

植物生态学报 ›› 2023, Vol. 47 ›› Issue (3): 361-373.DOI: 10.17521/cjpe.2021.0446
所属专题: 青藏高原植物生态学:生理生态学; 光合作用
师生波1,3,*( ), 周党卫1, 李天才1, 德科加2, 杲秀珍1, 马家麟1, 孙涛3, 王方琳3
), 周党卫1, 李天才1, 德科加2, 杲秀珍1, 马家麟1, 孙涛3, 王方琳3
收稿日期:2021-12-02
接受日期:2022-05-21
出版日期:2023-03-20
发布日期:2022-10-11
通讯作者:
师生波
作者简介:* E-mail: sbshi@nwipb.cas.cn基金资助:
SHI Sheng-Bo1,3,*( ), ZHOU Dang-Wei1, LI Tian-Cai1, DE Ke-Jia2, GAO Xiu-Zhen1, MA Jia-Lin1, SUN Tao3, WANG Fang-Lin3
), ZHOU Dang-Wei1, LI Tian-Cai1, DE Ke-Jia2, GAO Xiu-Zhen1, MA Jia-Lin1, SUN Tao3, WANG Fang-Lin3
Received:2021-12-02
Accepted:2022-05-21
Online:2023-03-20
Published:2022-10-11
Contact:
SHI Sheng-Bo
Supported by:摘要:
昼夜温差大是青藏高原的典型气候特征, 夜间低温作为植物生长季内非常频繁的非生物胁迫因子, 对典型高山植物日间光合生理功能的影响如何, 尚缺乏研究。该研究以采自青海大学-清华大学三江源高寒草地生态系统野外观测站的高山嵩草(Kobresia pygmaea)为材料, 应用叶绿素荧光图像分析手段, 研究了模拟夜间低温对叶片光系统II (PSII)非光化学猝灭中光诱导和非光诱导的量子产量, 及慢弛豫相和快弛豫相组分的影响。结果表明: 0 ℃夜间低温对日间PSII相对电子传递速率、PSII反应中心开放比率(qL)和PSII非光化学猝灭系数(qNP)的快速光响应曲线影响较小; 400和1 500 µmol·m-2·s-1稳态作用光强下的比较证实, 夜间低温并没有影响到光合机构活性及非光化学能量耗散过程。PSII反应中心激发能分配的量子通量分析表明, PSII实际光化学量子效率、PSII非光化学猝灭中非调节性和调节性能量耗散量子产量的相对比率在第3天高光强下, 对照组和夜间低温组分别为: 36:19:45和38:19:43; 较低光强下为66:22:12和66:23:11。非光化学猝灭(NPQ)中快弛豫相(NPQf)为主要组分, 而慢弛豫相(NPQs)所占份额(NPQs/NPQ)在对照植株的第1天和第3天分别为11%和10%, 夜间低温组则为13%和12%。因此, 0 ℃夜间低温后, 高山嵩草PSII反应中心发生光抑制的几率增大, 较低光强和夜间低温能导致光合诱导时间的延长; 但光化学能量转换和保护性的调节机制尚能有效分配吸收的光能, 夜间低温没有加剧过剩激发能难以调节耗散的趋势。
师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应. 植物生态学报, 2023, 47(3): 361-373. DOI: 10.17521/cjpe.2021.0446
SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau. Chinese Journal of Plant Ecology, 2023, 47(3): 361-373. DOI: 10.17521/cjpe.2021.0446
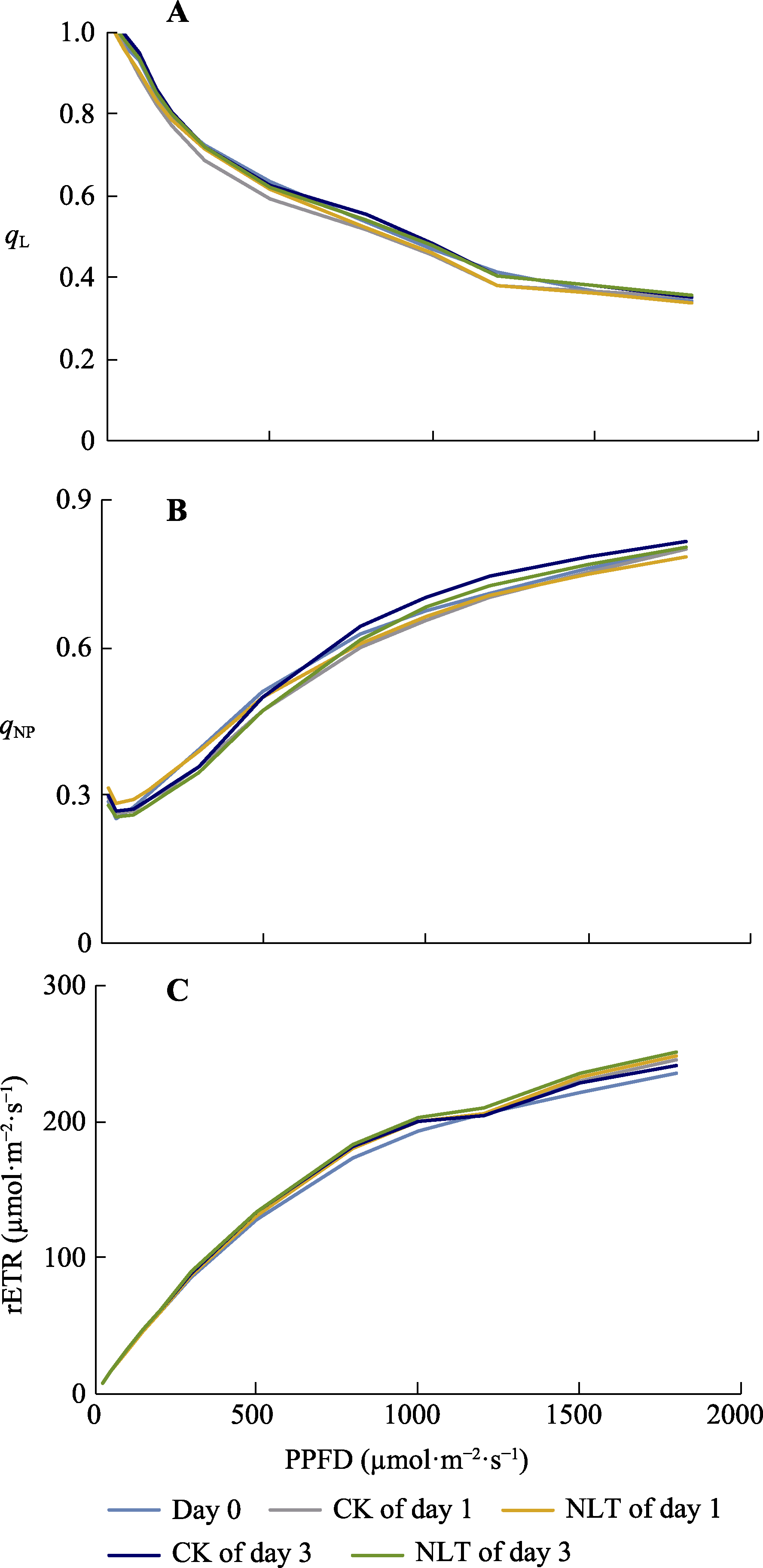
图1 高山嵩草叶片光系统II (PSII)反应中心开放比率(qL) (A)、PSII非光化学猝灭系数(qNP) (B)和PSII相对表观电子传递速率(rETR) (C)的快速光响应曲线。PPFD, 光合有效辐射通量密度。CK, 对照; NLT, 夜间低温。
Fig. 1 Rapid light-response curves of the fraction of open PSII centers (qL) (A), PSII non-photochemical quenching coefficient (qNP) (B), and relative electron transfer rate through PSII (rETR) (C) of Kobresia pygmaea leaves. PPFD, photosynthetical active photon flux density. CK, control; NLT, nocturnal low temperature treatment.
