

Chin J Plant Ecol ›› 2020, Vol. 44 ›› Issue (6): 677-686.DOI: 10.17521/cjpe.2020.0058
• Research Articles • Previous Articles Next Articles
LIU Li-Yan1,2, FENG Jin-Xia1, LIU Wen-Xin3, WAN Xian-Chong1,*( )
)
Received:2020-03-06
Accepted:2020-04-23
Online:2020-06-20
Published:2020-06-12
Contact:
WAN Xian-Chong
Supported by:LIU Li-Yan, FENG Jin-Xia, LIU Wen-Xin, WAN Xian-Chong. Effects of drought stress on photosynthesis, growth and root structure of transgenic PtPIP2;8 poplar 84K (Populus alba × P. glandulosa)[J]. Chin J Plant Ecol, 2020, 44(6): 677-686.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2020.0058
| 基因名称 Gene name | 上游引物 Forward primer | 下游引物 Reverse primer |
|---|---|---|
| PtACTINrt | 5′-AAACTGTAATGGTCCTCCCTCCG-3′ | 5′-GCATCATCACAATCACTCTCCGA-3′ |
| PtPIP2;8 | 5′-GAGACTGTGAGGGACTACCAGGA-3′ | 5′-AATACCAAGAATGCCAACACCAC-3′ |
Table 1 Primer sequences for real-time PCR
| 基因名称 Gene name | 上游引物 Forward primer | 下游引物 Reverse primer |
|---|---|---|
| PtACTINrt | 5′-AAACTGTAATGGTCCTCCCTCCG-3′ | 5′-GCATCATCACAATCACTCTCCGA-3′ |
| PtPIP2;8 | 5′-GAGACTGTGAGGGACTACCAGGA-3′ | 5′-AATACCAAGAATGCCAACACCAC-3′ |
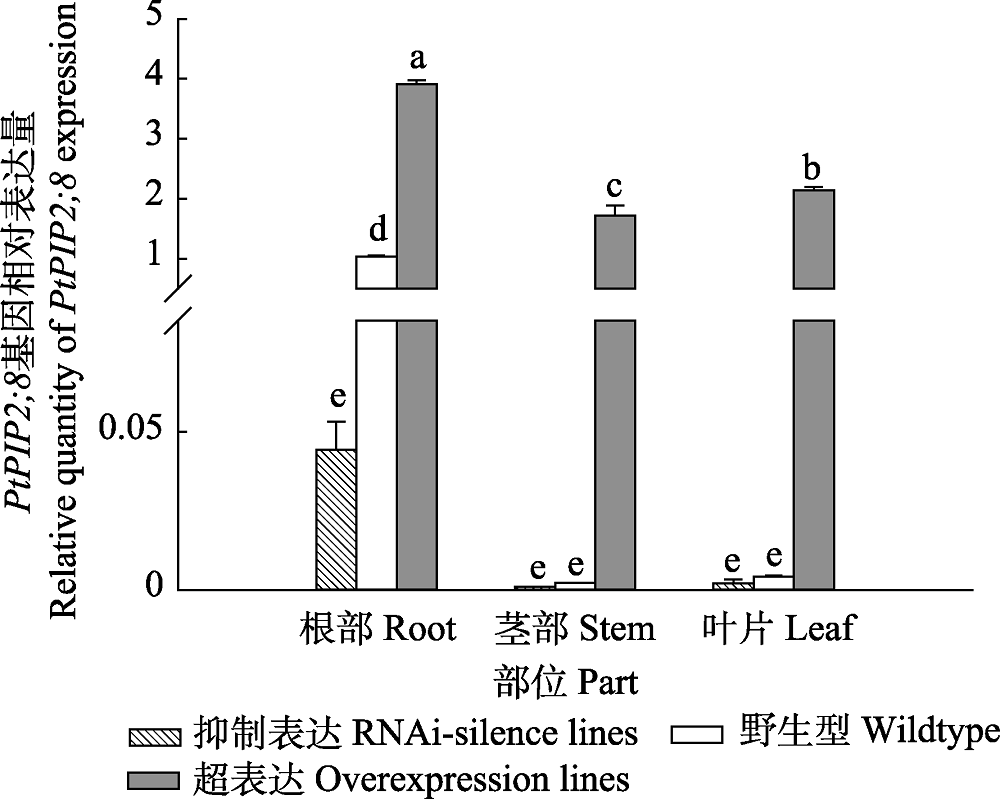
Fig. 1 Relative quantity of PtPIP2;8 gene expression in transgenic and wildtype poplar 84K (mean + SD). Different lowercase letters indicate significant differences in relative expression levels between different parts of transgenic and wildtype plants (p < 0.05).
