillum brasilense; D, control.
| [1] | Athar M, Johnson DA (1996). Influence of drought on competition between selected Rhizobium meliloti strains and naturalized soil rhizobia in alfalfa. Plant and Soil, 184, 231-241. |
| [2] | Bushby HVA, Marshall KC (1977). Some factors affecting the survival of root-nodule bacteria on desiccation. Soil Biology & Biochemistry, 9, 143-147. |
| [3] | Cai H, Tang HX (2011). Agricultural drought assessment and on wheat growth impact. Modern Agricultural Sciences and Technology, (8), 292-293. (in Chinese) |
| [ 蔡衡, 唐宏祥 (2011). 农业干旱的评估及对小麦生长的影响. 现代农业科技, (8), 292-293.] | |
| [4] | Chen SY, Lang NJ, Li JY, Jia LQ, Wu LY, Mi FD (2004). Changes of leaf relative water content, relative plasma membrane permeability and proline content of seedlings of three species under drought stress. Journal of West China Forestry Science, 33(3), 30-33, 41. (in Chinese with English abstract) |
| [ 陈少瑜, 郎南军, 李吉跃, 贾利强, 吴丽圆, 米方佃 (2004). 干旱胁迫下3树种苗木叶片相对含水量、质膜相对透性和脯氨酸含量的变化. 西部林业科学, 33(3), 30-33, 41.] | |
| [5] | Chi F (2006). Migration of Rhizobia in Plants and Proteome Analysis of Their Interaction. PhD dissertation, Institute of Botany, Chinese Academy of Sciences, Beijing. (in Chinese with English abstract) |
| [ 迟峰 (2006). 根瘤菌在植物内的迁移运动及其与植物相互作用的蛋白质组学研究. 博士学位论文, 中国科学院植物研究所, 北京.] | |
| [6] |
Chi F, Shen SH, Cheng HP, Jing YX, Yanni YG, Dazzo FB (2005). Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Applied and Environmental Microbiology, 71, 7271-7278.
URL PMID |
| [7] | de Oliveira AC, Varshney RK (2011). Root Genomics. Springer, New York. 1-10. |
| [8] | Döbereiner J, Day JM, Dart PJ (1972). Nitrogenase activity in the rhizosphere of sugar cane and some other tropical grasses. Plant and Soil, 37, 191-196. |
| [9] |
Fu B, Wang WW, Tang M, Chen XD (2009). Isolation and identification of hydrogen-oxidizing bacteria producing 1-aminocyclopropane-1- carboxylate deaminase and the determination of enzymatic activity. Acta Microbiologica Sinica, 49, 395-399. (in Chinese with English abstract)
URL PMID |
|
[ 付博, 王卫卫, 唐明, 陈兴都 (2009). 一株产1-氨基环丙烷-1-羧酸脱氨酶的氢氧化细菌的分离鉴定及酶活力测定及酶活力测定. 微生物学报, 49, 395-399.]
PMID |
|
| [10] | Fuhrmann J, Davey CB, Wollum AG (1986). Desiccation tolerance of clover rhizobia in sterile soils. Soil Science Society of America Journal, 50, 639-644. |
| [11] |
Glick BR, Bashan Y (1997). Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnology Advances, 15, 353-378.
URL PMID |
| [12] | Guo AG, Guo ZK (2007). Biochemical Experimental Technology. Higher Education Press, Beijing. (in Chinese) |
| [ 郭蔼光, 郭泽坤 (2007). 生物化学实验技术. 高等教育出版社, 北京.] | |
| [13] |
Ji KX, Chi F, Yang MF, Shen SH, Jing YX, Dazzo FB, Cheng HP (2010). Movement of rhizobia inside tobacco and lifestyle alternation from endophytes to free-living rhizobia on leaves. Journal of Microbiology and Biotechnology, 20, 238-244.
URL PMID |
| [14] |
Kang YJ, Cheng J, Mei LJ, Hu J, Piao Z, Yin SX (2010). Action mechanisms of plant growth-promoting rhizobacteria (PGPR): a review. Chinese Journal of Applied Ecology, 21, 232-238. (in Chinese with English abstract)
URL PMID |
|
[ 康贻军, 程洁, 梅丽娟, 胡健, 朴哲, 殷士学 (2010). 植物根际促生菌作用机制研究进展. 应用生态学报, 21, 232-238.]
