

植物生态学报 ›› 2021, Vol. 45 ›› Issue (9): 925-941.DOI: 10.17521/cjpe.2021.0111
• 综述 • 下一篇
收稿日期:2021-03-25
接受日期:2021-06-29
出版日期:2021-09-20
发布日期:2021-11-18
通讯作者:
王传宽
作者简介:ORCID: *王传宽: 0000-0003-3513-5426(wangck-cf@nefu.edu.cn)基金资助:
LUO Dan-Dan, WANG Chuan-Kuan( ), JIN Ying
), JIN Ying
Received:2021-03-25
Accepted:2021-06-29
Online:2021-09-20
Published:2021-11-18
Contact:
WANG Chuan-Kuan
Supported by:摘要:
干旱导致树木死亡对生态系统功能和碳平衡有重大影响。植物水分运输系统失调是引发树木死亡的主要机制。然而, 树木对干旱胁迫响应的多维性和复杂性, 使人们对植物水分运输系统在极端干旱条件下的响应以及植物死亡机理的认识还不清楚。该文首先评述衡量植物抗旱性的指标, 着重介绍可以综合评价植物干旱抗性特征的新参数——气孔安全阈值(SSM)。SSM越高, 表明气孔和水力性状之间的协调性越强, 木质部栓塞的可能性越低, 水力策略越保守。然后, 阐述木本植物应对干旱胁迫的一般响应过程。之后, 分别综述植物不同器官(叶、茎和根)对干旱胁迫的响应机制。植物达到死亡临界阈值的概率和时间, 取决于相关生理和形态学特征的相互作用。最后, 介绍木本植物水力恢复机制, 并提出3个亟待开展的研究问题: (1)改进叶片水分运输(木质部和木质部外水力导度)的测量方法, 量化4种不同途径的叶肉水分运输的相对贡献; (2)量化叶片表皮通透性变化, 以便更好地理解植物水分利用策略; (3)深入研究树木水碳耦合机制, 将个体结构和生理特征与群落/景观格局和过程相关联, 以便更好地评估和监测干旱诱导树木死亡的风险。
罗丹丹, 王传宽, 金鹰. 木本植物水力系统对干旱胁迫的响应机制. 植物生态学报, 2021, 45(9): 925-941. DOI: 10.17521/cjpe.2021.0111
LUO Dan-Dan, WANG Chuan-Kuan, JIN Ying. Response mechanisms of hydraulic systems of woody plants to drought stress. Chinese Journal of Plant Ecology, 2021, 45(9): 925-941. DOI: 10.17521/cjpe.2021.0111
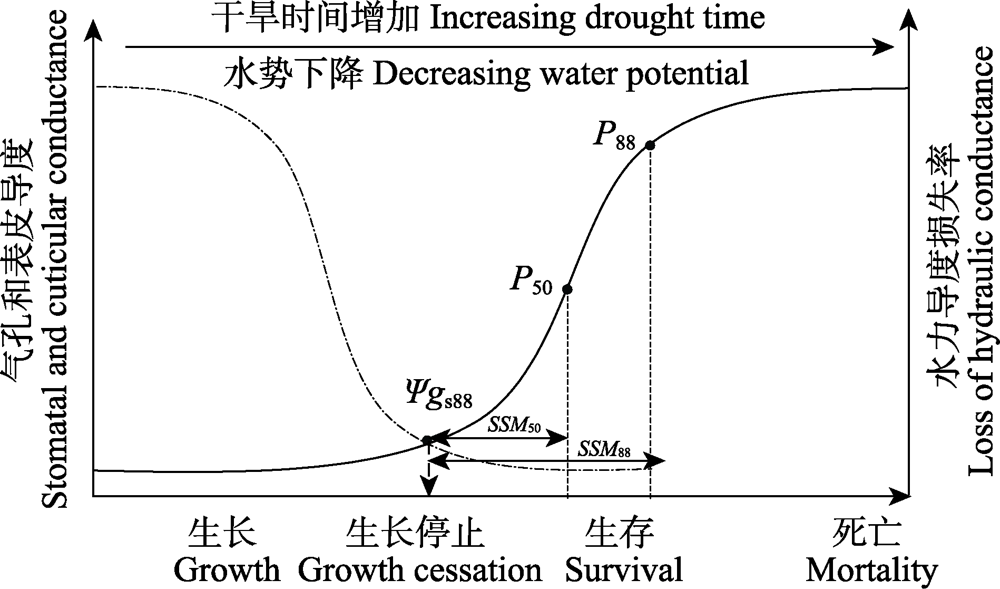
图1 植物对干旱胁迫的响应。随干旱胁迫增加, 虚线代表气孔和表皮导度变化趋势, 实线代表水力导度损失率; Ψgs88, 气孔导度下降88%的水势; P50和P88, 水力导度分别下降50%和88%的水势; SSM50和SSM88, 用Ψgs88分别减去P50和P88所计算的气孔安全阈值。
Fig. 1 Phases of drought response to drought stress in plants. With the increase of drought stress, the dotted curve represents the trend of stomatal and cuticular conductance, while the solid curve represents the loss of hydraulic conductance. Ψgs88, the water potential at 88% loss of stomatal conductance; P50 and P88, the water potential at 50% and 88% loss of hydraulic function, respectively; SSM50 and SSM88, the margins between Ψgs88 and P50 or P88, respectively.