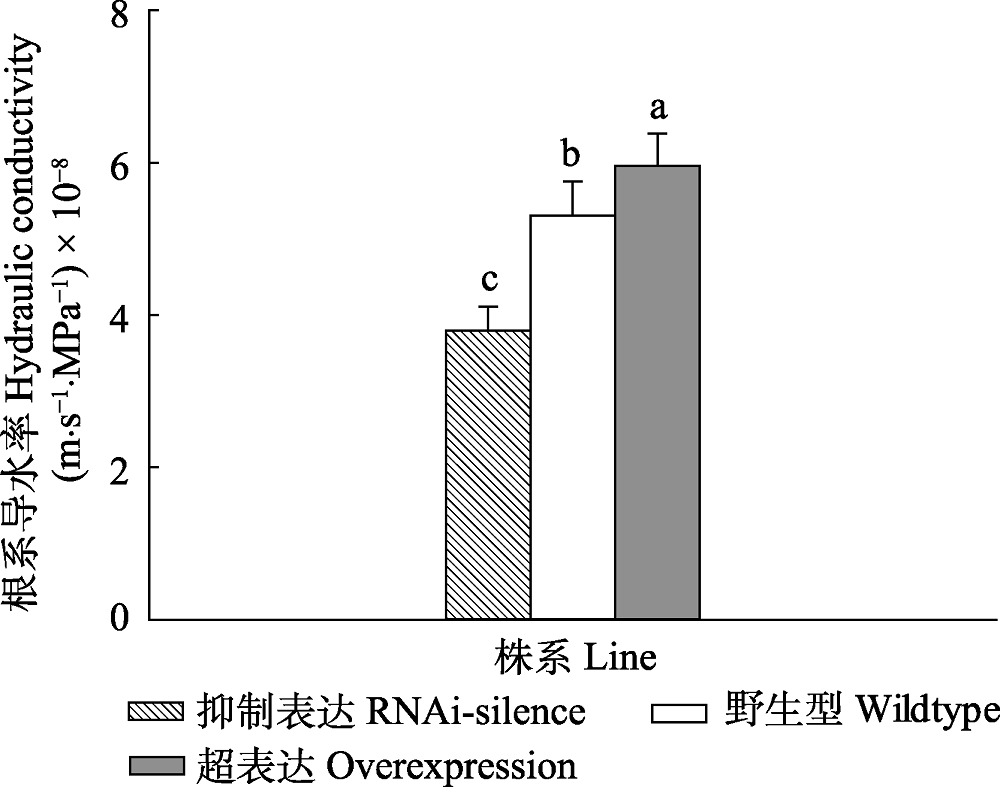
Fig. 2 Changes of root hydraulic conductivity of transgenic and wildtype poplar 84K under normal watering condition (mean + SD). Different lowercase letters indicate significant differences in transgenic and wildtype plants (p < 0.05).
| 株系 Line | 基径 Basal diameter (mm) | 苗高 Height (cm) | 根冠比 Root-shoot ratio | 叶面积 Leaf area (cm2) | 比叶面积 Specific leaf area (cm2·g-1) |
|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 4.93 ± 0.26c | 71.60 ± 4.57b | 0.54 ± 0.05a | 534.35 ± 32.47b | 254.33 ± 14.32a |
| 野生型 Wildtype | 5.62 ± 0.43a | 82.55 ± 4.70a | 0.50 ± 0.03b | 565.23 ± 25.05a | 262.57 ± 20.49a |
| 超表达 Overexpression | 5.02 ± 0.46bc | 83.85 ± 4.99a | 0.32 ± 0.02c | 576.75 ± 13.31a | 240.61 ± 11.19a |
Table 2 The change of biomass of transgenic and wildtype poplar 84K under normal water condition (mean ± SD)
| 株系 Line | 基径 Basal diameter (mm) | 苗高 Height (cm) | 根冠比 Root-shoot ratio | 叶面积 Leaf area (cm2) | 比叶面积 Specific leaf area (cm2·g-1) |
|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 4.93 ± 0.26c | 71.60 ± 4.57b | 0.54 ± 0.05a | 534.35 ± 32.47b | 254.33 ± 14.32a |
| 野生型 Wildtype | 5.62 ± 0.43a | 82.55 ± 4.70a | 0.50 ± 0.03b | 565.23 ± 25.05a | 262.57 ± 20.49a |