PMID |
|
| [15] | Kase H, Kochler M, Stuzel H (2004). Root growth and dry matter partitioning of cauliflower under drought stress conditions: measurement and simulation. European Journal of Agronomy, 20, 379-394. |
| [16] | Kozlowski TT, Pallardy SG (2002). Acclimation and adaptive responses of woody plants to environmental stresses. The Botanical Review, 68, 270-334. |
| [17] | Li XW, Li JM, Duan LS, Li ZH (2010). Primary study on inducing effect of coronatine on drought tolerance of winter wheat seedlings. Journal of Triticeae Crops, 30, 676-679. (in Chinese with English abstract) |
| [ 李相文, 李建民, 段留生, 李召虎 (2010). 冠菌素诱导冬小麦幼苗抗旱性的初步研究. 麦类作物学报, 30, 676-679.] | |
| [18] | Li YG, Zhou JC (2002). Root colonization of Sinorhizobium fredii pSym-cured strain HN01 in rhizosphere of Glycine max. Acta Ecologica Sinica, 22, 1420-1424. (in Chinese with English abstract) |
| [ 李友国, 周俊初 (2002). 消除共生质粒的费氏中华根瘤菌HND29SR在大豆根圈的定殖动态. 生态学报, 22, 1420-1424.] | |
| [19] | Liang Y, Chen SP, Gao YB, Ren AZ. (2002). Effects of endophyte infection on the growth of Lolium perenne L. under drought stress. Acta Phytoecologica Sinica, 26, 621-626. (in Chinese with English abstract) |
| [ 梁宇, 陈世苹, 高玉葆, 任安芝 (2002). 内生真菌感染对干旱胁迫下黑麦草生长的影响. 植物生态学报, 26, 621-626.] | |
| [20] | Liu HF, Gao YB, Zhang Q, Li X, Li CL (2004). Physio- ecological responses and their adaptation of different geographic Leymus chinensis populations to soil drought stress. Acta Scientiarum Naturalium Universitatis Nankaiensis (Natural Science Edition), 37(4), 105-110. (in Chinese with English abstract) |
| [ 刘慧芳, 高玉葆, 张强, 李欣, 李长林 (2004). 不同种群羊草幼苗对土壤干旱胁迫的生理生态响应. 南开大学学报(自然科学版), 37(4), 105-110.] | |
| [21] |
Liu HW, Sun C, Yang H, Lin XJ, Guo AG (2012). Promotion for wheat growth and root colonization after infecting wheat seeds with Azorhizobium caulinodans. Plant Nutrition and Fertilizer Science, 18, 210-217. (in Chinese with English abstract)
DOI URL |
| [ 刘华伟, 孙超, 杨呼, 林晓军, 郭蔼光 (2012). 田菁茎瘤固氮根瘤菌对小麦种子侵染的促生作用及其在根系内的定殖. 植物营养与肥料学报, 18, 210-217.] | |
| [22] | Liu HW, Wang QH, Zhang H, Wang R, Xiao HL, Guo AG (2009). Colonization of Azospirillum brasilense Yu62 in wheat via EGFP. Acta Botanica Boreali-Occidentalia Sinica, 29, 2367-2372. (in Chinese with English abstract) |
| [ 刘华伟, 王庆贺, 张宏, 王蕊, 肖红利, 郭蔼光 (2009). 巴西固氮螺菌Yu62的EGFP标记及其在小麦体内的定殖研究. 西北植物学报, 29, 2367-2372.] | |
| [23] | Lu YH, Zhang FS (2006). The advances in rhizosphere microbiology. Soils, 38, 113-121. (in Chinese with English abstract) |
| [ 陆雅海, 张福锁 (2006). 根际微生物研究进展. 土壤, 38, 113-121.] | |
| [24] | Pan PP, Zhou HB (1995). Plant hormones produced by Azorhizobium caulinodans ORS 571. Microbiology, 22, 10-13. (in Chinese with English abstract) |
| [ 潘佩平, 周鸿宾 (1995). 茎瘤固氮根瘤菌(Azorhizobium caulinodans) ORS571产生的植物激素. 微生物学通报, 22, 10-13.] | |
| [25] | Shen SH, Jing YX (2003). Present and prospect of nitrogen-fixing in China. Chinese Science Bulletin, 48, 535-540. (in Chinese) |
| [ 沈世华, 荆玉祥 (2003). 中国生物固氮研究现状和展望. 科学通报, 48, 535-540.] | |
| [26] | Shi Y, Lin Q, Wei DB, Yu ZW (1996). Impacts of soil water stress on photosynthesis and yield of winter wheat. Acta Agriculturae Boreali- Sinica, 11(4), 80-83. (in Chinese with English abstract) |
| [ 石研, 林琪, 魏东斌, 于振文 (1996). 土壤水分胁迫对冬小麦光合及产量的影响. 华北农学报, 11(4), 80-83.] | |
| [27] |
Timmusk S, Wagner EGH (1999). The plant growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Molecular Plant-Microbe Interactions, 12, 951-959.
