

植物生态学报 ›› 2011, Vol. 35 ›› Issue (4): 441-451.DOI: 10.3724/SP.J.1258.2011.00441
薛伟1,2,3,4, 李向义1,3,4,*( ), 林丽莎1,3,4, 王迎菊1,2,3,4, 李磊1,2,3,4
), 林丽莎1,3,4, 王迎菊1,2,3,4, 李磊1,2,3,4
收稿日期:2010-11-05
接受日期:2011-01-21
出版日期:2011-11-05
发布日期:2011-04-13
通讯作者:
李向义
作者简介:*E-mail: lixy@ms.xjb.ac.cn
XUE Wei1,2,3,4, LI Xiang-Yi1,3,4,*( ), LIN Li-Sha1,3,4, WANG Ying-Ju1,2,3,4, LI Lei1,2,3,4
), LIN Li-Sha1,3,4, WANG Ying-Ju1,2,3,4, LI Lei1,2,3,4
Received:2010-11-05
Accepted:2011-01-21
Online:2011-11-05
Published:2011-04-13
Contact:
LI Xiang-Yi
摘要:
生长在温带沙漠地区的植物在夏季时常遭受正午短时间的高温胁迫, 频繁和骤然的热胁迫在很大程度上限制了荒漠植物的光合作用。以塔克拉玛干沙漠南缘防风固沙的优势植物疏叶骆驼刺(Alhagi sparsifolia)为材料, 分别用叶绿素荧光诱导动力学和CO2响应方法分析热胁迫后光系统II (PSII)和RuBP羧化酶的热稳定性。结果表明: (1)在叶片温度超过43 ℃后PSII最大光化学量子产量、有活性反应中心数目、活力指数均出现明显的降低; 中高温度下PSII的电子供体侧比电子受体侧组分更容易受到热胁迫的伤害; 在58 ℃出现明显的K点(300 μs), 说明放氧复合体放氧结构受到破坏而失去活性。(2)随着叶片温度的上升, Rubisco活性先升高后降低, 在34 ℃时具有最高的活性水平。(3)叶片受到高温胁迫时, 细胞内氨态氮和活性氧分子等大量积累。(4)疏叶骆驼刺叶片处于短时间的高温环境时, 光合作用的光反应和暗反应阶段均表现出功能的不稳定性, 其中PSII和Rubisco是主要的热敏感位点。
薛伟, 李向义, 林丽莎, 王迎菊, 李磊. 短时间热胁迫对疏叶骆驼刺光系统II、Rubisco活性和活性氧化剂的影响. 植物生态学报, 2011, 35(4): 441-451. DOI: 10.3724/SP.J.1258.2011.00441
XUE Wei, LI Xiang-Yi, LIN Li-Sha, WANG Ying-Ju, LI Lei. Effects of short time heat stress on photosystem II, Rubisco activities and oxidative radicals in Alhagi sparsifolia. Chinese Journal of Plant Ecology, 2011, 35(4): 441-451. DOI: 10.3724/SP.J.1258.2011.00441
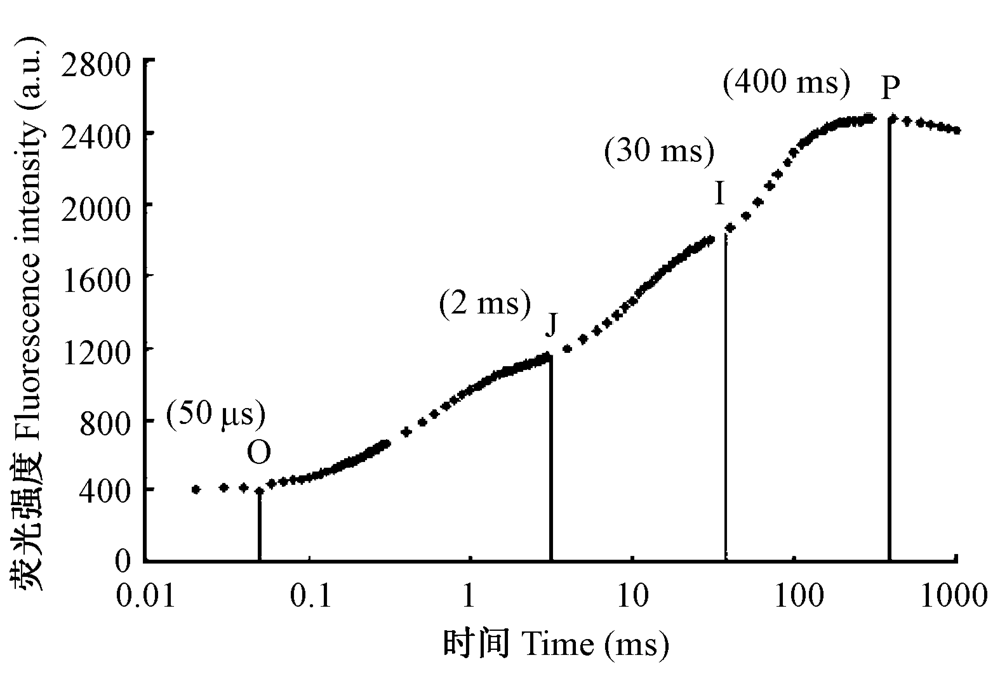
图2 骆驼刺正常叶片典型的多相叶绿素荧光上升曲线O-J-I-P。各点的含义详见李鹏民等(2005)。
Fig. 2 A typical chlorophyll polyphasic ?uorescence rise O-J-I-P for an untreated Alhagi sparsifolia leaf. The meaning of each point referred Li et al. (2005).