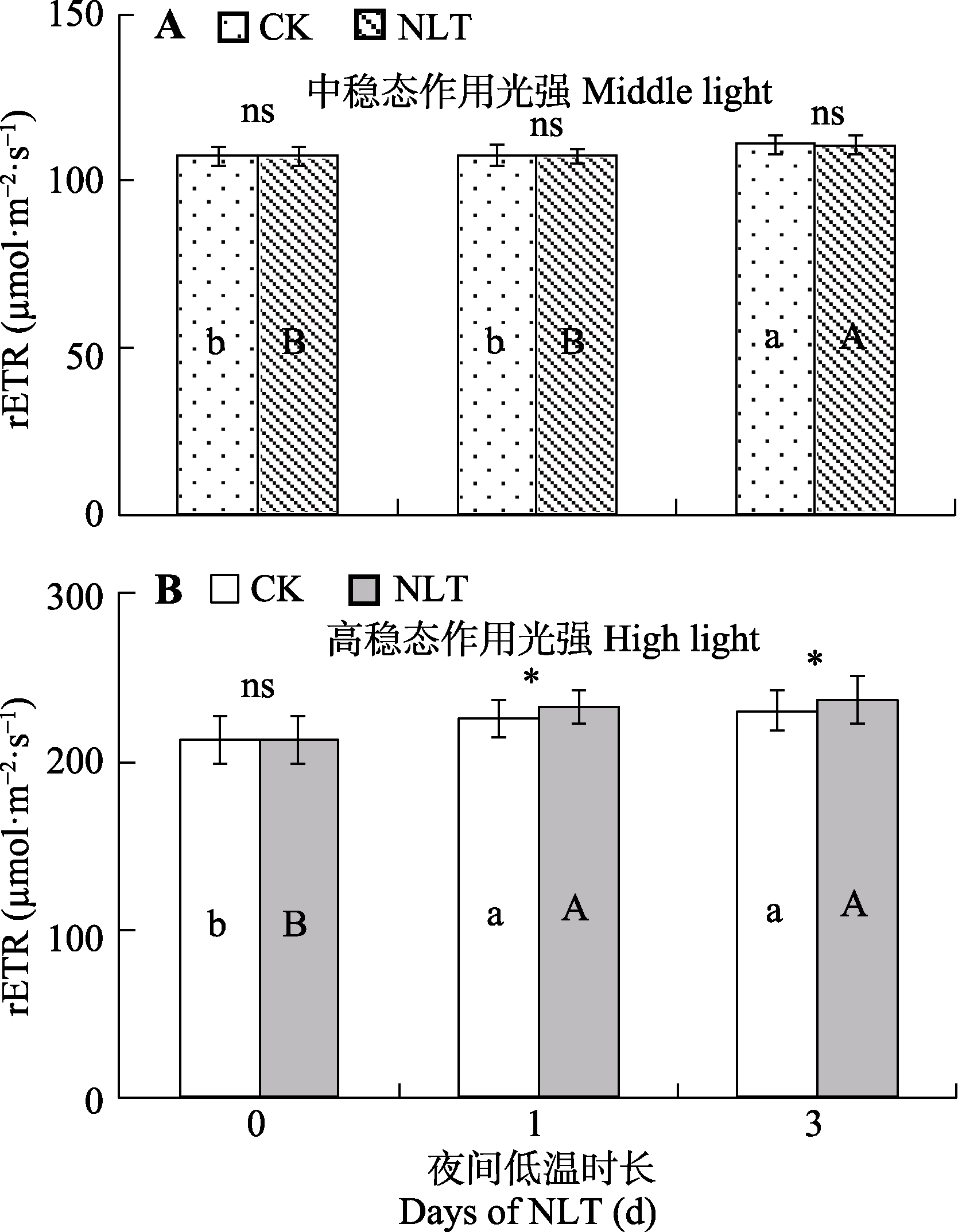
图2 稳态作用光强下高山嵩草叶片光系统II相对表观电子传递速率(rETR)的变化及对夜间低温(NLT)的响应(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照组和夜间低温组在不同时长间rETR的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05); *, 对照(CK)组和低温处理组间差异显著(p < 0.05)。
Fig. 2 Relative electron transfer rate through photosystem II (rETR) of Kobresia pygmaea leaves under nocturnal low-temperature (NLT) treatment at steady-state light intensities (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among different days of the treatment in the control and NLT groups, respectively (α = 0.05). ns, no significant difference between the control (CK) and NLT groups (p > 0.05); *, significant difference between the control and NLT groups (p < 0.05).