| 超表达 Overexpression | 5.02 ± 0.46bc | 83.85 ± 4.99a | 0.32 ± 0.02c | 576.75 ± 13.31a | 240.61 ± 11.19a |
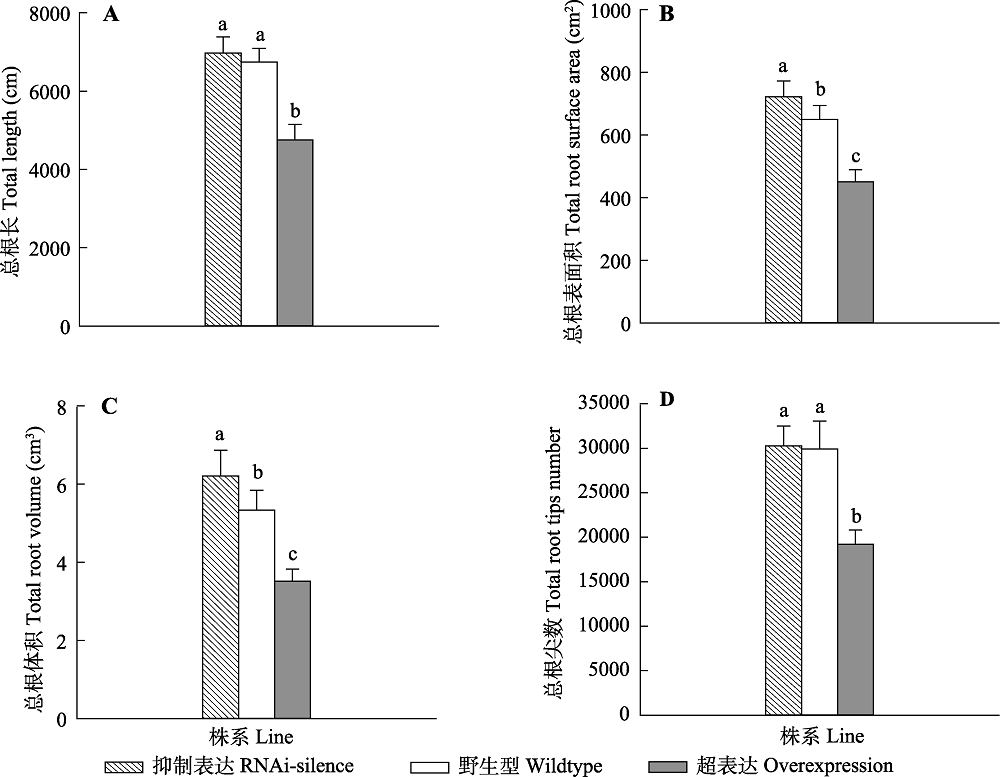
Fig. 3 Changes in root morphological traits of transgenic and wildtype poplar 84K under normal water condition (mean + SD). ferent lowercase letters indicate significant differences between transgenic and wildtype plants (p < 0.05).
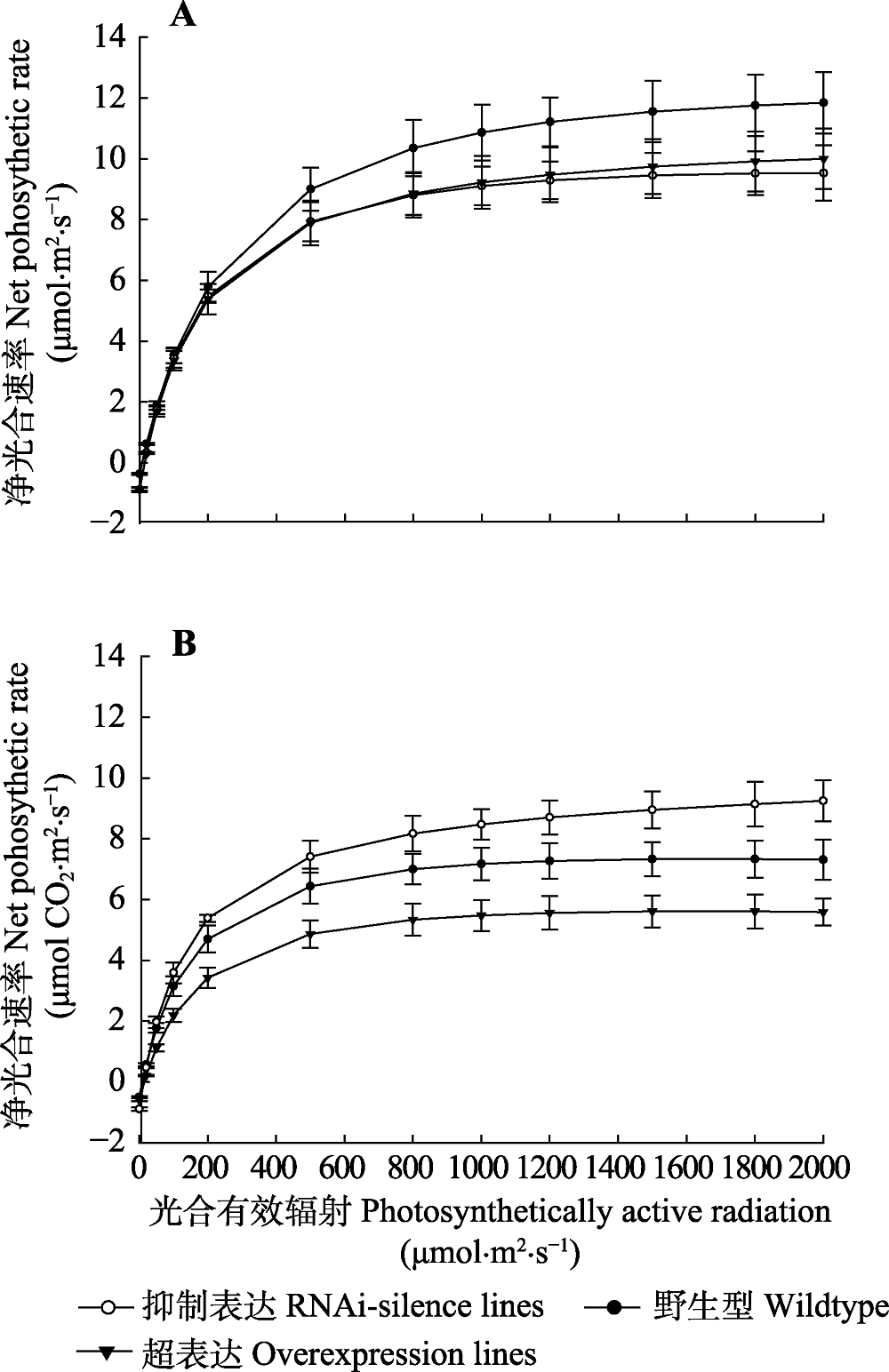
Fig. 4 Light response curve of net photosynthetic rate in transgenic and wildtype poplar 84K (mean ± SD). Light response of net photosynthetic rate under normal watering conditions. B, Light response of net photosynthetic rate after drought stress.
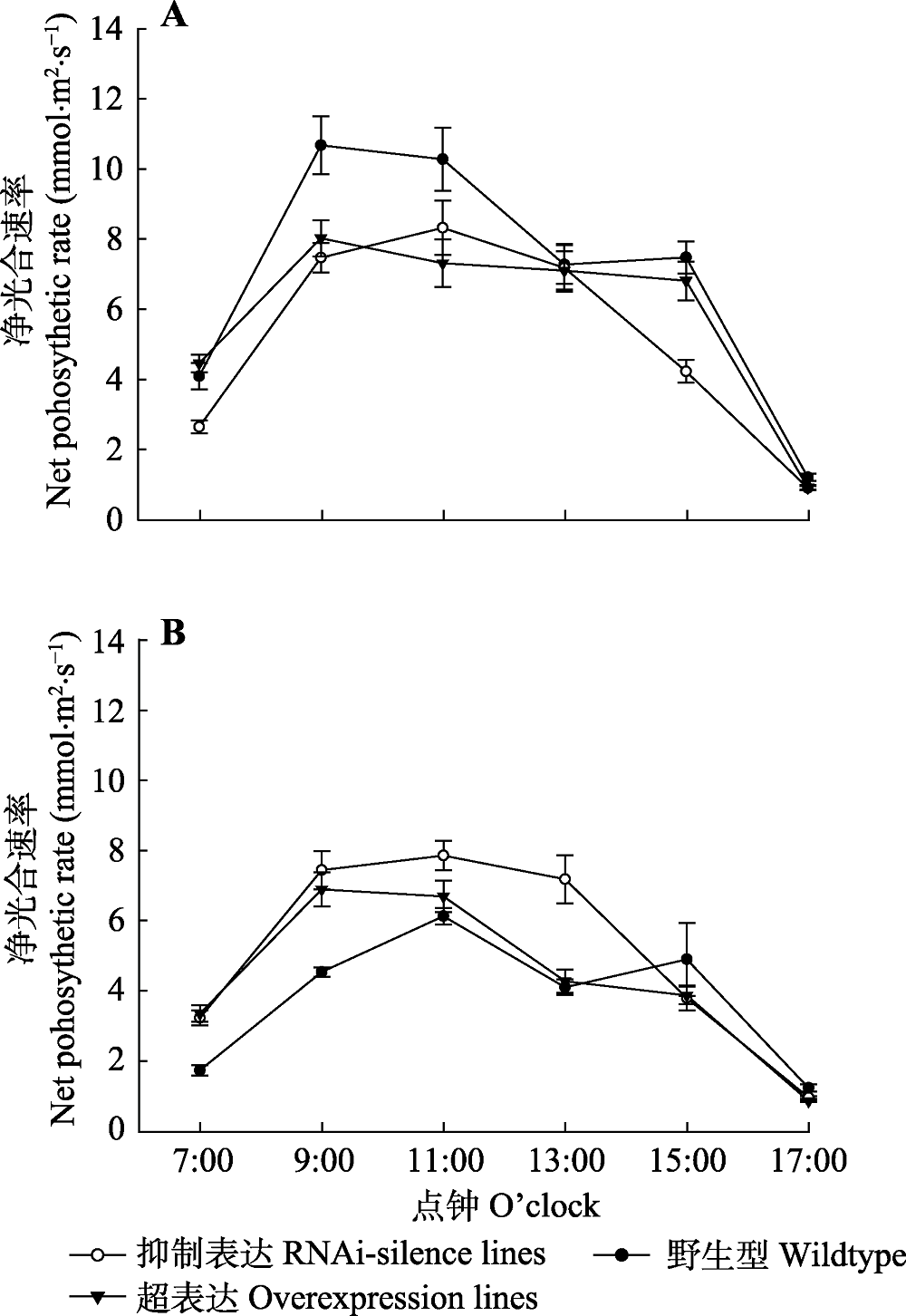
Fig. 5 Diurnal variations of net photosynthetic rate in transgenic and wildtype poplar 84K (mean ± SD). A, Diurnal variations of net photosynthetic rate under normal watering conditions. B, Diurnal variations of net photosynthetic rate after drought stress.
| 株系 Line | 基径 Basal diameter (%) | 苗高 Height (%) | 地上生物量 Aboveground biomass (%) | 地下生物量 Belowground biomass (%) | 根冠比 Root shoot ratio | 叶面积 Leaf area (%) |
|---|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 97 ± 3a | 94 ± 3a | 93 ± 2a | 84 ± 5a | 0.5 ± 0.02a | 68 ± 4a |