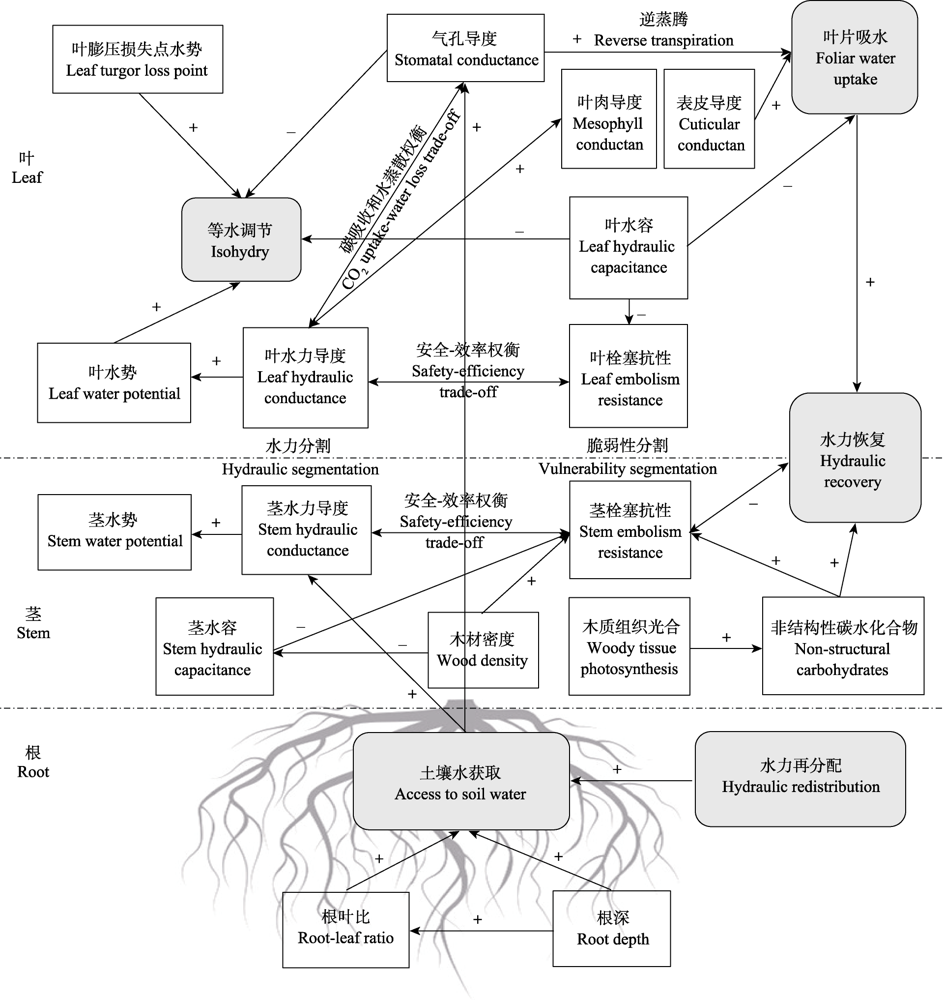
图2 植物水力学的影响因素及相互关系。图中“+”代表正相关, “-”代表负相关; 单箭头代表因果关系, 双箭头表示可能存在权衡关系; 虚线代表关系不确定。
Fig. 2 Factors influencing plant hydraulics and their correlations. “+” represents positive relationship, “-” represents negative relationship; single-ended arrows indicate causal relationship, whereas double-ended arrows indicate potential trade-off relationship; dashed lines represent uncertain relationship.
| [1] |
Adams HD, Zeppel MJB, Anderegg WRL, Hartmann H, Landhäusser SM, Tissue DT, Huxman TE, Hudson PJ, Franz TE, Allen CD, Anderegg LDL, Barron-Gafford GA, Beerling DJ, Breshears DD, Brodribb TJ, et al. (2017). A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature Ecology Evolution, 1, 1285-1291.
DOI URL |
| [2] |
Albuquerque C, Scoffoni C, Brodersen CR, Buckley TN, Sack L, McElrone AJ (2020). Coordinated decline of leaf hydraulic and stomatal conductances under drought is not linked to leaf xylem embolism for different grapevine cultivars. Journal of Experimental Botany, 71, 7286-7300.
DOI PMID |
| [3] | Allen CD, Breshears DD, McDowell NG (2015). On under- estimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere, 6, 1-55. |
| [4] |
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012). The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences of the United States of America, 109, 233-237.
DOI PMID |
| [5] |
Anderegg WRL, Flint A, Huang CY, Flint L, Berry JA, Davis FW, Sperry JS, Field CB (2015). Tree mortality predicted from drought-induced vascular damage. Nature Geoscience, 8, 367-371.
DOI URL |
| [6] |
Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AF, Choat B, Jansen S (2016). Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought- induced tree mortality across the globe. Proceedings of the National Academy of Sciences of the United States of America, 113, 5024-5029.
DOI PMID |
| [7] |
Ávila E, Herrera A, Tezara W (2014). Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species. Photosynthetica, 52, 3-15.
DOI URL |
| [8] | Barnard DM, Meinzer FC, Lachenbruch B, McCulloh KA, Johnson DM, Woodruff DR (2011). Climate-related trends in sapwood biophysical properties in two conifers: avoidance of hydraulic dysfunction through coordinated adjustments in xylem efficiency, safety and capacitance. Plant, Cell & Environment, 34, 643-654. |
| [9] |
Bartlett MK, Klein T, Jansen S, Choat B, Sack L (2016). The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proceedings of the National Academy of Sciences of the United States of America, 113, 13098-13103.
PMID |
| [10] | Berry ZC, Emery NC, Gotsch SG, Goldsmith GR (2019a). Foliar water uptake: processes, pathways, and integration into plant water budgets. Plant, Cell & Environment, 42, 410-423. |
| [11] |
Berry ZC, Espejel X, Williams-Linera G, Asbjornsen H (2019b). Linking coordinated hydraulic traits to drought and recovery responses in a tropical montane cloud forest. American Journal of Botany, 106, 1316-1326.