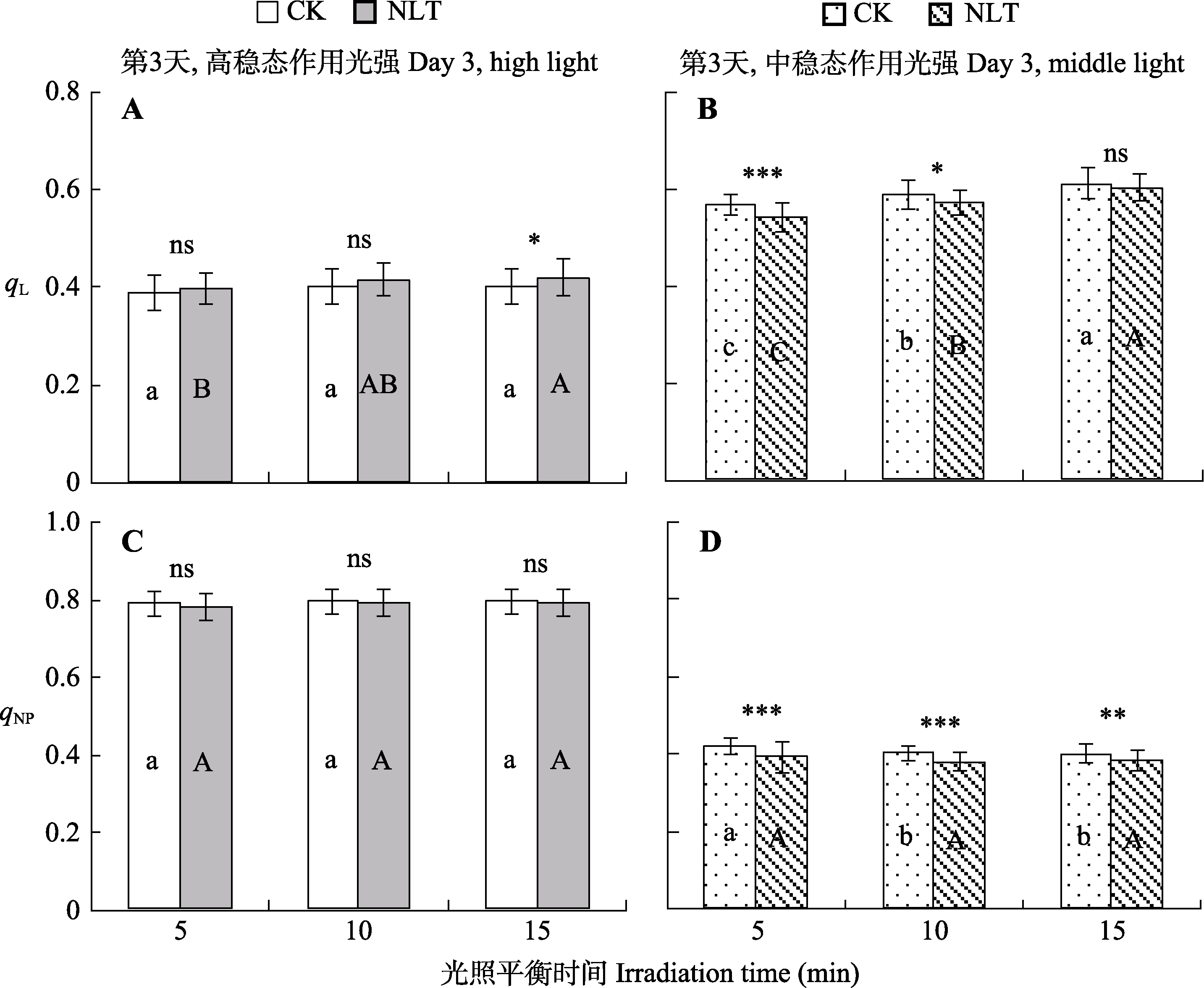
图3 夜间低温(NLT)对不同稳态光强下高山嵩草叶片光系统II (PSII)反应中心开放比率(qL) (A、B)和PSII非光化学猝灭系数(qNP) (C、D)的影响及光诱导平衡(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照(CK)组和夜间低温组在15 min的光照平衡期间qL和qNP的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05); *, 对照组和低温处理组间差异显著(p < 0.05); **和***, 对照组和低温处理组间差异极显著(p < 0.01和p < 0.001)。
Fig. 3 Fraction of open photosystem II (PSII) centers (qL) (A, B) and PSII non-photochemical quenching coefficient (qNP) (C, D) of Kobresia pygmaea leaves under necturnal low-temperature (NLT) treatment at steady-state light intensities (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among different irradiation times in the control (CK) and NLT groups, respectively (α = 0.05). ns, no significant difference between the control and NLT groups (p > 0.05); *, significant difference between the control and NLT groups (p < 0.05); ** and ***, highly significant difference between the control and NLT groups (p < 0.01 and p < 0.001).
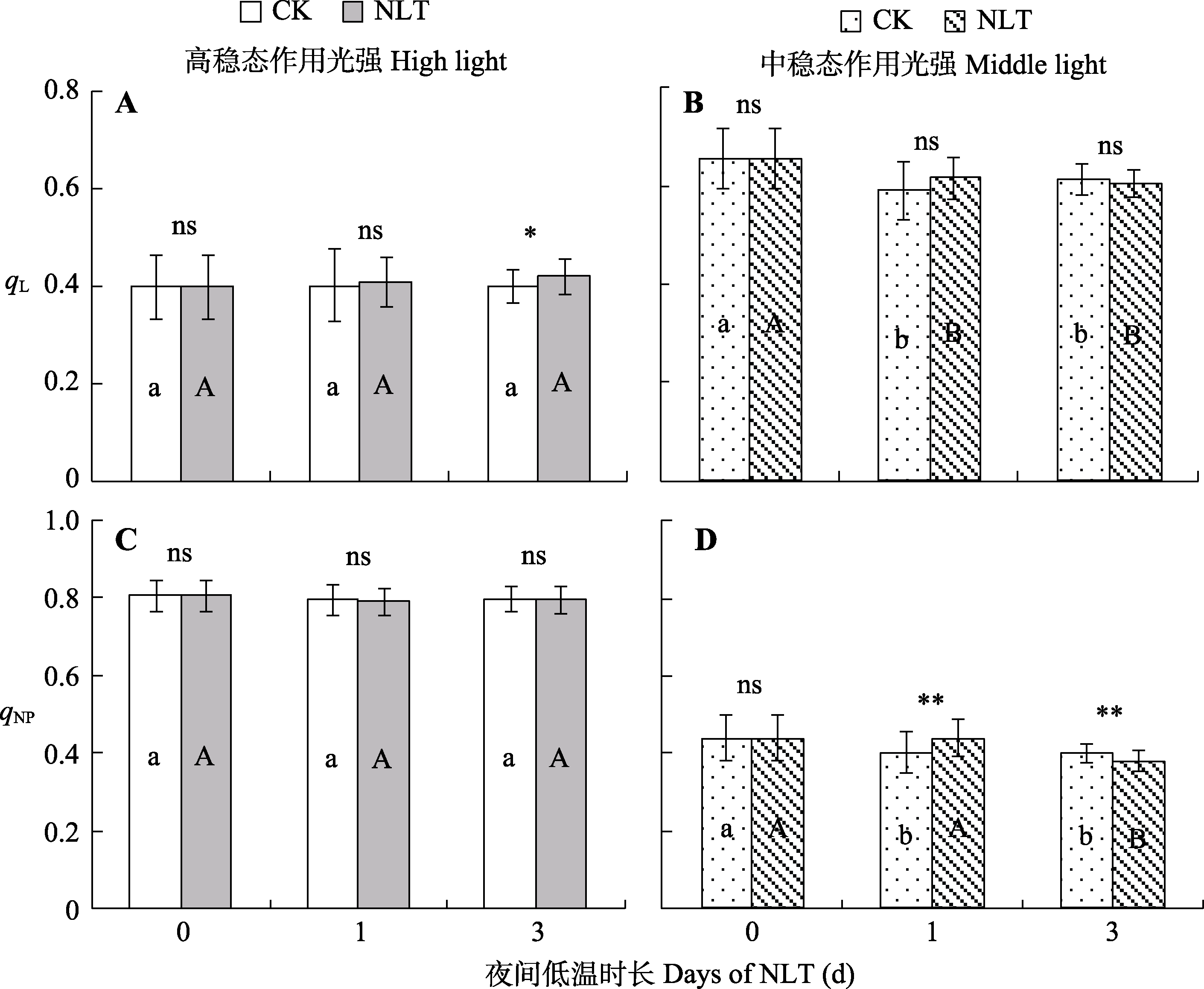
图4 不同时长高山嵩草叶片光系统II (PSII)反应中心开放比率(qL) (A、B)和PSII非光化学猝灭系数(qNP) (C、D)的变化及夜间低温(NLT)的影响(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照(CK)组和夜间低温组在不同处理时长间qL和qNP的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05); *, 对照组和低温处理组间差异显著(p < 0.05); **, 对照组和低温处理组间差异极显著(p < 0.01)。
Fig. 4 Fraction of open photosystem II (PSII) centers (qL) (A, B) and PSII non-photochemical quenching coefficient (qNP) (C, D) of Kobresia pygmaea leaves under different days of nocturnal low-temperature (NLT) treatment (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among different days of the treatment in the control (CK) and NLT groups, respectively (α = 0.05). ns, no significant difference between the control and NLT groups (p > 0.05); *, significant difference between the control group and NLT groups (p < 0.05); **, highly significant difference between the control and NLT groups (p < 0.01).