| 处理 Treatment | 初始荧光 Fo | 最大荧光 Fm | 可变荧光 Fv | PSII最大光化学量子产量 Fv/Fm |
|---|---|---|---|---|
| 30 ℃ | 435.70 ± 21.04 (0%) | 2 455.70 ± 147.46 (0%) | 2 020.00 ± 139.72 (0%) | 0.83 ± 0.01 (0%) |
| 38 ℃ | 468.13 ± 24.13 (7.44%) | 2 404.75 ± 134.34 (-2.07%) | 1 936.63 ± 144.42 (-4.13%) | 0.81 ± 0.02 (-2.41%) |
| 43 ℃ | 464.30 ± 27.45 (6.56%) | 2 318.40 ± 114.85 (-5.59%) | 1 854.10 ± 153.18 (-8.21%) | 0.80 ± 0.01 (-3.61%) |
| 48 ℃ | 602.00 ± 33.20 (38.17%) | 1 948.40 ± 103.69 (-20.66%) | 1 346.40 ± 62.77 (-33.35%) | 0.69 ± 0.06 (-16.87%) |
| 53 ℃ | 604.13 ± 33.42 (38.66%) | 1 840.75 ± 89.03 (-25.04%) | 1 236.63 ± 94.79 (-38.78%) | 0.65 ± 0.10 (-21.69%) |
| 58 ℃ | 849.40 ± 38.11 (94.95%) | 1 433.80 ± 69.32 (-41.61%) | 584.40 ± 25.67 (-71.07%) | 0.41 ± 0.10 (-50.60%) |
| 63 ℃ | 1 807.80 ± 40.02 (314.92%) | 1 809.20 ± 106.7 (-26.33%) | 1.40 ± 1.04 (-99.93%) | 0.000 7 ± 0.000 1 (-99.92%) |
表1 热胁迫对叶绿素荧光参数的影响(平均值±标准误差)
Table 1 Effects of heat stress on chlorophyll ?uorescence parameters (mean ± SE)
| 处理 Treatment | 初始荧光 Fo | 最大荧光 Fm | 可变荧光 Fv | PSII最大光化学量子产量 Fv/Fm |
|---|---|---|---|---|
| 30 ℃ | 435.70 ± 21.04 (0%) | 2 455.70 ± 147.46 (0%) | 2 020.00 ± 139.72 (0%) | 0.83 ± 0.01 (0%) |
| 38 ℃ | 468.13 ± 24.13 (7.44%) | 2 404.75 ± 134.34 (-2.07%) | 1 936.63 ± 144.42 (-4.13%) | 0.81 ± 0.02 (-2.41%) |
| 43 ℃ | 464.30 ± 27.45 (6.56%) | 2 318.40 ± 114.85 (-5.59%) | 1 854.10 ± 153.18 (-8.21%) | 0.80 ± 0.01 (-3.61%) |
| 48 ℃ | 602.00 ± 33.20 (38.17%) | 1 948.40 ± 103.69 (-20.66%) | 1 346.40 ± 62.77 (-33.35%) | 0.69 ± 0.06 (-16.87%) |