DOI URL |
| [12] |
Berry ZC, White JC, Smith WK (2014). Foliar uptake, carbon fluxes and water status are affected by the timing of daily fog in saplings from a threatened cloud forest. Tree Physiology, 34, 459-470.
DOI URL |
| [13] | Binks O, Coughlin I, Mencuccini M, Meir P (2020). Equivalence of foliar water uptake and stomatal conductance? Plant, Cell & Environment, 43, 524-528. |
| [14] |
Binks O, Mencuccini M, Rowland L, da Costa ACL, de Carvalho CJR, Bittencourt P, Eller C, Teodoro GS, Carvalho EJM, Soza A, Ferreira L, Vasconcelos SS, Oliveira R, Meir P,(2019). Foliar water uptake in Amazonian trees: evidence and consequences. Global Change Biology, 25, 2678-2690.
DOI URL |
| [15] |
Blackman CJ, Brodribb TJ, Jordan GJ (2012). Leaf hydraulic vulnerability influences species' bioclimatic limits in a diverse group of woody angiosperms. Oecologia, 168, 1-10.
DOI PMID |
| [16] |
Boanares D, Ferreira BG, Kozovits AR, Sousa HC, Isaias RMS, França MGC (2018). Pectin and cellulose cell wall composition enables different strategies to leaf water uptake in plants from tropical fog mountain. Plant Physiology and Biochemistry, 122, 57-64.
DOI PMID |
| [17] |
Brodersen CR, McElrone AJ (2013). Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Frontiers in Plant Science, 4, 108. DOI: 10.3389/fpls.2013.00108.
DOI PMID |
| [18] |
Brodribb TJ, Bowman DJMS, Nichols S, Delzon S, Burlett R (2010). Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist, 188, 533-542.
DOI PMID |
| [19] |
Brodribb TJ, Cochard H (2009). Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology, 149, 575-584.
DOI PMID |
| [20] |
Brodribb TJ, Feild TS, Jordan GJ (2007). Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology, 144, 1890-1898.
PMID |
| [21] |
Brodribb TJ, McAdam SAM (2011). Passive origins of stomatal control in vascular plants. Science, 331, 582-585.
DOI PMID |
| [22] |
Brodribb TJ, McAdam SAM (2017). Evolution of the stomatal regulation of plant water content. Plant Physiology, 174, 639-649.
DOI PMID |
| [23] |
Brodribb TJ, McAdam SAM, Jordan GJ, Martins SCV (2014). Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proceedings of the National Academy of Sciences of the United States of America, 111, 14489-14493.
DOI PMID |
| [24] |
Buckley TN (2019). How do stomata respond to water status? New Phytologist, 224, 21-36.
DOI PMID |
| [25] |
Buckley TN, John GP, Scoffoni C, Sack L (2015). How does leaf anatomy influence water transport outside the xylem? Plant Physiology, 168, 1616-1635.
DOI PMID |
| [26] |
Cardoso AA, Randall JM, McAdam SAM (2019). Hydraulics regulate stomatal responses to changes in leaf water status in the fern Athyrium filix-femina. Plant Physiology, 179, 533-543.
DOI PMID |
| [27] | Cassana FF, Eller CB, Oliveira RS, Dillenburg LR (2016). Effects of soil water availability on foliar water uptake of Araucaria angustifolia. Plant and Soil, 399, 147-157. |
| [28] |
Cernusak LA, Cheesman AW (2015). The benefits of recycling: How photosynthetic bark can increase drought tolerance. New Phytologist, 208, 995-997.
DOI PMID |
| [29] | Chen WL, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016). Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proceedings of the National Academy of Sciences of the United States of America, 113, 8741-8746. |
| [30] |
Chen ZC, Li S, Luan JW, Zhang YT, Zhu SD, Wan XC, Liu SR (2019). Prediction of temperate broadleaf tree species mortality in arid limestone habitats with stomatal safety margins. Tree Physiology, 39, 1428-1437.
DOI URL |
| [31] |
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018). Triggers of tree mortality under drought. Nature, 558, 531-539.
DOI URL |
| [32] |
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez-Vilalta J, Mayr S, et al. (2012). Global convergence in the vulnerability of forests to drought. Nature, 491, 752-755.
DOI URL |
| [33] |
Clark JS, Iverson L, Woodall CW, Allen CD, Bell DM, Bragg DC, D'Amato AW, Davis FW, Hersh MH, Ibanez I, Jackson ST, Matthews S, Pederson N, Peters M, Schwartz MW et al. (2016). The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Global Change Biology, 22, 2329-2352.
DOI URL |
| [34] | Creek D, Blackman C, Brodribb TJ, Choat B, Tissue DT (2018). Coordination between leaf, stem and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant, Cell & Environment, 41, 2869- 2881. |
| [35] |
Creek D, Lamarque LJ, Torres-Ruiz JM, Parise C, Burlett R, Tissue DT, Delzon S (2020). Xylem embolism in leaves does not occur with open stomata: evidence from direct observations using the optical visualization technique. Journal of Experimental Botany, 71, 1151-1159.
DOI URL |
| [36] |
Dawson TE, Goldsmith GR (2018). The value of wet leaves. New Phytologist, 219, 1156-1169.
DOI PMID |
| [37] |
De Baerdemaeker NJF, Salomón RL, De Roo L, Steppe K (2017). Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytologist, 216, 720-727.
DOI PMID |
| [38] |
De Guzman ME, Acosta-Rangel A, Winter K, Meinzer FC, Bonal D, Santiago LS (2021). Hydraulic traits of Neotropical canopy liana and tree species across a broad range of wood density: implications for predicting drought mortality with models. Tree Physiology, 41, 24-34.