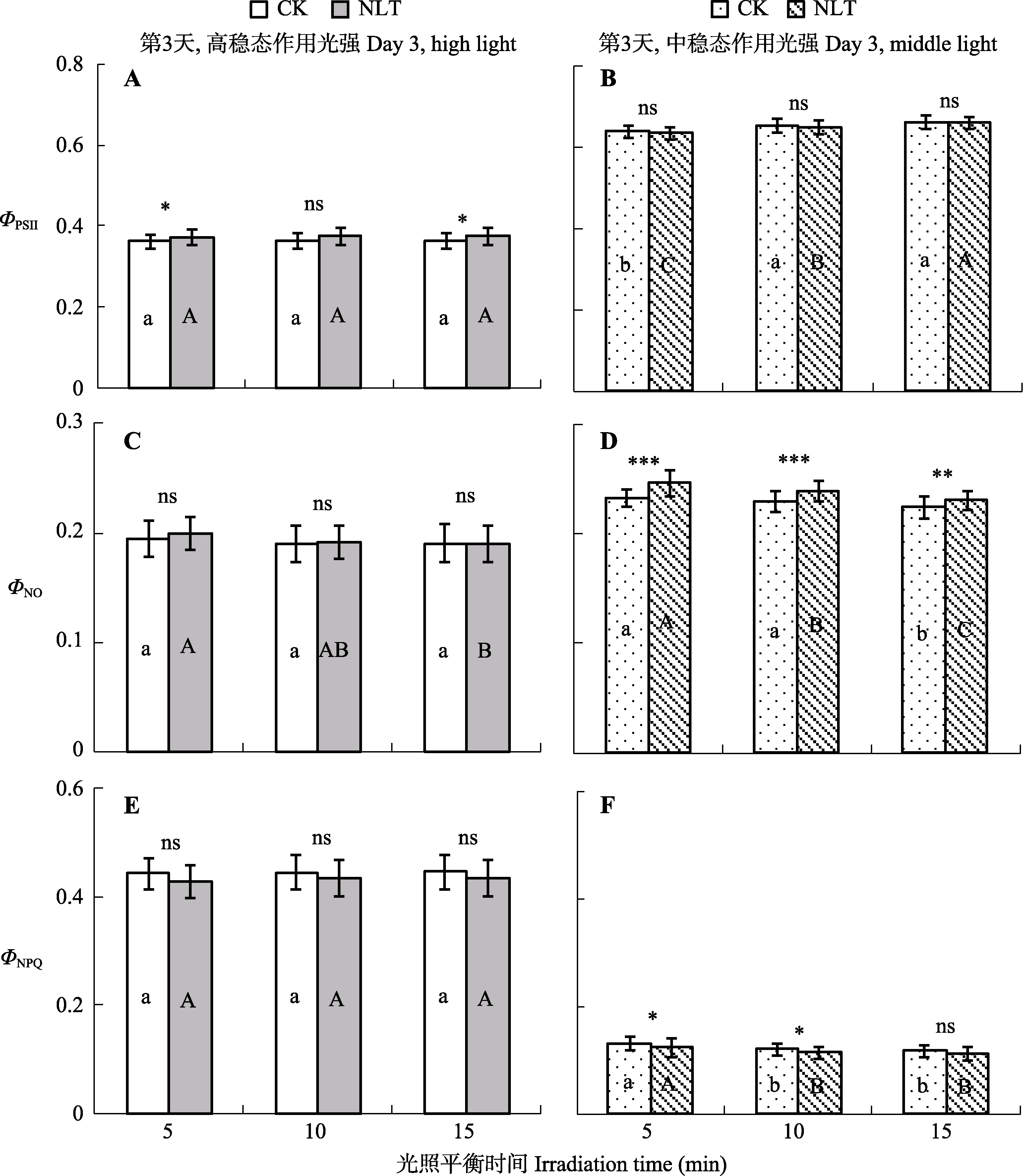
图5 稳态作用光强下夜间低温(NLT)处理对高山嵩草叶片光系统II (PSII)实际光化学量子效率(ΦPSII) (A、B)、非调节性能量耗散量子产量(ΦNO) (C、D)和调节性能量耗散量子产量(ΦNPQ) (E、F)的影响及随光诱导时间的变化(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照(CK)组和夜间低温组在15 min的光照平衡期间ΦPSII、ΦNO和ΦNPQ的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05); *, 对照组和低温处理组间差异显著(p < 0.05); **和***, 对照组和低温处理组间差异极显著(p < 0.01和p < 0.001)。
Fig. 5 Photosystem II (PSII) actual photochemical efficiency (ΦPSII) (A, B), and the quantum yield of non-regulated energy dissipation (ΦNO) (C, D) and regulated energy dissipation (ΦNPQ) (E, F) of Kobresia pygmaea leaves under different days of nocturnal low-temperature (NLT) treatment (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among different days of the treatment in the control (CK) and NLT groups, respectively (α = 0.05). ns, no significant difference between the control and NLT groups (p > 0.05); *, significant difference between the control and NLT groups (p < 0.05); **, highly significant difference between the control group and NLT groups (p < 0.01).