| 53 ℃ | 604.13 ± 33.42 (38.66%) | 1 840.75 ± 89.03 (-25.04%) | 1 236.63 ± 94.79 (-38.78%) | 0.65 ± 0.10 (-21.69%) |
| 58 ℃ | 849.40 ± 38.11 (94.95%) | 1 433.80 ± 69.32 (-41.61%) | 584.40 ± 25.67 (-71.07%) | 0.41 ± 0.10 (-50.60%) |
| 63 ℃ | 1 807.80 ± 40.02 (314.92%) | 1 809.20 ± 106.7 (-26.33%) | 1.40 ± 1.04 (-99.93%) | 0.000 7 ± 0.000 1 (-99.92%) |
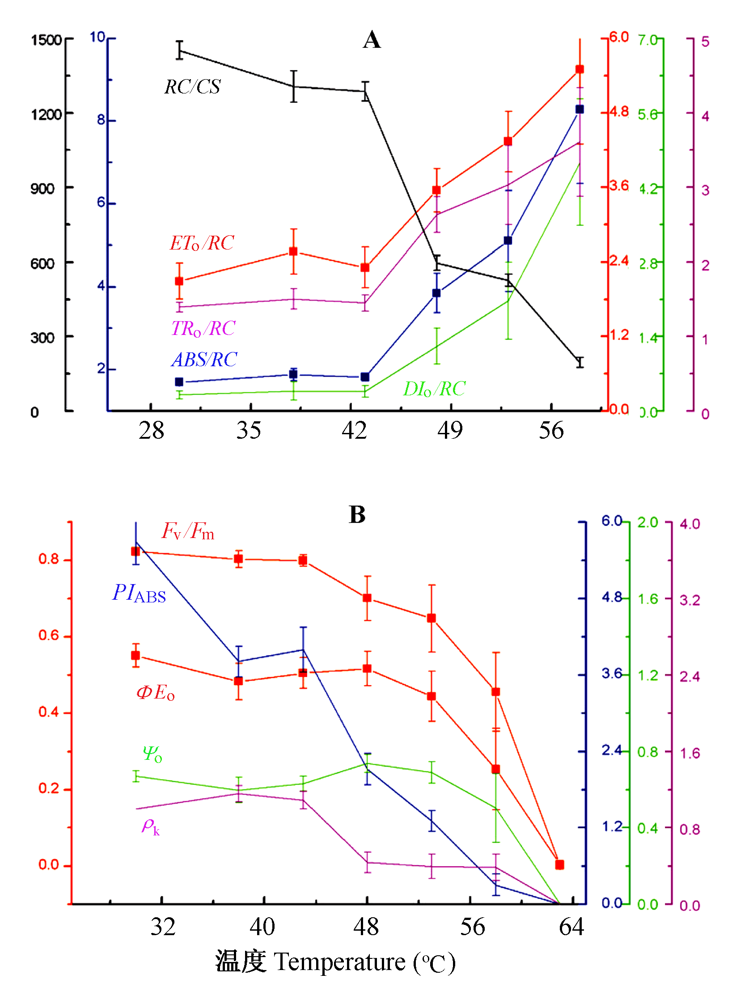
图4 热胁迫对骆驼刺叶片PSII能量流动(A)和能量利用效率(B)的影响(平均值±标准误差)。图中曲线的颜色与纵坐标颜色相对应。ABS/RC, 天线色素吸收的能量; DIo/RC, 用于热耗散的能量; ETo/RC, 用于电子传递的能量; Fv/Fm, PSII最大光化学量子产量; PIABS, 叶片性能指数; RC/CS, 单位面积的光合机构被激活反应中心的数量; TRo/RC, 反应中心捕获的能量; ρk, 放氧复合体放氧活性; ΦEo, 电子传递的量子产额; ψo, 捕获的激发能导致电子传递的效率。
Fig. 4 Effects of heat stress on PSII energy flux (A) and energy use efficiency (B) of Alhagi sparsifolia leaves (mean ± SE). In graph the color of curve is correspondence with the color of y-axis. The specific energy fluxes (per reaction centers, RC) for absorption (ABS/RC), trapping (TRo/RC), electron transport (ETo/RC) and dissipation (DIo/RC); the flux ratios or yield, i.e. the maximum quantum yield of primary photochemistry (Fv/Fm), the efficiency with which a trapped exciton can move an electron into the electron transport chain further than OA- (ψo), and the quantum yield of electron transport (ΦEo); the fraction of O2 evolving centers in comparison with the control sample (ρk); the amount of active PSII reaction centers per excited cross section (RC/CS), and the performance index (PIABS).