| 野生型 Wildtype | 92 ± 3b | 88 ± 4b | 86 ± 7b | 77 ± 4b | 0.4 ± 0.03b | 44 ± 2b |
| 超表达 Overexpression | 92 ± 2b | 87 ± 4b | 77 ± 3c | 76 ± 5b | 0.3 ± 0.02c | 43 ± 2b |
Table 3 Changes of relative growth rate and biomass of transgenic and wildtype poplar 84K after drought stress (mean ± SD)
| 株系 Line | 基径 Basal diameter (%) | 苗高 Height (%) | 地上生物量 Aboveground biomass (%) | 地下生物量 Belowground biomass (%) | 根冠比 Root shoot ratio | 叶面积 Leaf area (%) |
|---|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 97 ± 3a | 94 ± 3a | 93 ± 2a | 84 ± 5a | 0.5 ± 0.02a | 68 ± 4a |
| 野生型 Wildtype | 92 ± 3b | 88 ± 4b | 86 ± 7b | 77 ± 4b | 0.4 ± 0.03b | 44 ± 2b |
| 超表达 Overexpression | 92 ± 2b | 87 ± 4b | 77 ± 3c | 76 ± 5b | 0.3 ± 0.02c | 43 ± 2b |
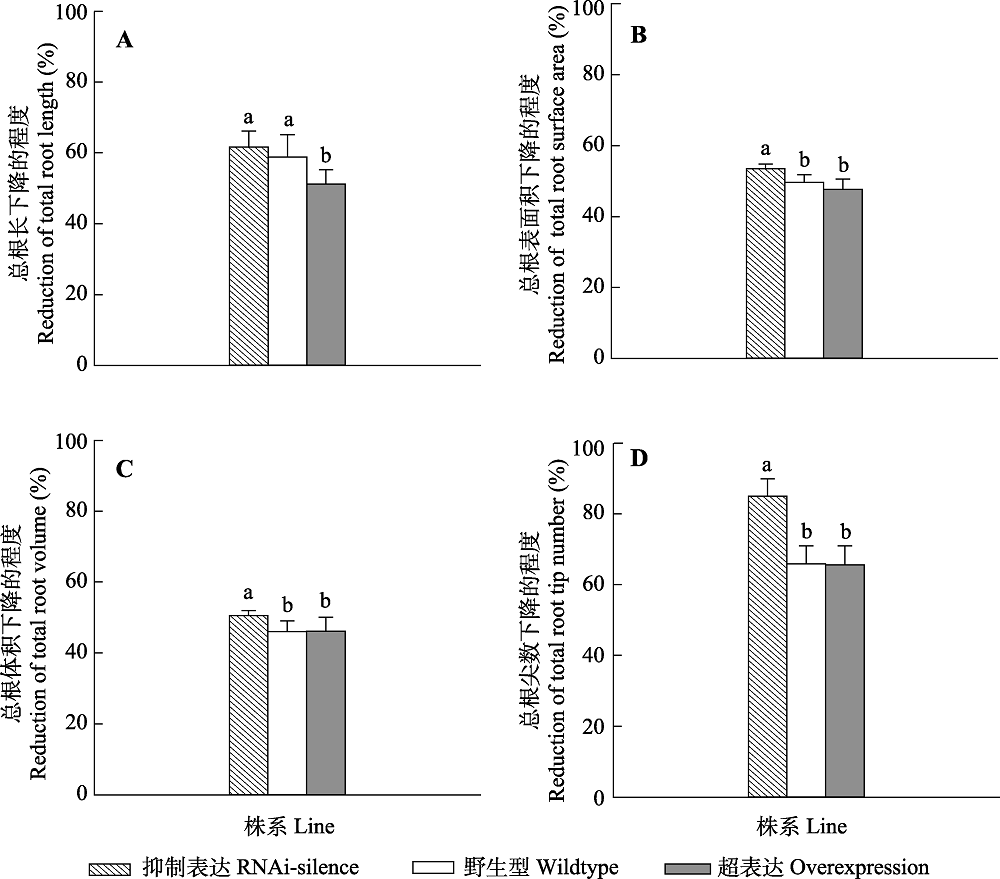
Fig. 6 Changes of root morphological traits of transgenic and wildtype poplar 84K after drought dress (mean + SD). Different lowercase letters indicate significant differences between transgenic and wildtype plants (p < 0.05).
| [1] |
Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003). Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. The Plant Cell, 15, 1-9.
DOI URL PMID |
| [2] |
Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005). Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology, 59, 469-484.