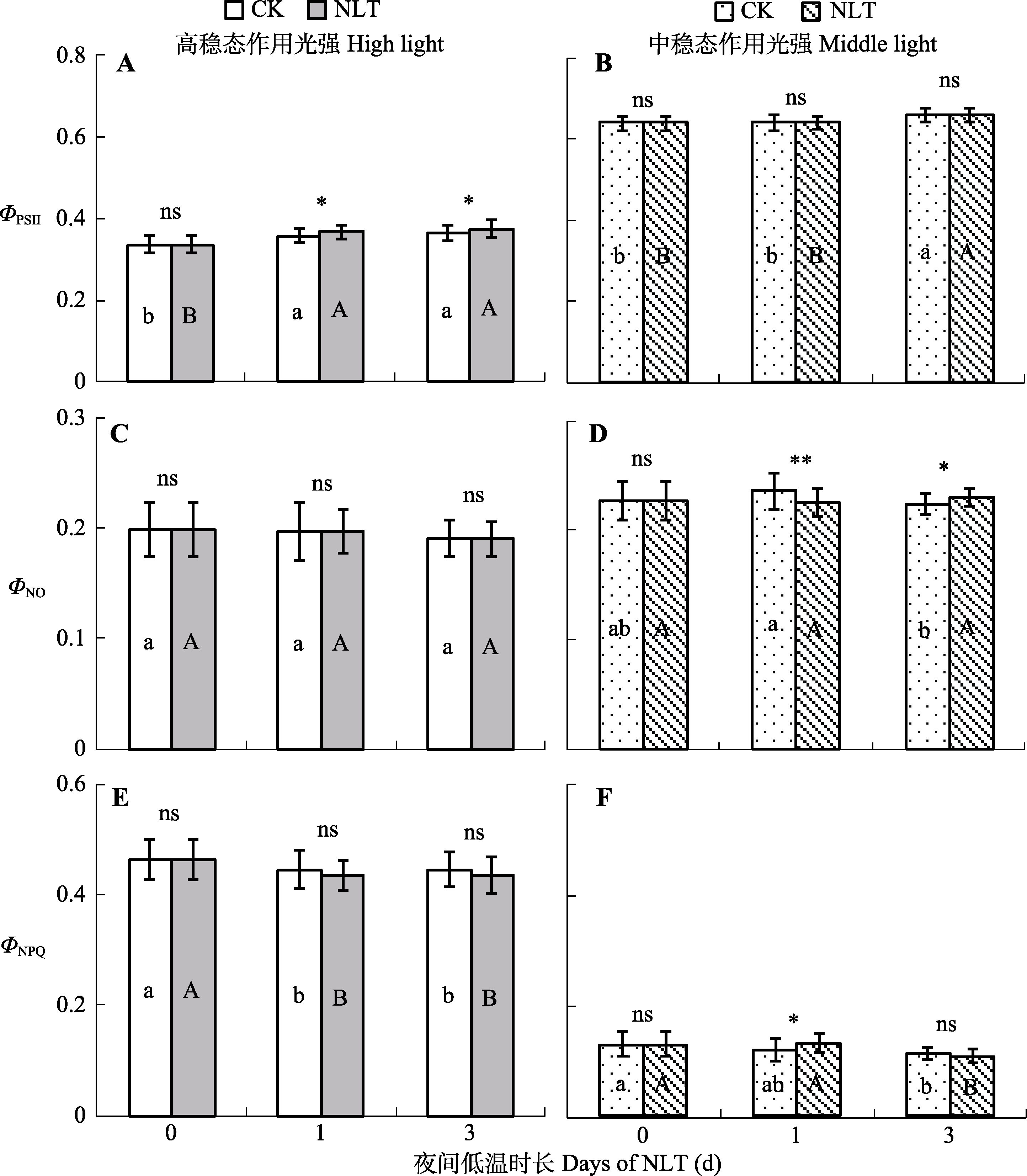
图6 不同时长夜间低温(NLT)处理高山嵩草叶片光系统II (PSII)实际光化学量子效率(ΦPSII) (A、B)、非调节性能量耗散量子产量(ΦNO) (C、D)和调节性能量耗散量子产量(ΦNPQ) (E、F)的变化及对夜间低温的响应(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照(CK)组和夜间低温组在不同处理时长间ΦPSII、ΦNO和ΦNPQ的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05); *, 对照组和低温处理组间差异显著(p < 0.05); **, 对照组和低温处理组间差异极显著(p < 0.01)。
Fig. 6 Photosystem II (PSII) actual photochemical efficiency (ΦPSII) (A, B), and the quantum yield of non-regulated energy dissipation (ΦNO) (C, D) and regulated energy dissipation (ΦNPQ) (E, F) of Kobresia pygmaea leaves under nocturnal low-temperature (NLT) treatment at steady-state light intensities (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among irradiation times in the control (CK) and NLT groups, respectively (α = 0.05). ns, no significant difference between the control and NLT groups (p > 0.05); *, significant difference between the control and NLT groups (p < 0.05); ** and ***, highly significant difference between the control and NLT groups (p < 0.01 and p < 0.001).