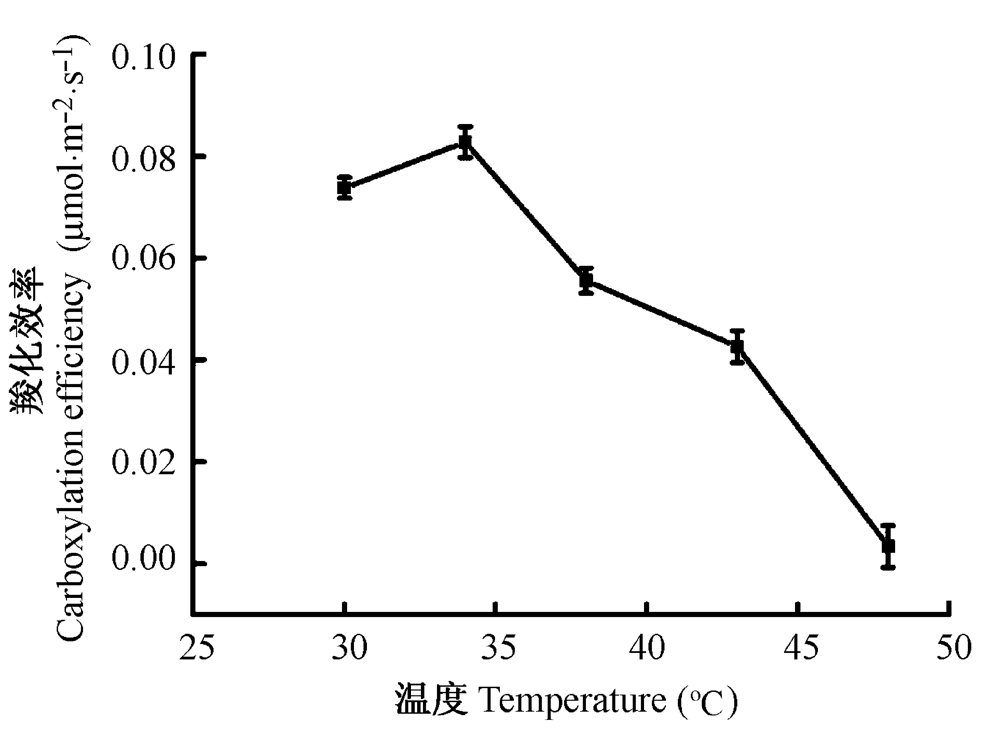
图5 高温胁迫对骆驼刺叶片RuBP羧化效率的影响(平均值±标准误差)。计算初始斜率时采用前6个点进行拟合。
Fig. 5 Effects of elevated temperature on RuBP carboxylation efficiency of Alhagi sparsifolia leaves (mean ± SE). Parameters of linear fitting based on the initial stage of Pn-Ci cruves which only contains the six foremost data points.
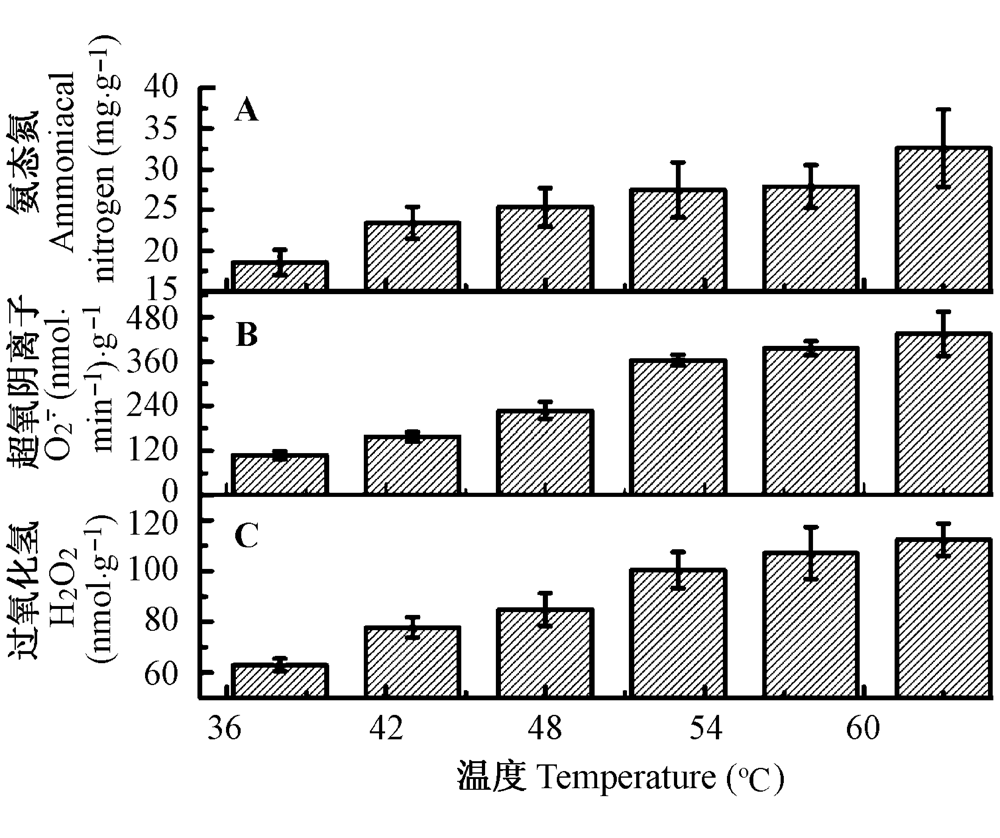
图6 热胁迫对疏叶骆驼刺叶片中氨态氮(A)、活性氧分子O2-· (B)和H2O2 (C)的影响(平均值±标准误差)。
Fig. 6 Effects of heat stress on ammoniacal nitrogen (A) and reactive oxygen molecules O2-· (B) and H2O2 (C) concentration of Alhagi sparsifolia leaves (mean ± SE).