DOI URL |
| [39] |
De Roo L, Salomón RL, Oleksyn J, Steppe K (2020a). Woody tissue photosynthesis delays drought stress in Populus tremula trees and maintains starch reserves in branch xylem tissues. New Phytologist, 228, 70-81.
DOI URL |
| [40] | De Roo L, Salomón RL, Steppe K (2020b). Woody tissue photosynthesis reduces stem CO2 efflux by half and remains unaffected by drought stress in young Populus tremula trees. Plant, Cell & Environment, 43, 981-991. |
| [41] |
Domec JC, Warren JM, Meinzer FC, Brooks JR, Coulombe R (2004). Native root xylem embolism and stomatal closure in stands of Douglas-fir and ponderosa pine: mitigation by hydraulic redistribution. Oecologia, 141, 7-16.
PMID |
| [42] |
Duursma RA, Blackman CJ, Lopéz R, Martin-StPaul NK, Cochard H, Medlyn BE (2019). On the minimum leaf conductance: Its role in models of plant water use, and ecological and environmental controls. New Phytologist, 221, 693-705.
DOI PMID |
| [43] |
Eller CB, Lima AL, Oliveira RS (2016). Cloud forest trees with higher foliar water uptake capacity and anisohydric behavior are more vulnerable to drought and climate change. New Phytologist, 211, 489-501.
DOI URL |
| [44] |
Flexas J, Carriquí M, Nadal M (2018). Gas exchange and hydraulics during drought in crops: Who drives whom? Journal of Experimental Botany, 69, 3791-3795.
DOI PMID |
| [45] |
Fort F, Cruz P, Lecloux E, Bittencourt de Oliveira L, Stroia C, Theau JP, Jouany C, Pugnaire F (2016). Grassland root functional parameters vary according to a community-level resource acquisition-conservation trade-off. Journal of Vegetation Science, 27, 749-758.
DOI URL |
| [46] |
Fort F, Volaire F, Guilioni L, Barkaoui K, Navas ML, Roumet C (2017). Root traits are related to plant water-use among rangeland Mediterranean species. Functional Ecology, 31, 1700-1709.
DOI URL |
| [47] |
Fu XL, Meinzer FC (2019). Metrics and proxies for stringency of regulation of plant water status (iso/anisohydry): a global data set reveals coordination and trade-offs among water transport traits. Tree Physiology, 39, 122-134.
DOI URL |
| [48] | Fu XL, Meinzer FC, Woodruff DR, Liu YY, Smith DD, McCulloh KA, Howard AR (2019). Coordination and trade-offs between leaf and stem hydraulic traits and stomatal regulation along a spectrum of isohydry to anisohydry. Plant, Cell & Environment, 42, 2245-2258. |
| [49] | Fuenzalida TI, Bryant CJ, Ovington LI, Yoon HJ, Oliveira RS, Sack L, Ball MC (2019). Shoot surface water uptake enables leaf hydraulic recovery in Avicennia marina. New Phytologist, 224, 1504-1511. |
| [50] |
Garcia-Forner N, Sala AN, Biel C, Savé R, Martínez-Vilalta J (2016). Individual traits as determinants of time to death under extreme drought in Pinus sylvestris L. Tree Physiology, 36, 1196-1209.
PMID |
| [51] |
Goldsmith GR, Lehmann MM, Cernusak LA, Arend M, Siegwolf RTW (2017). Inferring foliar water uptake using stable isotopes of water. Oecologia, 184, 763-766.
DOI PMID |
| [52] |
Gotsch SG, Nadkarni N, Darby A, Glunk A, Dix M, Davidson K, Dawson TE (2015). Life in the treetops: ecophysiological strategies of canopy epiphytes in a tropical montane cloud forest. Ecological Monographs, 85, 393-412.
DOI URL |
| [53] |
Greenwood S, Ruiz-Benito P, Martínez-Vilalta J, Lloret F, Kitzberger T, Allen CD, Fensham R, Laughlin DC, Kattge J, Bönisch G, Kraft NJB, Jump AS (2017). Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecology Letters, 20, 539-553.
DOI PMID |
| [54] |
Hartmann H, Link RM, Schuldt B (2021). A whole-plant perspective of isohydry: stem-level support for leaf-level plant water regulation. Tree Physiology, 41, 901-905.
DOI PMID |
| [55] |
Hochberg U, Windt CW, Ponomarenko A, Zhang YJ, Gersony J, Rockwell FE, Holbrook NM (2017). Stomatal closure, basal leaf embolism, and shedding protect the hydraulic integrity of grape stems. Plant Physiology, 174, 764-775.
DOI PMID |
| [56] |
Hoffmann WA, Marchin RM, Abit P, Lau OL (2011). Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Change Biology, 17, 2731-2742.
DOI URL |
| [57] |
Ishida A, Nakano T, Yazaki K, Matsuki S, Koike N, Lauenstein DL, Shimizu M, Yamashita N (2008). Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia, 156, 193-202.
DOI URL |
| [58] |
Jiang PP, Meinzer FC, Fu XL, Kou L, Dai XQ, Wang HM (2021). Trade-offs between xylem water and carbohydrate storage among 24 coexisting subtropical understory shrub species spanning a spectrum of isohydry. Tree Physiology, 41, 403-415.
DOI URL |
| [59] |
Jin Y, Wang CK, Zhou ZH (2016). Mechanisms of xylem embolism repair in woody plants: research progress and questions. Chinese Journal of Plant Ecology, 40, 834-846.