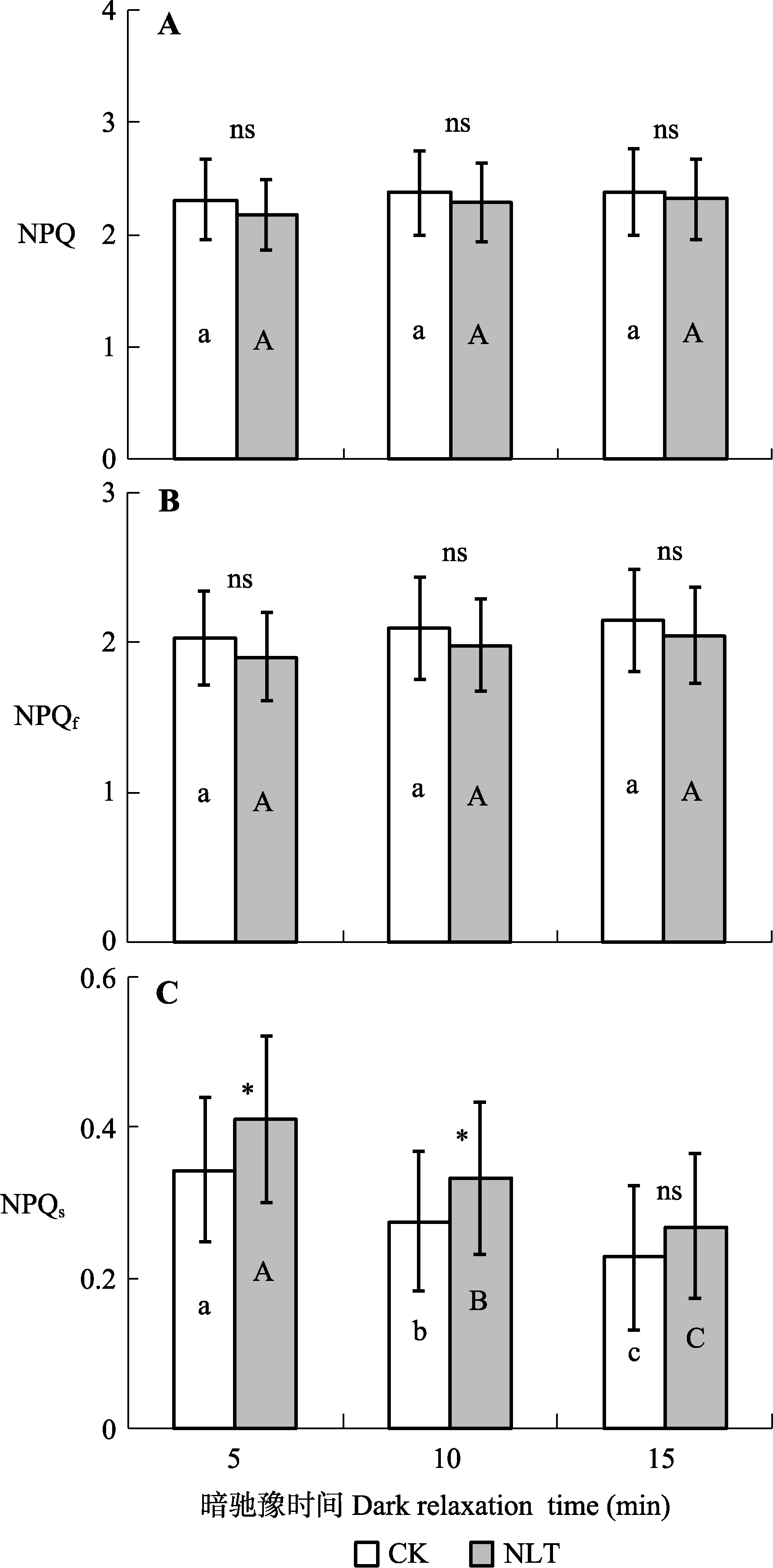
图7 夜间低温(NLT)对高山嵩草叶片光系统II (PSII)非光化学猝灭(NPQ) (A)和快弛豫与慢弛豫组分(NPQf和NPQs) (B、C)的影响及随暗驰豫时间的变化(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照(CK)组和夜间低温组在15 min的光照平衡期间NPQ、NPQf和NPQs的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05); *, 对照组和低温处理组间差异显著(p < 0.05)。
Fig. 7 Photosystem II (PSII) non-photochemical quenching (NPQ) (A) and its fast and slow components (NPQf and NPQs) (B, C) of Kobresia pygmaea leaves under nocturnal low-temperature (NLT) treatment and their variation with dark relaxation time (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among different dark relaxation times in the control (CK) and NLT groups, respectively (α = 0.05). ns, no significant difference between the control and NLT groups (p > 0.05); *, significant difference between the control and NLT groups (p < 0.05).
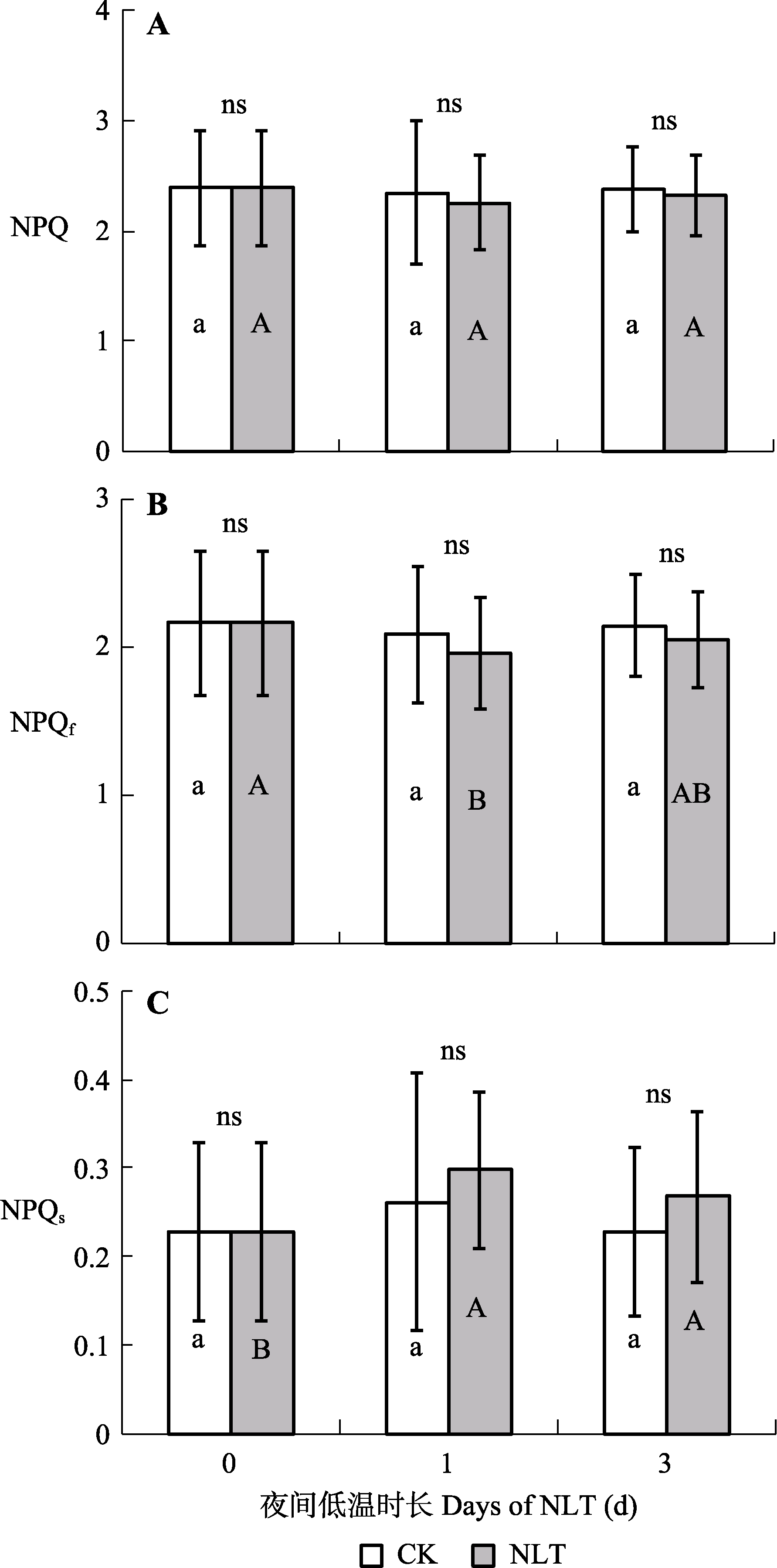
图8 不同时长夜间低温(NLT)处理高山嵩草叶片光系统II (PSII)非光化学猝灭(NPQ) (A)和快弛豫与慢弛豫组分(NPQf和NPQs) (B、C)的变化及对夜间低温的响应(平均值±标准差, n = 30)。不同小写字母和大写字母分别表示对照(CK)组和夜间低温组在不同处理时长间NPQ、NPQf和NPQs的差异显著性(α = 0.05)。ns, 对照组和低温处理组间差异不显著(p > 0.05)。
Fig. 8 Photosystem II (PSII) non-photochemical quenching (NPQ) (A) and its fast and slow components (NPQf and NPQs) (B, C) of Kobresia pygmaea leaves under different days of nocturnal low-temperature (NLT) treatment (mean ± SD, n = 30). Different lowercase and uppercase letters indicate significant differences among different days of the treatment in the control (CK) and NLT groups, respectively (α = 0.05). ns, no significant difference between the control and NLT groups (p > 0.05).
| [1] |
Baker NR (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89-113.