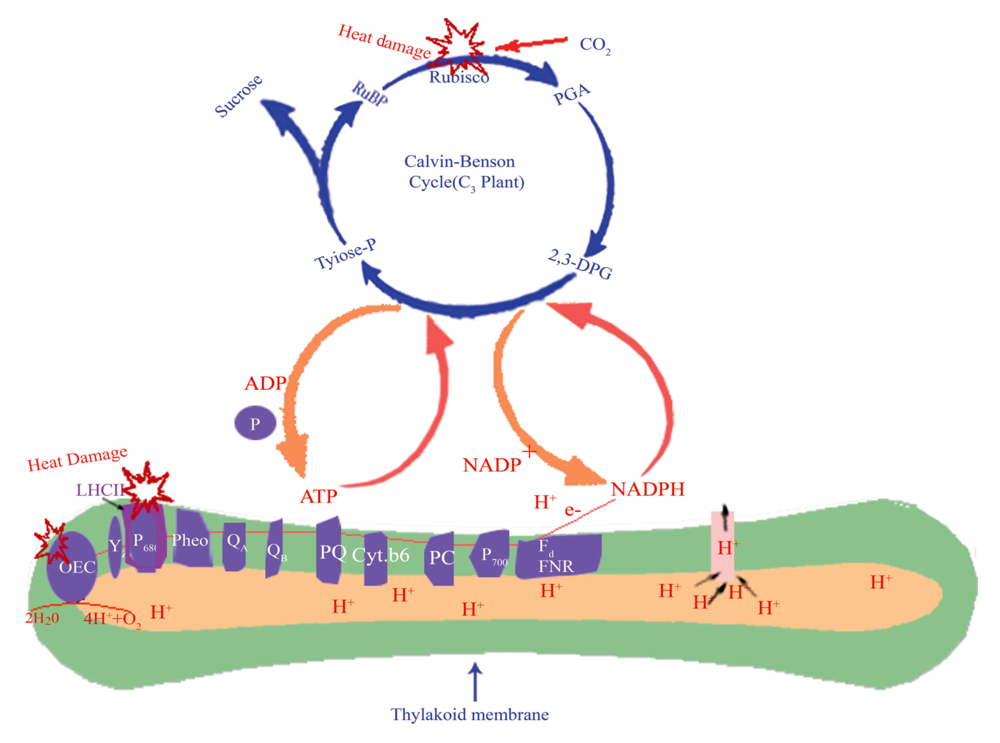
图7 光合系统主要的热胁迫伤害位点(绘制结构参考于http://i.hudong.com/profile.do?useriden=nfEACAwQFWUICDQJ_和http://tupian.hudong.com/a3_42_21_01300000332400124108219709521_jpg.html。但光合反应的生理学过程经过了本文作者思考加工)。2,3-DPG, 二磷酸甘油酸; Cyt.b6, 细胞色素b6-f复合物; Fd, 铁氧还原蛋白; LHCII, 光系统II捕光色素系统; OEC, 放氧复合体; P680, 光系统II反应中心色素; P700, 光系统I反应中心色素; PC, 质体蓝素; PGA, 磷酸甘油酸; Pheo, 去镁叶绿素; PQ, 质体醌; QA, 初级醌受体; QB, 次级醌受体; Sucrose, 蔗糖; Tyiose-P, 磷酸丙糖; Yz, 原初电子供体。
Fig. 7 The heat damaged parts of photosynthesis which includes light reaction phase mainly PSII and dark reaction phase under high temperature. When green leaves were exposed to high temperature (e.g. over 43 ℃), three important components given above oxygen evolving complex (OEC), light-harvesting system and Rubisco were more vulnerable than others components. 2,3-DPG, 2, 3-diphosphoglycerate; Cyt.b6, cytochrome b6-f complex; Fd, ferredoxin; LHCII, light-harvesting complex in photosystem II; P680, PSII reaction centre; P700, photosystem I reaction centre; PC, plastocyanin; PGA, phosphoglycerate; Pheo, pheophytin; PQ, plastoquinone; QA, the primary quinone acceptor; QB, the secondary quinone acceptor; Tyiose-P, triose phosphate; Yz, tyrosine Z.
| [1] |
Appenroth KJ, Stöckel J, Srivastava A, Strasser RJ (2001). Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environmental Pollution, 115, 49-64.