DOI URL |
|
[ 金鹰, 王传宽, 周正虎 (2016). 木本植物木质部栓塞修复机制: 研究进展与问题. 植物生态学报, 40, 834-846.]
DOI |
|
| [60] |
Jin Y, Wang CK, Zhou ZH (2019). Conifers but not angiosperms exhibit vulnerability segmentation between leaves and branches in a temperate forest. Tree Physiology, 39, 454-462.
DOI URL |
| [61] |
Jin Y, Wang CK, Zhou ZH, Li ZM (2016). Co-ordinated performance of leaf hydraulics and economics in 10 Chinese temperate tree species. Functional Plant Biology, 43, 1082- 1090.
DOI URL |
| [62] | Johnson DM, Domec JC, Carter Berry Z, Schwantes AM, McCulloh KA, Woodruff DR, Wayne Polley H, Wortemann R, Swenson JJ, Scott Mackay D, McDowell NG, Jackson RB (2018). Co-occurring woody species have diverse hydraulic strategies and mortality rates during an extreme drought. Plant, Cell & Environment, 41, 576-588. |
| [63] |
Johnson DM, Wortemann R, McCulloh KA, Jordan-Meille L, Ward E, Warren JM, Palmroth S, Domec JC (2016). A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiology, 36, 983-993.
DOI PMID |
| [64] |
Klein T (2014). The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Functional Ecology, 28, 1313-1320.
DOI URL |
| [65] | Klein T, Zeppel MJB, Anderegg WRL, Bloemen J, Kauwe MG, Hudson P, Ruehr NK, Powell TL, Arx G, Nardini A (2018). Xylem embolism refilling and resilience against drought- induced mortality in woody plants: processes and trade- offs. Ecological Research, 33, 839-855. |
| [66] |
Lanning M, Wang LX, Novick KA (2020). The importance of cuticular permeance in assessing plant water-use strategies. Tree Physiology, 40, 425-432.
DOI URL |
| [67] |
Laur J, Hacke UG (2014). Exploring Picea glauca aquaporins in the context of needle water uptake and xylem refilling. New Phytologist, 203, 388-400.
DOI URL |
| [68] |
Lechthaler S, Colangeli P, Gazzabin M, Anfodillo T (2019). Axial anatomy of the leaf midrib provides new insights into the hydraulic architecture and cavitation patterns of Acer pseudoplatanus leaves. Journal of Experimental Botany, 70, 6195-6201.
DOI PMID |
| [69] |
Lehmann MM, Goldsmith GR, Schmid L, Gessler A, Saurer M, Siegwolf RTW (2018). The effect of 18O-labelled water vapour on the oxygen isotope ratio of water and assimilates in plants at high humidity. New Phytologist, 217, 105-116.
DOI PMID |
| [70] | Leigh A, Sevanto S, Close JD, Nicotra AB (2017). The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant, Cell & Environment, 40, 237-248. |
| [71] |
Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S (2011). Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist, 190, 709-723.
DOI URL |
| [72] |
Li XM, Blackman CJ, Peters JMR, Choat B, Rymer PD, Medlyn BE, Tissue DT (2019). More than iso/anisohydry: hydroscapes integrate plant water use and drought tolerance traits in 10 eucalypt species from contrasting climates. Functional Ecology, 33, 1035-1049.
DOI URL |
| [73] |
Liang XY, Ye Q, Liu H, Brodribb TJ (2021). Wood density predicts mortality threshold for diverse trees. New Phytologist, 229, 3053-3057.
DOI URL |
| [74] | Liu JX, Gu L, Yu YC, Huang P, Wu ZG, Zhang Q, Qian YQ, Wan XC, Sun ZY (2019a). Corticular photosynthesis drives bark water uptake to refill embolized vessels in dehydrated branches of Salix matsudana. Plant, Cell & Environment, 42, 2584-2596. |
| [75] |
Liu XR, Liu H, Gleason SM, Goldstein G, Zhu SD, He PC, Hou H, Li RH, Ye Q (2019b). Water transport from stem to stomata: the coordination of hydraulic and gas exchange traits across 33 subtropical woody species. Tree Physiology, 39, 1665-1674.
DOI URL |
| [76] |
López R, Cano FJ, Martin-StPaul NK, Cochard H, Choat B (2021). Coordination of stem and leaf traits define different strategies to regulate water loss and tolerance ranges to aridity. New Phytologist, 230, 497-509.
DOI URL |
| [77] | Luo DD, Wang CK, Jin Y (2017). Plant water-regulation strategies: isohydric versus anisohydric behavior. Chinese Journal of Plant Ecology, 41, 1021-1032. |
|
[ 罗丹丹, 王传宽, 金鹰 (2017). 植物水分调节对策: 等水与非等水行为. 植物生态学报, 41, 1020-1032.]
DOI |
|
| [78] | Luo DD, Wang CK, Jin Y (2019). Stomatal regulation of plants in response to drought stress. Chinese Journal of Applied Ecology, 30, 4333-4343. |
| [ 罗丹丹, 王传宽, 金鹰 (2019). 植物应对干旱胁迫的气孔调节. 应用生态学报, 30, 4333-4343.] | |
| [79] |
Martin-Stpaul N, Delzon S, Cochard H (2017). Plant resistance to drought depends on timely stomatal closure. Ecology Letters, 20, 1437-1447.
DOI PMID |
| [80] |
Martínez-Vilalta J, Anderegg WRL, Sapes G, Sala AN (2019). Greater focus on water pools may improve our ability to understand and anticipate drought-induced mortality in plants. New Phytologist, 223, 22-32.