DOI PMID |
| [2] |
Bertamini M, Muthuchelian K, Rubinigg M, Zorer R, Velasco R, Nedunchezhian N (2006). Low-night temperature increased the photoinhibition of photosynthesis in grapevine (Vitis vinifera L. cv. Riesling) leaves. Environmental and Experimental Botany, 57, 25-31.
DOI URL |
| [3] |
Bilger W, Björkman O (1990). Role of the xanthophyll cycle photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research, 25, 173-185.
DOI PMID |
| [4] |
Dyer MI, Turner CL, Seastedt TR (1991). Mowing and fertilization effect on productivity and spectral reflectance in Bromus inermis plots. Ecological Applications, 1, 443-452.
DOI URL |
| [5] | Gao J, Li QF, Xue JQ, Zhang RH (2016). Physiological compensation mechanism of photosystem II in maize leaves induced by drought stress and re-watering condition. Plant Physiology Journal, 52, 1413-1420. |
| [高杰, 李青风, 薛吉全, 张仁和 (2016). 干旱复水激发玉米叶片光系统Ⅱ性能的生理补偿机制. 植物生理学报, 52, 1413-1420.] | |
| [6] |
Gaudet CL, Keddy PA (1988). A comparative approach to predicting competitive ability from plant traits. Nature, 334, 242-243.
DOI |
| [7] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [8] |
Guarini JM, Moritz C (2009). Modelling the dynamics of the electron transport rate measured by PAM fluorimetry during rapid light curve experiments. Photosynthetica, 47, 206-214.
DOI URL |
| [9] |
Hendrickson L, Furbank RT, Chow WS (2004). A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynthesis Research, 82, 73-81.
DOI PMID |
| [10] |
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research, 79, 209-218.
DOI PMID |
| [11] | Larcher W (1980). Physiological Plant Ecology. 2nd ed. Springer-Verlag, New York. 5-60. |
| [12] | Li YK, Lin L, Zhang FW, Liang DY, Wang X, Cao GM (2010). Kobresia pygmaea community—Disclimax of alpine meadow zonal vegetation in the pressure of grazing. Journal of Mountain Science, 28, 257-265. |
| [李以康, 林丽, 张法伟, 梁东营, 王溪, 曹广民 (2010). 小嵩草群落——高寒草甸地带性植被放牧压力下的偏途顶极群落. 山地学报, 28, 257-265.] | |
| [13] |
Lima Neto MC, Lobo AKM, Martins MO, Fontenele AV, Silveira JAG (2014). Dissipation of excess photosynthetic energy contributes to salinity tolerance: a comparative study of salt-tolerant Ricinus communis and salt-sensitive Jatropha curcas. Journal of Plant Physiology, 171, 23-30.
DOI URL |
| [14] | Liu ZG, Sun WC, Fang Y, Li XC, Yang NN, Wu JY, Zeng XC, Wang Y (2015). Effects of low nocturnal temperature on photosynthetic apparatus of winter rapeseed (Brassica campestris L.). Scientia Agricultura Sinica, 48, 672-682. |
|
[刘自刚, 孙万仓, 方彦, 李学才, 杨宁宁, 武军艳, 曾秀存, 王月 (2015). 夜间低温对白菜型冬油菜光合机构的影响. 中国农业科学, 48, 672-682.]
DOI |
|
| [15] |
Masini L, Grenville-Briggs LJ, Andreasson E, Råberg L, Lankinen Å (2019). Tolerance and overcompensation to infection by Phytophthora infestans in the wild perennial climber Solanum dulcamara. Ecology and Evolution, 9, 4557-4567.
DOI |
| [16] |
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence—A practical guide. Journal of Experimental Botany, 51, 659-668.
DOI PMID |
| [17] | Miehe G, Miehe S, Kaiser K, Liu J, Zhao X, Zhou H (2008). Status and dynamics of the Kobresia pygmaea ecosystem on the Tibetan Plateau. Ambio, 37, 258-265. |
| [18] |
Miehe G, Schleuss PM, Seeber E, Babel W, Biermann T, Braendle M, Chen F, Coners H, Foken T, Gerken T, Graf HF, Guggenberger G, Hafner S, Holzapfel M, Ingrisch J, et al. (2019). The Kobresia pygmaea ecosystem of the Tibetan highlands—Origin, functioning and degradation of the world’s largest pastoral alpine ecosystem: Kobresia pastures of Tibet. Science of the Total Environment, 648, 754-771.
DOI URL |
| [19] |
Murchie EH, Niyogi KK (2011). Manipulation of photoprotection to improve plant photosynthesis. Plant Physiology, 155, 86-92.
DOI PMID |
| [20] |
Oxborough K, Baker NR (1997). Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynthesis Research, 54, 135-142.
DOI URL |
| [21] |
Peng SM, Du QY, Lin AW, Hu B, Xiao K, Xi YL (2015). Observation and estimation of photosynthetically active radiation in Lhasa (Tibetan Plateau). Advances in Space Research, 55, 1604-1612.
DOI URL |
| [22] | Sáez PL, Bravo LA, Latsague MI, Toneatti MJ, Sánchez-Olate M, Ríos DG (2013). Light energy management in micropropagated plants of Castanea sativa, effects of photoinhibition. Plant Science, 201- 202, 12-24. |
| [23] | Schreiber U, Bilger W, Neubauer C (1995). Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis//Schulze ED, Caldwell MM. Ecophysiology of Photosynthesis. Springer-Verlag, Berlin. 49-70. |
| [24] | Shi SB, Li TC, Li M, Liu SZ, Li AD, Ma JP (2015). Interaction effect analysis of soil drought and strong light on PSII non-photochemical quenching in Kobresia pygmaea leaves. Plant Physiology Journal, 51, 1678-1686. |
| [师生波, 李天才, 李妙, 刘世增, 李爱德, 马剑平 (2015). 土壤干旱和强光对高山嵩草叶片PSII反应中心非光化学猝灭的交互影响分析. 植物生理学报, 51, 1678-1686.] | |
| [25] |
Shi SB, Shang YX, Zhu PJ, Yang L, Zhang B (2011). Effects of solar UV-B radiation on the efficiency of PSII photochemistry in the alpine plant Saussurea superba under different weather conditions in the Qinghai-Tibet Plateau of China. Chinese Journal of Plant Ecology, 35, 741-750.