DOI URL PMID |
| [3] | Bao ZLT, Gao L, Wang SM (2017). Physiological functions of plant aquaporin. Plant Physiology Journal, 53, 1171-1178. |
| [ 包珠拉太, 高丽, 王锁民 (2017). 植物水通道蛋白及其生理功能. 植物生理学报, 53, 1171-1178.] | |
| [4] | Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005a). PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. Journal of Experimental Botany, 56, 113-121. |
| [5] | Bots M, Vergeldt F, Wolters-Arts M, Weterings K, van As H, Mariani C (2005b). Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiology, 137, 1049-1056. |
| [6] |
Boughalleb F, Hajlaoui H (2011). Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiologiae Plantarum, 33, 53-65.
DOI URL |
| [7] | Cai Q, Ding GJ, Wen XP (2016). Cloning of thePmPIP1 gene from Pinus massoniana and its expression with drought stress. Journal of Zhejiang A&F University, 33, 191-200. |
| [ 蔡琼, 丁贵杰, 文晓鹏 (2016). 马尾松水通道蛋白PmPIP1基因克隆及在干旱胁迫下的表达分析. 浙江农林大学学报, 33, 191-200.] | |
| [8] |
Chaumont F, Tyerman SD (2014). Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology, 164, 1600-1618.
DOI URL PMID |
| [9] |
Fotiadis D, Jenö P, Mini T, Wirtz S, Müller SA, Fraysse L, Kjellbom P, Engel A (2001). Structural characterization of two aquaporins isolated from native spinach leaf plasma membranes. Journal of Biological Chemistry, 276, 1707-1714.