DOI URL |
| [81] | Martínez-Vilalta J, Garcia-Forner N (2017). Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell & Environment, 40, 962-976. |
| [82] | Martins SCV, McAdam SAM, Deans RM, DaMatta FM, Brodribb TJ (2016). Stomatal dynamics are limited by leaf hydraulics in ferns and conifers: results from simultaneous measurements of liquid and vapour fluxes in leaves. Plant, Cell & Environment, 39, 694-705. |
| [83] | Mason Earles J, Sperling O, Silva LCR, McElrone AJ, Brodersen CR, North MP, Zwieniecki MA (2016). Bark water uptake promotes localized hydraulic recovery in coastal redwood crown. Plant, Cell & Environment, 39, 320-328. |
| [84] | McCulloh KA, Domec JC, Johnson DM, Smith DD, Meinzer FC (2019). A dynamic yet vulnerable pipeline: integration and coordination of hydraulic traits across whole plants. Plant, Cell & Environment, 42, 2789-2807. |
| [85] | McCulloh KA, Johnson DM, Meinzer FC, Woodruff DR (2014). The dynamic pipeline: hydraulic capacitance and xylem hydraulic safety in four tall conifer species. Plant, Cell & Environment, 37, 1171-1183. |
| [86] |
McDowell NG (2011). Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology, 155, 1051-1059.
DOI PMID |
| [87] |
McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011). The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology & Evolution, 26, 523-532.
DOI URL |
| [88] |
McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178, 719-739.
DOI PMID |
| [89] |
McDowell NG, Ryan MG, Zeppel MJB, Tissue DT (2013). Feature: improving our knowledge of drought-induced forest mortality through experiments, observations, and modeling. New Phytologist, 200, 289-293.
DOI PMID |
| [90] |
Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR (2009). Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Functional Ecology, 23, 922-930.
DOI URL |
| [91] | Meinzer FC, Smith DD, Woodruff DR, Marias DE, McCulloh KA, Howard AR, Magedman AL (2017). Stomatal kinetics and photosynthetic gas exchange along a continuum of isohydric to anisohydric regulation of plant water status. Plant, Cell & Environment, 40, 1618-1628. |
| [92] |
Meinzer FC, Woodruff DR, Marias DE, Smith DD, McCulloh KA, Howard AR, Magedman AL (2016). Mapping “hydroscapes” along the iso- to anisohydric continuum of stomatal regulation of plant water status. Ecology Letters, 19, 1343-1352.
DOI URL |
| [93] |
Mitchell PJ, O'Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA (2013). Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist, 197, 862-872.
DOI PMID |
| [94] | Nardini A, Savi T, Trifilò P, Lo Gullo MA,(2018). Drought stress and the recovery from xylem embolism in woody plants. Progress in Botany, 79, 197-231. |
| [95] |
Ogasa M, Miki NH, Murakami Y, Yoshikawa K (2013). Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiology, 33, 335-344.
DOI URL |
| [96] | Pivovaroff AL, Cook VMW, Santiago LS (2018). Stomatal behaviour and stem xylem traits are coordinated for woody plant species under exceptional drought conditions. Plant, Cell & Environment, 41, 2617-2626. |
| [97] |
Pivovaroff AL, Pasquini SC, De Guzman ME, Alstad KP, Stemke JS, Santiago LS (2016). Multiple strategies for drought survival among woody plant species. Functional Ecology, 30, 517-526.
DOI URL |
| [98] |
Pivovaroff AL, Sack L, Santiago LS (2014). Coordination of stem and leaf hydraulic conductance in southern California shrubs: a test of the hydraulic segmentation hypothesis. New Phytologist, 203, 842-850.
DOI URL |
| [99] |
Powell TL, Wheeler JK, de Oliveira AAR, da Costa ACL, Saleska SR, Meir P, Moorcroft PR,(2017). Differences in xylem and leaf hydraulic traits explain differences in drought tolerance among mature Amazon rainforest trees. Global Change Biology, 23, 4280-4293.
DOI URL |
| [100] | Pratt RB, Jacobsen AL (2017). Conflicting demands on angiosperm xylem: tradeoffs among storage, transport and biomechanics. Plant, Cell & Environment, 40, 897-913. |
| [101] | Roddy AB, Simonin KA, McCulloh KA, Brodersen CR, Dawson TE (2018). Water relations of Calycanthus flowers: hydraulic conductance, capacitance, and embolism resistance. Plant, Cell & Environment, 41, 2250-2262. |
| [102] |
Rodriguez-Dominguez CM, Carins Murphy MR, Lucani C, Brodribb TJ (2018). Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytologist, 218, 1025-1035.
DOI PMID |
| [103] |
Roman DT, Novick KA, Brzostek ER, Dragoni D, Rahman F, Phillips RP (2015). The role of isohydric and anisohydric species in determining ecosystem-scale response to severe drought. Oecologia, 179, 641-654.
DOI PMID |
| [104] | Sack L, Ball MC, Brodersen C, Davis SD, Des Marais DL, Donovan LA, Givnish TJ, Hacke UG, Huxman T, Jansen S, Jacobsen AL, Johnson DM, Koch GW, Maurel C, McCulloh KA, et al. (2016). Plant hydraulics as a central hub integrating plant and ecosystem function: meeting report for “Emerging Frontiers in Plant Hydraulics” (Washington DC, May 2015). Plant, Cell & Environment, 39, 2085-2094. |
| [105] | Salomón RL, Steppe K, Ourcival JM, Villers S, Rodríguez- Calcerrada J, Schapman R, Limousin JM (2020). Hydraulic acclimation in a Mediterranean oak subjected to permanent throughfall exclusion results in increased stem hydraulic capacitance. Plant, Cell & Environment, 43, 1528-1544. |
| [106] | Saveyn A, Steppe K, Ubierna N, Dawson TE (2010). Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant, Cell & Environment, 33, 1949-1958. |
| [107] |
Savi T, Casolo V, Luglio J, Bertuzzi S, Trifilo P, Lo Gullo MA, Nardini A (2016). Species-specific reversal of stem xylem embolism after a prolonged drought correlates to endpoint concentration of soluble sugars. Plant Physiology Biochemistry, 106, 198-207.