URL PMID |
| [28] | Wang WE, Li Y, Su N, Yu WC (2011). The effects of salt stress on seed germination of Indigofera amblyantha. Hubei Agricultural Sciences, 50, 321-324. (in Chinese with English abstract) |
| [ 王文恩, 李颖, 苏农, 余文成 (2011). 盐胁迫对多花木蓝种子萌发的影响. 湖北农业科学, 50, 321-324.] | |
| [29] | Xu XL, Guan XQ, Liu GS (2003). Effects of cooperation between associative azotobacteria and rhizobia on wheat seedling. Chinese Journal of Eco-Agriculture, 11(3), 66-68. (in Chinese with English abstract) |
| [ 徐兴良, 关秀清, 刘公社 (2003). 联合固氮菌与根瘤菌协同作用对小麦幼苗的影响. 中国生态农业学报, 11(3), 66-68.] | |
| [30] | Ye AH, Yuan Y, Yang L, Cai YP, Tian SN (2003). A comparison of the effects of two arbuscular mycorrhizal fungal species on photosynthesis, transpiration and water use efficiency of wheat. Chinese Agricultural Science Bulletin, 19(3), 18-20. (in English with Chinese abstract) |
| [ 叶爱华, 袁艺, 杨莉, 蔡永萍, 田胜尼 (2003). 两种丛枝菌根菌抗旱效应的比较. 中国农学通报, 19(3), 18-20.] | |
| [31] |
Yu CQ (2011). China’s water crisis needs more than words. Nature, 470, 307.
DOI URL PMID |
| [32] | Zhang DZ, Wang PH, Zhao HX (1990). Determination of the content of free proline in wheat leaves. Plant Physiology Communications, (4), 62-65. (in Chinese with English abstract) |
| [ 张殿忠, 王沛洪, 赵会贤 (1990). 测定小麦叶片游离脯氨酸含量的方法. 植物生理学通讯, (4), 62-65.] | |
| [33] | Zhao SJ, Li SL, Wang ZQ (2004). Effects of VA mycorrhizal fungi on drought resistance of spring wheat under non-irrigation. Journal of Inner Mongolia Institute of Agriculture and Animal Husbandry, 25(4), 43-46. (in Chinese with English abstract) |
| [ 赵士杰, 李树林, 王志强 (2004). VA菌根真菌对旱作春小麦抗旱性的影响. 内蒙古农业大学学报, 25(4), 43-46.] | |
| [34] | Zhou LP, Liu WJ, Ma HC, Wu JR (2010). Research progress on drought resistance of rhizobium strains and plants inoculated with rhizobium. Journal of Anhui Agricultural Sciences, 38, 11978-11980, 11983. (in Chinese with English abstract) |
| [ 周利平, 刘文杰, 马焕成, 伍建榕 (2010). 根瘤菌菌株和接种根瘤菌植株的耐旱性研究进展. 安徽农业科学, 38, 11978-11980, 11983.] |
| [1] | 罗丹丹, 王传宽, 金鹰. 木本植物水力系统对干旱胁迫的响应机制[J]. 植物生态学报, 2021, 45(9): 925-941. |
| [2] | 安东升, 曹娟, 黄小华, 周娟, 窦美安. 基于Lake模型的叶绿素荧光参数在甘蔗苗期抗旱性研究中的应用[J]. 植物生态学报, 2015, 39(4): 398-406. |
| [3] | 邱权,潘昕,李吉跃,王军辉,马建伟,杜坤. 青藏高原20种灌木抗旱形态和生理特征[J]. 植物生态学报, 2014, 38(6): 562-575. |
| [4] | 李涛, 陈保冬. 丛枝菌根真菌通过上调根系及自身水孔蛋白基因表达提高玉米抗旱性[J]. 植物生态学报, 2012, 36(9): 973-981. |
| [5] | 张智猛, 万书波, 戴良香, 宋文武, 陈静, 石运庆. 花生抗旱性鉴定指标的筛选与评价[J]. 植物生态学报, 2011, 35(1): 100-109. |
| [6] | 贺学礼, 张焕仕, 赵丽莉. 不同土壤中水分胁迫和AM真菌对油蒿抗旱性的影响[J]. 植物生态学报, 2008, 32(5): 994-1001. |
| [7] | 李君, 周守标, 王春景, 李金花. 野生和栽培马蹄金抗旱性比较及其抗旱机制初探[J]. 植物生态学报, 2007, 31(3): 521-527. |
| [8] | 刘颖慧, 高琼, 贾海坤. 半干旱地区3种植物叶片水平的抗旱耐旱特性分析——两个气孔导度模型的应用和比较[J]. 植物生态学报, 2006, 30(1): 64-70. |
| [9] | 关军锋, 刘海龙, 李广敏. 干旱胁迫下小麦幼苗根、叶多胺含量和多胺氧化酶活性的变化[J]. 植物生态学报, 2003, 27(5): 655-660. |
| [10] | 王孟本, 李洪建, 柴宝峰. 柠条(Caragana korshinskii)的水分生理生态学特性[J]. 植物生态学报, 1996, 20(6): 494-501. |
| [11] | 徐萌, 山仑. 天机营养对春小麦抗旱适应性的影响[J]. 植物生态学报, 1991, 15(1): 79-87. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19