DOI URL PMID |
| [10] | Huang J, Chen C, Zhang WX, Ding CJ, Su XH, Huang QJ (2017). Effects of drought stress on anatomical structure and photosynthetic characteristics of transgenic JERF36Populus alba × P. berolinensis seedling leaves. Scientia Silvae Sinicae, 53(5), 8-15. |
| [ 黄绢, 陈存, 张伟溪, 丁昌俊, 苏晓华, 黄秦军 (2017). 干旱胁迫对转JERF36银中杨苗木叶片解剖结构及光合特性的影响. 林业科学, 53(5), 8-15.] | |
| [11] |
Javot H, Maurel C (2002). The role of aquaporins in root water uptake. Annals of Botany, 90, 301-313.
DOI URL PMID |
| [12] | Jiang LJ, Chen CH, Yan X, Yang SM (2018). Research progress on responsive mechanism of aquaporins to drought stress in plants. Guihaia, 38, 672-680. |
| [ 江林娟, 陈春华, 颜旭, 杨世民 (2018). 植物水通道蛋白的干旱应答机制研究进展. 广西植物, 38, 672-680.] | |
| [13] |
Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P (2000). The role of aquaporins in cellular and whole plant water balance. Biochimica et Biophysica Acta, 1465, 324-342.
DOI URL PMID |
| [14] | Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U (1998). Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. The Plant Journal, 14, 121-128. |
| [15] |
Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression. The Plant Journal, 6, 187-199.