DOI URL |
| [108] |
Schmitz N, Egerton J, Lovelock C, Ball M (2012). Light- dependent maintenance of hydraulic function in mangrove branches: Do xylary chloroplasts play a role in embolism repair? New Phytologist, 195, 40-46.
DOI PMID |
| [109] |
Scoffoni C, Albuquerque C, Brodersen CR, Townes SV, John GP, Bartlett MK, Buckley TN, McElrone AJ, Sack L (2017). Outside-xylem vulnerability, not xylem embolism, controls leaf hydraulic decline during dehydration. Plant Physiology, 173, 1197-1210.
DOI URL |
| [110] | Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT (2014). How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell & Environment, 37, 153-161. |
| [111] |
Skelton RP, Dawson TE, Thompson SE, Shen YZ, Weitz AP, Ackerly D (2018). Low vulnerability to xylem embolism in leaves and stems of north American oaks. Plant Physiology, 177, 1066-1077.
DOI PMID |
| [112] |
Skelton RP, West AG, Dawson TE (2015). Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proceedings of the National Academy of Sciences of the United States of America, 112, 5744-5749.
DOI PMID |
| [113] | Sperry JS, Meinzer FC, McCullon KA (2008). Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant, Cell & Environment, 31, 632-645. |
| [114] |
Steppe K, Vandegehuchte MW, van de Wal BAE, Hoste P, Guyot A, Lovelock CE, Lockington DA (2018). Direct uptake of canopy rainwater causes turgor-driven growth spurts in the mangrove Avicennia marina. Tree Physiology, 38, 979-991.
DOI PMID |
| [115] |
Taneda H, Sperry JS (2008). A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiology, 28, 1641-1651.
DOI URL |
| [116] |
Tardieu F, Simonneau T (1998). Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany, 49, 419-432.
DOI URL |
| [117] |
Tomasella M, Casolo V, Aichner N, Petruzzellis F, Savi T, Trifilò P, Nardini A (2019a). Non-structural carbohydrate and hydraulic dynamics during drought and recovery in Fraxinus ornus and Ostrya carpinifolia saplings. Plant Physiology Biochemistry, 145, 1-9.
DOI URL |
| [118] |
Tomasella M, Häberle KH, Nardini A, Hesse B, Machlet A, Matyssek R (2017). Post-drought hydraulic recovery is accompanied by non-structural carbohydrate depletion in the stem wood of Norway spruce saplings. Scientific Reports, 7, 14308. DOI: 10.1038/s41598-017-14645-w.
DOI PMID |
| [119] | Tomasella M, Nardini A, Hesse BD, Machlet A, Matyssek R, Häberle KH (2019b). Close to the edge: effects of repeated severe drought on stem hydraulics and non-structural carbohydrates in European beech saplings. Tree Physiology, 39, 717-728. |
| [120] |
Tombesi S, Nardini A, Farinelli D, Palliotti A (2014). Relationships between stomatal behavior, xylem vulnerability to cavitation and leaf water relations in two cultivars of Vitis vinifera. Physiologia Plantarum, 152, 453-464.
DOI URL |
| [121] |
Tombesi S, Nardini A, Frioni T, Soccolini M, Zadra C, Farinelli D, Poni S, Palliotti A (2015). Stomatal closure is induced by hydraulic signals and maintained by ABA in drought- stressed grapevine. Scientific Reports, 5, 12449. DOI: 10.1038/srep12449.
DOI PMID |
| [122] |
Trifilò P, Casolo V, Raimondo F, Petrussa E, Boscutti F, Lo Gullo MA, Nardini A (2017). Effects of prolonged drought on stem non-structural carbohydrates content and post- drought hydraulic recovery in Laurus nobilis L.: the possible link between carbon starvation and hydraulic failure. Plant Physiology Biochemistry, 120, 232-241.
DOI URL |
| [123] |
Trifilò P, Kiorapostolou N, Petruzzellis F, Vitti S, Petit G, Lo Gullo MA, Nardini A, Casolo V (2019). Hydraulic recovery from xylem embolism in excised branches of twelve woody species: relationships with parenchyma cells and non-structural carbohydrates. Plant Physiology and Biochemistry, 139, 513-520.
DOI URL |
| [124] |
Trifilò P, Nardini A, Lo Gullo MA, Barbera PM, Savi T, Raimondo F (2015). Diurnal changes in embolism rate in nine dry forest trees: relationships with species-specific xylem vulnerability, hydraulic strategy and wood traits. Tree Physiology, 35, 694-705.
DOI URL |
| [125] |
Trifiló P, Raimondo F, Savi T, Lo Gullo MA, Nardini A (2016). The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. Journal of Experimental Botany, 67, 5029-5039.
DOI URL |
| [126] |
Tyree MT, Ewers FW (1991). The hydraulic architecture of trees and other woody plants. New Phytologist, 119, 345-360.
DOI URL |
| [127] |
Tyree MT, Sperry JS (1988). Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant physiology, 88, 574-580.
PMID |
| [128] | Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S (2013). Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology, 33, 672-683. |
| [129] |
Vandegehuchte MW, Bloemen J, Vergeynst LL, Steppe K (2015). Woody tissue photosynthesis in trees: salve on the wounds of drought? New Phytologist, 208, 998-1002.
DOI PMID |
| [130] |
Venturas MD, Sperry JS, Hacke UG (2017). Plant xylem hydraulics: What we understand, current research, and future challenges. Journal of Integrative Plant Biology, 59, 356- 389.
DOI |
| [131] |
Vesala T, Sevanto S, Grönholm T, Salmon Y, Nikinmaa E, Hari P, Hölttä T (2017). Effect of leaf water potential on internal humidity and CO2 dissolution: reverse transpiration and improved water use efficiency under negative pressure. Frontiers in Plant Science, 8, 54. DOI: 10.3389/ fpls.2017.00054.
DOI PMID |
| [132] |
Wang AY, Han SJ, Zhang JH, Wang M, Yin XH, Fang LD, Yang D, Hao GY (2018a). The interaction between nonstructural carbohydrate reserves and xylem hydraulics in Korean pine trees across an altitudinal gradient. Tree physiology, 38, 1792-1804.
DOI URL |
| [133] |
Wang XX, Du TT, Huang JL, Peng SB, Xiong DL (2018b). Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice. Journal of Experimental Botany, 69, 4033-4045.
DOI URL |
| [134] | Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM (2013). Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant, Cell & Environment, 36, 1938-1949. |
| [135] |
Yang B, Wen XF, Sun XM (2015). Seasonal variations in depth of water uptake for a subtropical coniferous plantation subjected to drought in an East Asian monsoon region. Agricultural and Forest Meteorology, 201, 218-228.
DOI URL |
| [136] |
Yoshimura K, Saiki ST, Yazaki K, Ogasa MY, Shirai M, Nakano T, Yoshimura J, Ishida A (2016). The dynamics of carbon stored in xylem sapwood to drought-induced hydraulic stress in mature trees. Scientific Reports, 6, 24513. DOI: 10.1038/srep24513.
DOI PMID |
| [137] |
Zeppel MJB, Harrison SP, Adams HD, Kelley DI, Li G, Tissue DT, Dawson TE, Fensham R, Medlyn BE, Palmer A, West AG, McDowell NG (2015). Drought and resprouting plants. New Phytologist, 206, 583-589.
DOI URL |
| [138] |
Zhang YJ, Rockwell FE, Graham AC, Alexander T, Holbrook NM (2016). Reversible leaf xylem collapse: a potential “circuit breaker” against cavitation. Plant Physiology, 172, 2261-2274.
DOI URL |
| [139] |
Zhu SD, Liu H, Xu QY, Cao KF, Ye Q (2016). Are leaves more vulnerable to cavitation than branches? Functional Ecology, 30, 1740-1744.
DOI URL |
| [140] |
Zwieniecki MA, Holbrook NM (2009). Confronting Maxwell's demon: biophysics of xylem embolism repair. Trends in Plant Science, 14, 530-534.
DOI PMID |
| [1] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [2] | 任金培, 李俊鹏, 王卫锋, 代永欣, 王林. 八个树种叶水力性状对水分条件的响应及其驱动因素[J]. 植物生态学报, 2021, 45(9): 942-951. |
| [3] | 罗丹丹, 王传宽, 金鹰. 植物水分调节对策: 等水与非等水行为[J]. 植物生态学报, 2017, 41(9): 1020-1032. |
| [4] | 陈志成, 万贤崇. 虫害叶损失造成的树木非结构性碳减少与树木生长、死亡的关系研究进展[J]. 植物生态学报, 2016, 40(9): 958-968. |
| [5] | 金鹰, 王传宽, 周正虎. 木本植物木质部栓塞修复机制: 研究进展与问题[J]. 植物生态学报, 2016, 40(8): 834-846. |
| [6] | 金鹰, 王传宽. 九种不同材性的温带树种叶水力性状及其权衡关系[J]. 植物生态学报, 2016, 40(7): 702-710. |
| [7] | 李荣, 姜在民, 张硕新, 蔡靖. 木本植物木质部栓塞脆弱性研究新进展[J]. 植物生态学报, 2015, 39(8): 838-848. |
| [8] | 安东升, 曹娟, 黄小华, 周娟, 窦美安. 基于Lake模型的叶绿素荧光参数在甘蔗苗期抗旱性研究中的应用[J]. 植物生态学报, 2015, 39(4): 398-406. |
| [9] | 金鹰, 王传宽. 植物叶片水力与经济性状权衡关系的研究进展[J]. 植物生态学报, 2015, 39(10): 1021-1032. |
| [10] | 邱权,潘昕,李吉跃,王军辉,马建伟,杜坤. 青藏高原20种灌木抗旱形态和生理特征[J]. 植物生态学报, 2014, 38(6): 562-575. |
| [11] | 刘华伟, 林晓军, 孙超, 李强, 杨呼, 郭蔼光. 接种两种固氮菌增强小麦幼苗抗渗透胁迫及生长能力[J]. 植物生态学报, 2013, 37(1): 70-79. |
| [12] | 李涛, 陈保冬. 丛枝菌根真菌通过上调根系及自身水孔蛋白基因表达提高玉米抗旱性[J]. 植物生态学报, 2012, 36(9): 973-981. |
| [13] | 张智猛, 万书波, 戴良香, 宋文武, 陈静, 石运庆. 花生抗旱性鉴定指标的筛选与评价[J]. 植物生态学报, 2011, 35(1): 100-109. |
| [14] | 黄菊莹, 余海龙, 张硕新. 施水和钾素添加对元宝枫和女贞木质部栓塞的影响[J]. 植物生态学报, 2009, 33(6): 1199-1207. |
| [15] | 贺学礼, 张焕仕, 赵丽莉. 不同土壤中水分胁迫和AM真菌对油蒿抗旱性的影响[J]. 植物生态学报, 2008, 32(5): 994-1001. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19