DOI URL |
|
[师生波, 尚艳霞, 朱鹏锦, 杨莉, 张波 (2011). 不同天气类型下UV-B辐射对高山植物美丽风毛菊叶片PSII光化学效率的影响分析. 植物生态学报, 35, 741-750.]
DOI |
|
| [26] | Sun BG, Long RJ, Wang CT (2007). A study on the plant population phenology in Qinghai-Tibet Plateau Kobrecia pygmaea meadow. Pratacultural Science, 24(8), 16-20. |
| [孙步功, 龙瑞军, 王长庭 (2007). 青藏高原冷龙岭南麓高寒小嵩草草甸植物种群物候学研究. 草业科学, 24(8), 16-20.] | |
| [27] |
Sun HL, Zheng D, Yao TD, Zhang YL (2012). Protection and construction of the national ecological security shelter zone on Tibetan Plateau. Acta Geographica Sinica, 67, 3-12.
DOI |
|
[孙鸿烈, 郑度, 姚檀栋, 张镱锂 (2012). 青藏高原国家生态安全屏障保护与建设. 地理学报, 67, 3-12.]
DOI |
|
| [28] |
Tikkanen M, Mekala NR, Aro EM (2014). Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochimica et Biophysica Acta, 1837, 210-215.
DOI PMID |
| [29] |
Valizadeh-Kamran R, Toorchi M, Mogadam M, Mohammadi H, Pessarakli M (2018). Effects of freeze and cold stress on certain physiological and biochemical traits in sensitive and tolerant barley (Hordeum vulgare) genotypes. Journal of Plant Nutrition, 41, 102-111.
DOI URL |
| [30] |
Krüger GHJ (2004). Dark chilling inhibition of photosynthesis and symbiotic nitrogen fixation in soybean during pod filling. Journal of Plant Physiology, 161, 599-609.
PMID |
| [31] | Wang CT, Long RJ, Ding LM (2004). Study of alpine meadow of basic characteristic in Qinghai Tibet Plateau. Pratacultural Science, 21(8), 16-19. |
| [王长庭, 龙瑞军, 丁路明 (2004). 青藏高原高寒嵩草草甸基本特征的研究. 草业科学, 21(8), 16-19.] | |
| [32] | Wang LJ, Li TL, Hao JH, Chen WZ (2010). Effects of low night temperature in short time on photosynthesis of tomato. Journal of Agricultural University of Hebei, 33(4), 46-50. |
| [王丽娟, 李天来, 郝敬虹, 陈伟芝 (2010). 短期低夜温处理对番茄光合作用的影响. 河北农业大学学报, 33(4), 46-50.] | |
| [33] | Wang WY, Wang QJ, Deng ZF (1998). Communities structural characteristic and plant distribution pattern in alpine Kobresia meadow, Haibei region of Qinghai Province. Acta Phytoecologica Sinica, 22, 336-343. |
| [王文颖, 王启基, 邓自发 (1998). 青海海北地区高山嵩草草甸植物群落的结构特征及其分布格局. 植物生态学报, 22, 336-343.] | |
| [34] | Xu DQ (2002). Photosynthetic Efficiency. Shanghai Scientific and Technical Press, Shanghai. |
| [许大全 (2002). 光合作用效率. 上海科学技术出版社, 上海.] | |
| [35] |
Yamori W, Hikosaka K, Way DA (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research, 119, 101-117.
DOI PMID |
| [36] |
Yu BH, Lü CH (2011). Assessment of ecological vulnerability on the Tibetan Plateau. Geographical Research, 30, 2289-2295.
DOI |
| [于伯华, 吕昌河 (2011). 青藏高原高寒区生态脆弱性评价. 地理研究, 30, 2289-2295.] | |
| [37] | Zhou XM (2001). Chinese Kobresia Meadow. Science Press, Beijing. |
| [周兴民 (2001). 中国嵩草草甸. 科学出版社, 北京.] |
| [1] | 赵艳超, 陈立同. 土壤养分对青藏高原高寒草地生物量响应增温的调节作用[J]. 植物生态学报, 2023, 47(8): 1071-1081. |
| [2] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [3] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [4] | 林马震, 黄勇, 李洋, 孙建. 高寒草地植物生存策略地理分布特征及其影响因素[J]. 植物生态学报, 2023, 47(1): 41-50. |
| [5] | 朱玉英, 张华敏, 丁明军, 余紫萍. 青藏高原植被绿度变化及其对干湿变化的响应[J]. 植物生态学报, 2023, 47(1): 51-64. |
| [6] | 魏瑶, 马志远, 周佳颖, 张振华. 模拟增温改变青藏高原植物繁殖物候及植株高度[J]. 植物生态学报, 2022, 46(9): 995-1004. |
| [7] | 金伊丽, 王皓言, 魏临风, 侯颖, 胡景, 吴铠, 夏昊钧, 夏洁, 周伯睿, 李凯, 倪健. 青藏高原植物群落样方数据集[J]. 植物生态学报, 2022, 46(7): 846-854. |
| [8] | 卢晶, 马宗祺, 高鹏斐, 樊宝丽, 孙坤. 祁连山区演替先锋物种西藏沙棘的种群结构及动态对海拔梯度的响应[J]. 植物生态学报, 2022, 46(5): 569-579. |
| [9] | 胡潇飞, 魏临风, 程琦, 吴星麒, 倪健. 青藏高原地区气候图解数据集[J]. 植物生态学报, 2022, 46(4): 484-492. |
| [10] | 吴赞, 彭云峰, 杨贵彪, 李秦鲁, 刘洋, 马黎华, 杨元合, 蒋先军. 青藏高原高寒草地退化对土壤及微生物化学计量特征的影响[J]. 植物生态学报, 2022, 46(4): 461-472. |
| [11] | 郑周涛, 张扬建. 1982-2018年青藏高原水分利用效率变化及归因分析[J]. 植物生态学报, 2022, 46(12): 1486-1496. |
| [12] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [13] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| [14] | 刘宁, 彭守璋, 陈云明. 气候因子对青藏高原植被生长的时间效应[J]. 植物生态学报, 2022, 46(1): 18-26. |
| [15] | 聂秀青, 王冬, 周国英, 熊丰, 杜岩功. 三江源地区高寒湿地土壤微生物生物量碳氮磷及其化学计量特征[J]. 植物生态学报, 2021, 45(9): 996-1005. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19