DOI URL PMID |
| [16] |
Katsuhara M, Koshio K, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K (2003). Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant and Cell Physiology, 44, 1378-1383.
DOI URL PMID |
| [17] |
Knapp AK, Smith MD (2001). Variation among biomes in temporal dynamics of aboveground primary production. Science, 291, 481-484.
DOI URL PMID |
| [18] | Knipfer T, Besse M, Verdeil JL, Fricke W (2011). Aquaporin- facilitated water uptake in barley (Hordeum vulgare L.) roots. Journal of Experimental Botany, 62, 4115-4126. |
| [19] | Leng HN (2012). Cloning and Expression of PIPs Gene in Populus and Aquaporins Role in Embolism Recovery. PhD dissertation, Chinese Academy of Forestry, Beijing. 23-95. |
| [ 冷华妮 (2012). 植物栓塞修复机制与质膜内在水通道蛋白基因的克隆、表达和转基因研究. 博士学位论文, 中国林业科学研究院, 北京. 23-95.] | |
| [20] | Lopez F, Bousser A, Sissoëff I, Gaspar M, Lachaise B, Hoarau J, Mahé A (2003). Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant and Cell Physiology, 44, 1384-1395. |
| [21] | Lovisolo C, Secchi F, Nardini A, Salleo S, Buffa R, Schubert A (2007). Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance. Physiologia Plantarum, 130, 543-551. |
| [22] | Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ (2002). Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology, 130, 2101-2110. |
| [23] | Martre P, North GB, Nobel PS (2001). Hydraulic conductance and mercury-sensitive water transport for roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiology, 126, 352-362. |
| [24] | Matsunami M, Toyofuku K, Ishikawa-Sakurai J, Ogawa A, Matsunami T, Kokubun M (2016). Root development and the expression of aquaporin genes in rice seedlings under osmotic stress. Plant Production Science, 19, 315-322. |
| [25] | O’Brien M, Bertrand C, Matton DP (2002). Characterization of a fertilization-induced and developmentally regulated plasma-membrane aquaporin expressed in reproductive tissues, in the wild potato Solanum chacoense Bitt. Planta, 215, 485-493. |
| [26] |
Prieto I, Armas C, Pugnaire FI (2012). Water release through plant roots: new insights into its consequences at the plant and ecosystem level. New Phytologist, 193, 830-841.
DOI URL PMID |
| [27] | Schuurmans JAMJ, van Dongen JT, Rutjens BPW, Boonman A, Pieterse CMJ, Borstlap AC (2003). Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Molecular Biology, 53, 655-667. |
| [28] |
Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002). PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. The Plant Cell, 14, 869-876.
DOI URL PMID |
| [29] | Steudle E, Peterson CA (1998). How does water get through roots? Journal of Experimental Botany, 49, 775-788. |
| [30] |
Suga S, Imagawa S, Maeshima M (2001). Specificity of the accumulation of mRNAs and proteins of the plasma membrane and tonoplast aquaporins in radish organs. Planta, 212, 294-304.
DOI URL PMID |
| [31] |
Weig A, Deswarte C, Chrispeels MJ (1997). The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiology, 114, 1347-1357.
DOI URL PMID |
| [32] | Yao QQ, Xie GS (2005). The photosynthetic stomatal and nonstomatal limitation under drought stress. Chinese Journal of Tropical Agriculture, 25(4), 80-85. |
| [ 姚庆群, 谢贵水 (2005). 干旱胁迫下光合作用的气孔与非气孔限制. 热带农业科学, 25(4), 80-85.] | |
| [33] |
Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F (2007). FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proceedings of the National Academy of Sciences of the United States of America, 104, 12359-12364.