DOI URL |
| [2] |
Björkman O, Demmig B (1987). Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta, 170, 489-504.
DOI URL PMID |
| [3] | Bloom AJ (1997). Nitrogen as a limiting factor: crop acquisition of ammonium and nitrate. In: Jackson LE ed. Ecology in Agriculture. Academic Press, San Diego, USA. 145-172. |
| [4] |
Bruelheide H, Vonlanthen B, Jandt U, Thomas FM, Foetzki A, Gries D, Wang G, Zhang XM, Runge M (2010). Life on the edge—to which degree does phreatic water sustain vegetation in the periphery of the Taklamakan Desert? Applied Vegetation Science, 13, 56-71.
DOI URL |
| [5] |
Čajánek M, Štroch M, Lachetová I, Kalina J, Spunda V (1998). Characteization of the photosystem II inactivation of heat-stressed barley leaves as monitored by the various parameters of chlorophyll a fluorescence and delayed fluorescence. Journal of Photochemistry and Photobiology B: Biology, 47, 39-45.
DOI URL |
| [6] |
Costa ES, Bressan-Smith R, Oliveira JG, Campostrini E, Pimentel C (2002). Photochemical efficiency in bean plants (Phaseolus vulgaris L. and Vigna unguiculata L. Walp) during recovery from high temperature stress. Brazilian Journal of Plant Physiology, 14, 105-110.
DOI URL |
| [7] |
Crafts-Brandner SJ, Salvucci ME (2002). Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiology, 129, 1773-1780.
DOI URL PMID |
| [8] | Deng X (邓雄), Li XM (李小明), Zhang XM (张希明), Ye WH (叶万辉), Zhao Q (赵强) (2002). Relationship between gas exchange of four desert plants and environmental factors in Taklamakan. Chinese Journal of Applied and Environmental Biology (应用与环境生物学报), 8, 445-452. |
| [9] |
Ferguson IB, Watkins CB, Harman JE (1983). Inhibition by calcium of senescence of detached cucumber cotyledons: effect on ethylene and hydroperoxide production. Plant Physiology, 71, 182-186.
DOI URL PMID |
| [10] | Foyer CH, Noctor G (2005). Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell & Environment, 28, 1056-1071. |
| [11] | Guissé B, Srivastava A, Strasser RJ (1995). The polyphasic rise of the chlorophyll a fluorescence (O-K-J-I-P) in heat- stressed leaves. Archives des Sciences Genève, 48, 147-160. |
| [12] | Haldimann P, Feller U (2004). Inhibition of photosynthesis by high temperature in oak ( Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant, Cell & Environment, 27, 1169-1183. |
| [13] | Institute of Plant Physiology & Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, (中国科学院上海生命科学研究院植物生理生态研究所), (2004). Experimental Guide of Modern Plant Physiology (现代植物生理学实验指南). Science Press, Beijing. 138-309. (in Chinese) |
| [14] | IPCC (Intergovernmental Panel on Climate Change) (2007). Climate Change: Impacts, Adaptation and Vulnerability: Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK. |
| [15] | Jiang GM (蒋高明) (2007). Plant Ecophysiology 2nd edn (植物生理生态学第二版). Higher Education Press, Beijing. 178-179. (in Chinese) |
| [16] |
Komayama K, Khatoon M, Takenaka D, Horie J, Yamashita A, Yoshioka M, Nakayama Y, Yoshida M, Ohira S, Morita N, Velitchkova M, Enami I, Yamamoto Y (2007). Quality control of photosystem II: cleavage and aggregation of heat-damaged D1 protein in spinach thylakoids. Biochimica et Biophysica Acta-Bioenergetics, 1767, 838-846.
DOI URL |
| [17] |
Krause GH, Weis E (1984). Chlorophyll fluorescence as a tool in plant physiology II. Interpretation of fluorescence signals. Photosynthesis Research, 5, 139-157.
DOI URL PMID |
| [18] |
Krause GH, Weis E (1991). Chlorophyll fluorescence and photosynthesis: the basics. Annual Review of Plant Physiology and Plant Molecular Biology, 42, 313-349.
DOI URL |
| [19] |
Kubien DS, Sage RF (2008). The temperature response of photosynthesis in tobacco with reduced amounts of Rubisco. Plant, Cell & Environment, 31, 407-418.
DOI URL PMID |
| [20] | Lambers H, Chapin FS, Pons TL (1998). Plant Physiological Ecology. Springer-Verlag, New York . 8-68. |
| [21] | Lazár D, Schansker G (2009). Models of chlorophyll a fluorescence transients. In: Laisk A, Nedbal L, Govindjee eds. Photosynthesis in Silico: Understanding Complexity from Molecules to Ecosystems. Springer, Dordrecht, the Netherlands. 85-123. |
| [22] | Li PM (李鹏民), Gao HY (高辉远), Strasser RJ (2005). Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Journal of Plant Physiology and Molecular Biology (植物生理与分子生物学学报), 31, 559-566. (in Chinese with English abstract) |
| [23] | Li XY, Zhang XM, Zeng FJ, Foetzki A, Thomas FM, Li XM, Runge M, He XY (2002). Water relations on Alhagi sparsifolia in the southern fringe of Taklamakan Desert. Acta Botanica Sinica, 44, 1219-1224. |
| [24] |
Lichtenthaler HK (1998). The stress concept in plants: an introduction. Annals of the New York Academy of Sciences, 851, 187-198.
DOI URL PMID |
| [25] | Liu FH (刘凤红), Ye XH (叶学华), Yu HF (于飞海), Dong M (董鸣) (2006). Clonal integration modifies responses of Hedysarum laeve to local sand burial in MU Us sandland. Journal of Plant Ecology (Chinese Version) (植物生态学报), 30, 278-285. (in Chinese with English abstract) |
| [26] |
Mathur S, Jajoo A, Mehta P, Bharti S (2011). Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves ( Triticum astivum). Plant Biology, 13, 1-6.
DOI URL |
| [27] |
Musil CF, van Heerden PDR, Cilliers CD, Schmiedel U (2009). Mild experimental climate warming induces metabolic impairment and massive mortalities in southern African quartz field succulents. Environmental and Experimental Botany, 66, 79-87
DOI URL |
| [28] |
op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim CH, Danon A, Wagner D, Hideg É, Göbel C, Feussner I, Nater M, Apel K (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in arabidopsis. The Plant Cell, 15, 2320-2332.
DOI URL PMID |
| [29] |
Oukarroum A, Madidi SE, Schansker G, Strasser RJ (2007). Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environmental and Experimental Botany, 60, 438-446.
DOI URL |
| [30] |
Pospíšil P, Šnyrychová I, Nauš J (2007). Dark production of reactive oxygen species in photosystem II membrane particles at elevated temperature: EPR spin-trapping study. Biochimica et Biophysica Acta-Bioenergetics, 1767, 854-859.
DOI URL |
| [31] |
Schreiber U, Berry JA (1977). Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthesis apparatus. Planta, 136, 233-238.
DOI URL PMID |
| [32] |
Sharkey TD (2005). Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, Rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell & Environment, 28, 269-277.
DOI URL |
| [33] | Shi YF (施雅风), Shen YP (沈永平) (2003). Signa, impact and outlook of climatic shift from warm-dry to warm-humid in Northwest China. Science and Technology Review (科技导报), (2), 54-57. (in Chinese) |
| [34] |
Somersalo S, Krause GH (1989). Photoinhibition at chilling temperature: fluorescence characteristics of unhardened and cold-acclimated spinach leaves. Planta, 177, 409-416.
DOI URL PMID |
| [35] |
Stasik O, Jones HG (2007). Response of photosynthetic apparatus to moderate high temperature in contrasting wheat cultivars at different oxygen concentrations. Journal of Experimental Botany, 58, 2133-2143.
DOI URL PMID |
| [36] | Strasser BJ, Strasser RJ(1995). Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P ed. Photosynthesis: From Light to Biosphere. Kluwer Academic Publishers, Dordrecht, the Netherlands. 977-980. |
| [37] | Strasser RJ (1981). The grouping model of plant photosynthesis: heterogeneity of photosynthetic units in thylakoids. In: Akoyunoglou G ed. Photosynthesis III. Structure and Molecular Organisation of the Photosynthetic Apparatus. Balaban International Science Serve, Philadelphia. 727-737. |
| [38] | Strasser RJ, Tsimilli-Michael M, Srivastava A (2004). Analysis of the chlorophyll a fluorescence transient. In: Papage- orgiou GC, Govindjee eds. Chlorophyll Fluorescence: A Signature of Photosynthesis (Advances in Photosynthesis and Respiration). Springer, Dordrecht, the Netherlands. 321-362. |
| [39] |
Tang YL, Chen M, Xu YN, Kuang TY (2007). Changes in thermostability of photosystem II and leaf lipid composi- tion of rice mutant with deficiency of light-harvesting chlorophyll a/b protein complexes. Journal of Integrative Plant Biology, 49, 515-522.
DOI URL |
| [40] |
van Heerden PDR, Strasser RJ, Krüger GHJ (2004). Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiologia Plantarum, 121, 239-249.
DOI URL PMID |
| [41] |
van Heerden PDR, Tsimilli-Michael M, Krüger GHJ, Strasser RJ (2003). Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiologia Plantarum, 117, 476-491.
DOI URL PMID |
| [42] |
von Caemmerer S, Evans JR, Hudson GS, Andrews TJ (1994). The kinetics of ribulose-1, 5-bisphosphate carboxylase/ oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta, 195, 88-97.
DOI URL |
| [43] |
Wahid A, Gelani S, Ashraf M, Foolad MR (2007). Heat tolerance in plants: an overview. Environmental and Experimental Botany, 61, 199-223.
DOI URL |
| [44] | Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004). Electron transport is the functional limitation of photosynthesis in field-grown pima cotton plants at high temperature. Plant, Cell & Environment, 27, 717-724 |
| [45] | Xu DQ (许大全), Shen YG (沈允钢) (1998). Plant Physiology and Molecular Biology (植物生理与分子生物学). Science Press, Beijing. 262-276. (in Chinese) |
| [46] |
Yin Y, Li SM, Liao WQ, Lu QT, Wen XG, Lu CM (2010). Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. Journal of Plant Physiology, 167, 959-966.
DOI URL PMID |
| [47] |
Zeng J, Zeng FJ, Arndt SK, Guo HF, Yan HL, Xing WJ, Liu B (2008). Growth, physiological characteristics and ion distribution of NaCl stressed Alhagi sparsifolia seedlings. Chinese Science Bulletin, 53, 169-176.
DOI URL |
| [48] |
Zhang L, Xu H, Yang JC, Li WD, Jiang GM, Li YG (2010). Photosynthetic characteristics of diploid honeysuckle ( Lonicera japonica Thunb.) and its autotetraploid cultivar subjected to elevated ozone exposure. Photosynthetica, 48, 87-95.
DOI URL |
| [49] |
Zhou HH, Chen YN, Li WH, Chen YP (2010a). Photosynthesis of Populus euphratica in relation to groundwater depths and high temperature in arid environment, northwest China. Photosynthetica, 48, 257-268.
DOI URL |
| [50] |
Zhou SB, Liu K, Zhang D, Li QF, Zhu GP (2010b). Photos- ynthetic performance of Lycoris radiate var. radiata to shade treatments. Photosynthetica, 48, 241-248.
DOI URL |
| [51] |
Zhu BQ, Yang XP (2007). The ion chemistry of surface and ground waters in the Taklamakan Desert of Tarim Basin, western China. Chinese Science Bulletin, 52, 2123-2129.
DOI URL |
| [1] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [2] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [3] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [4] | 叶子飘, 胡文海, 闫小红. 光系统II实际光化学量子效率对光的响应模型的比较[J]. 植物生态学报, 2016, 40(11): 1208-1217. |
| [5] | 李志真, 刘东焕, 赵世伟, 姜闯道, 石雷. 环境强光诱导玉簪叶片光抑制的机制[J]. 植物生态学报, 2014, 38(7): 720-728. |
| [6] | 罗维成, 曾凡江, 刘波, 宋聪, 彭守兰, Stefan K. ARNDT. 疏叶骆驼刺母株与子株间的水分整合[J]. 植物生态学报, 2013, 37(2): 164-172. |
| [7] | 罗维成, 曾凡江, 刘波, 张利刚, 宋聪, 彭守兰, StefanK.ARNDT. 疏叶骆驼刺根系对土壤异质性和种间竞争的响应[J]. 植物生态学报, 2012, 36(10): 1015-1023. |
| [8] | 薛伟, 李向义, 朱军涛, 林丽莎, 王迎菊. 遮阴对疏叶骆驼刺叶形态和光合参数的影响[J]. 植物生态学报, 2011, 35(1): 82-90. |
| [9] | 张宗申, 利容千, 王建波. 外源Ca2+预处理对高温胁迫下辣椒叶片细胞膜透性和GSH、AsA含量及Ca2+分布的影响[J]. 植物生态学报, 2001, 25(2): 230-234. |
| [10] | 金启宏. K、Na、Ca、Mg 4种元素在疏叶骆驼刺体内含量分布特点的研究[J]. 植物生态学报, 1996, 20(1): 80-84. |
| [11] | 金启宏. 疏叶骆驼刺种群性质与植物群落演替[J]. 植物生态学报, 1995, 19(3): 255-260. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19