DOI URL PMID |
| [34] | Zhao T, Cheng L, Wang C, You JQ, Zhu YF, Wang YX (2018). Effect of different apple scion-rootstock combinations on growth and photosynthesis characteristics. Acta Botanica Boreali-Occidentalia Sinica, 38, 1707-1716. |
| [ 赵通, 程丽, 王城, 游继权, 朱燕芳, 王延秀 (2018). 不同苹果砧穗组合的生长及光合特性. 西北植物学报, 38, 1707-1716.] | |
| [35] | Zhou XY (2013). Research on 84K Populus Regeneration and Genetic Transformation System. Master degree dissertation, Northeast Forestry University, Harbin. 1-8. |
| [ 周熙莹 (2013). 84K杨再生和遗传转化体系的研究. 硕士学位论文, 东北林业大学, 哈尔滨. 1-8.] |
| [1] | FAN Hong-Kun, ZENG Tao, JIN Guang-Ze, LIU Zhi-Li. Leaf trait variation and trade-offs among growth types of broadleaf plants in Xiao Hinggan Mountains [J]. Chin J Plant Ecol, 2024, 48(3): 364-376. |
| [2] | CHEN Tu-Qiang, XU Gui-Qing, LIU Shen-Si, LI Yan. Hydraulic traits adjustments and nonstructural carbohydrate dynamics of Haloxylon ammodendron under drought stress [J]. Chin J Plant Ecol, 2023, 47(10): 1407-1421. |
| [3] | LI Bian-Bian, ZHANG Feng-Hua, ZHAO Ya-Guang, SUN Bing-Nan. Effects of different clipping degrees on non-structural carbohydrate metabolism and biomass of Cyperus esculentus [J]. Chin J Plant Ecol, 2023, 47(1): 101-113. |
| [4] | ZHOU Jie, YANG Xiao-Dong, WANG Ya-Yun, LONG Yan-Xin, WANG Yan, LI Bo-Rui, SUN Qi-Xing, SUN Nan. Difference in adaptation strategy between Haloxylon ammodendron and Alhagi sparsifolia to drought [J]. Chin J Plant Ecol, 2022, 46(9): 1064-1076. |
| [5] | ZHANG Xiao-Yan, WEE Kim Shan Alison, KAJITA Tadashi, CAO Kun-Fang. Effects of provenance on leaf structure and function of two mangrove species: the genetic adaptation to temperature [J]. Chin J Plant Ecol, 2021, 45(11): 1241-1250. |
| [6] | LUO Jin-Huan, TAN Zhao-Yuan, CHEN Bin, CHEN Guang-Wu, JIANG Kai, HEI Qi-Fang, ZHANG Hui. Key characteristics for facilitating Leucaena leucocephala to successfully invade pioneer communities of tropical rain forests [J]. Chin J Plant Ecol, 2020, 44(12): 1215-1223. |
| [7] | XU Li-Jiao, HAO Zhi-Peng, XIE Wei, LI Fang, CHEN Bao-Dong. Transmembrane H + and Ca 2+ fluxes through extraradical hyphae of arbuscular mycorrhizal fungi in response to drought stress [J]. Chin J Plant Ecol, 2018, 42(7): 764-773. |
| [8] | CHENG Han-Ting, LI Qin-Fen, LIU Jing-Kun, YAN Ting-Liang, ZHANG Qiao-Yan, WANG Jin-Chuang. Seasonal changes of photosynthetic characteristics of Alpinia oxyphylla growing under Hevea brasiliensis [J]. Chin J Plant Ecol, 2018, 42(5): 585-594. |
| [9] | Xi WANG,Hong-Ling HU,Ting-Xing HU,Cheng-Hao ZHANG,Xin WANG,Dan LIU. Effects of drought stress on the osmotic adjustment and active oxygen metabolism of Phoebe zhennan seedlings and its alleviation by nitrogen application [J]. Chin J Plan Ecolo, 2018, 42(2): 240-251. |
| [10] | Dan-Dan LUO, Chuan-Kuan WANG, Ying JIN. Plant water-regulation strategies: Isohydric versus anisohydric behavior [J]. Chin J Plan Ecolo, 2017, 41(9): 1020-1032. |
| [11] | Zhan-Wei ZHAI, Ji-Rui GONG, Qin-Pu LUO, Yan PAN, Taogetao BAOYIN, Sha XU, Min LIU, Li-Li YANG. Effects of nitrogen addition on photosynthetic characteristics of Leymus chinensis in the temperate grassland of Nei Mongol, China [J]. Chin J Plant Ecol, 2017, 41(2): 196-208. |
| [12] | Yu CEN, Mei-Zhen LIU. Effects of dew on eco-physiological traits and leaf structures of Leymus chinensis and Agropyron cristatum grown under drought stress [J]. Chin J Plan Ecolo, 2017, 41(11): 1199-1207. |
| [13] | Rui GUO, Ji ZHOU, Fan YANG, Feng LI, Hao-Ru LI, Xu XIA, Qi LIU. Growth metabolism of wheat under drought stress at the jointing-booting stage [J]. Chin J Plan Ecolo, 2016, 40(12): 1319-1327. |
| [14] | ZOU Chang-Ming,WANG Yun-Qing,LIU Ying,ZHANG Xiao-Hong,TANG Shan. Responses of photosynthesis and growth to weak light regime in four legume species [J]. Chin J Plan Ecolo, 2015, 39(9): 909-916. |
| [15] | AN Dong-Sheng,CAO Juan,HUANG Xiao-Hua,ZHOU Juan,DOU Mei-An. Application of Lake-model based indices from chlorophyll fluorescence on sugarcane seedling drought resistance study [J]. Chin J Plan Ecolo, 2015, 39(4): 398-406. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn