

Chin J Plant Ecol ›› 2018, Vol. 42 ›› Issue (7): 764-773.DOI: 10.17521/cjpe.2018.0089
Special Issue: 菌根真菌
• Research Articles • Previous Articles Next Articles
XU Li-Jiao1,2, HAO Zhi-Peng1, XIE Wei1,2, LI Fang1,2, CHEN Bao-Dong1,2,*( )
)
Online:2018-07-20
Published:2018-06-11
Contact:
Bao-Dong CHEN
Supported by:XU Li-Jiao, HAO Zhi-Peng, XIE Wei, LI Fang, CHEN Bao-Dong. Transmembrane H + and Ca 2+ fluxes through extraradical hyphae of arbuscular mycorrhizal fungi in response to drought stress[J]. Chin J Plant Ecol, 2018, 42(7): 764-773.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2018.0089
| 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) | 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) |
|---|---|---|---|
| Mg2+ | 70.8 | Fe2+ | 0.1 |
| SO42- | 280.1 | Mn2+ | 1.6 |
| K+ | 61 | Zn2+ | 0.6 |
| NO3- | 230 | BO33- | 0.24 |
| Cl- | 37.2 | 甘氨酸 Glycine | 3 |
| H2PO4- | 4.8 | 维生素B1 Vitamin B1 | 0.1 |
| Ca2+ | 49 | 维生素B6 Vitamin B6 | 0.1 |
| I- | 0.58 | 烟酸 Nicotinic acid | 0.5 |
| Na+ | 0.43 | 肌醇 Inositol | 50 |
Table 1 Formulation of the M medium
| 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) | 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) |
|---|---|---|---|
| Mg2+ | 70.8 | Fe2+ | 0.1 |
| SO42- | 280.1 | Mn2+ | 1.6 |
| K+ | 61 | Zn2+ | 0.6 |
| NO3- | 230 | BO33- | 0.24 |
| Cl- | 37.2 | 甘氨酸 Glycine | 3 |
| H2PO4- | 4.8 | 维生素B1 Vitamin B1 | 0.1 |
| Ca2+ | 49 | 维生素B6 Vitamin B6 | 0.1 |
| I- | 0.58 | 烟酸 Nicotinic acid | 0.5 |
| Na+ | 0.43 | 肌醇 Inositol | 50 |
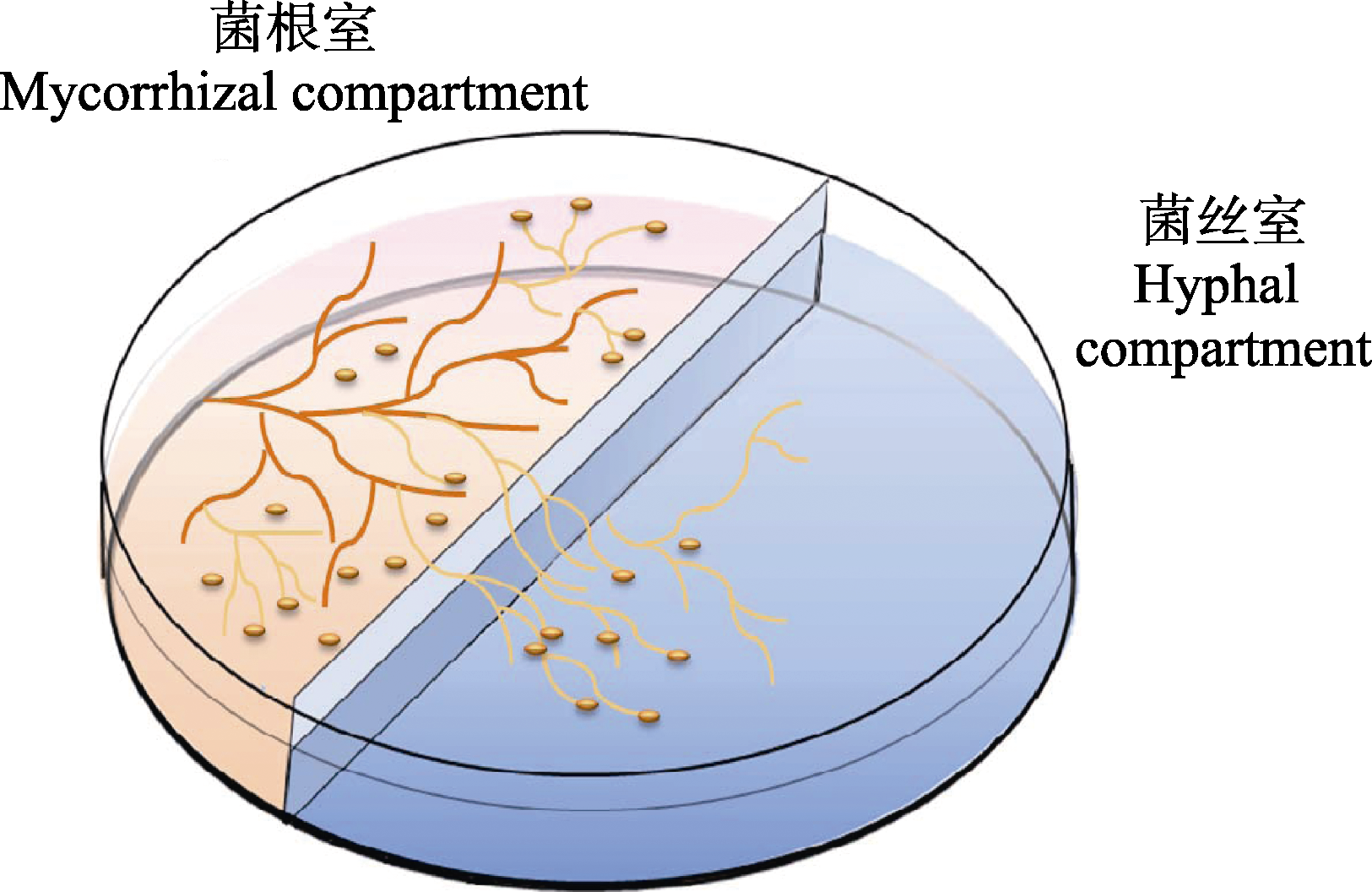
Fig. 1 Diagram of the two-compartments in vitro culture system of arbuscular mycorrhizal fungi with hairy carrot root. Mycorrhizal compartment was filled with solid M medium gelled with 0.4% phytagel, allowing development of mycorrhizal roots; extraradical mycelium ramified into hyphal compartment filled with liquid M medium without sucrose and phytagel, and the roots that crossed the central wall were trimmed to prevent their growth in hyphal compartment (referred to St-Arnaud et al., 1996).
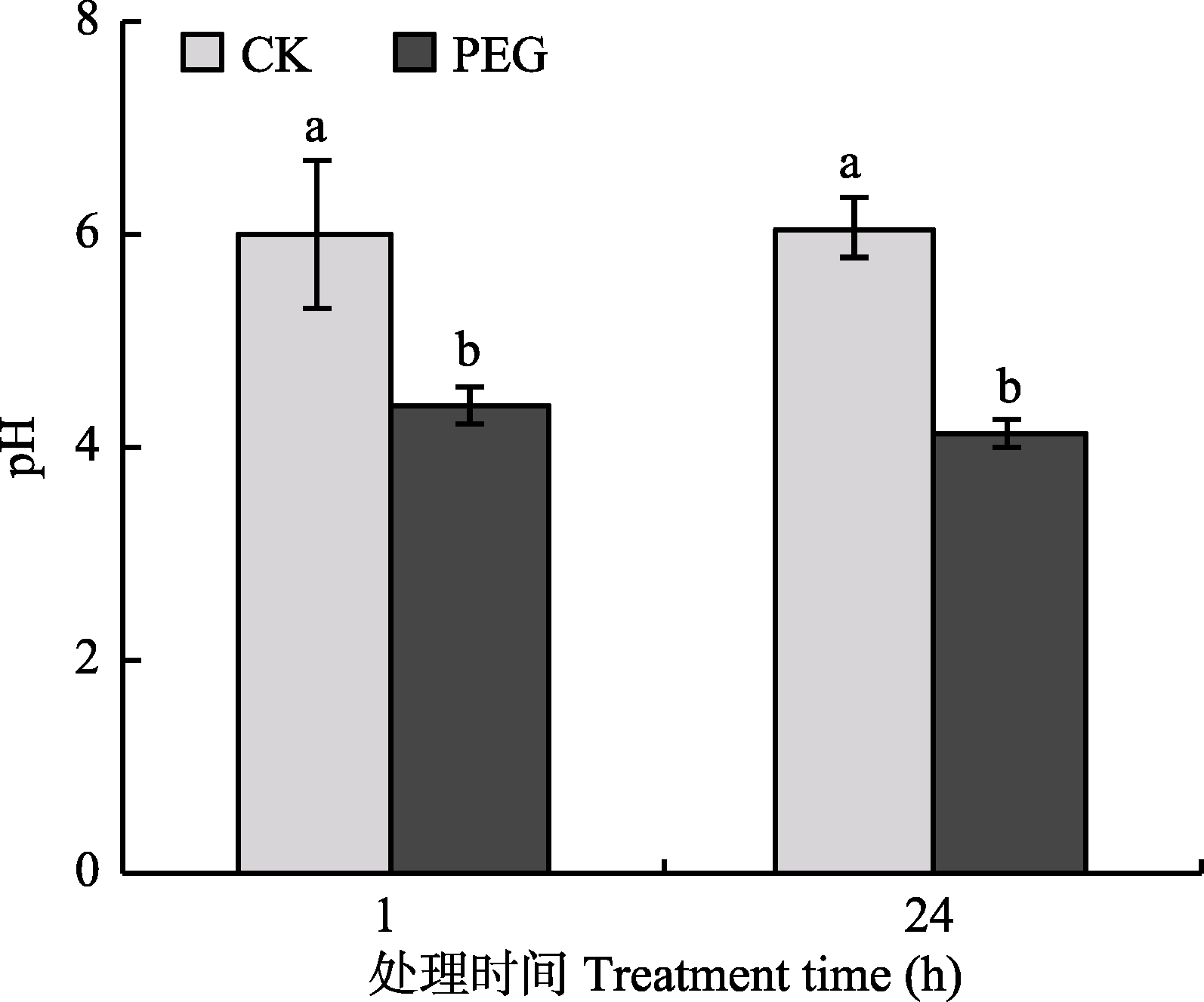
Fig. 2 The pH value of culture medium in hyphal compartment after treatment by PEG (mean ± SD, n = 6). Different lowercase letters indicate significant difference between treatments (Duncan’s multiple range test, p < 0.05).
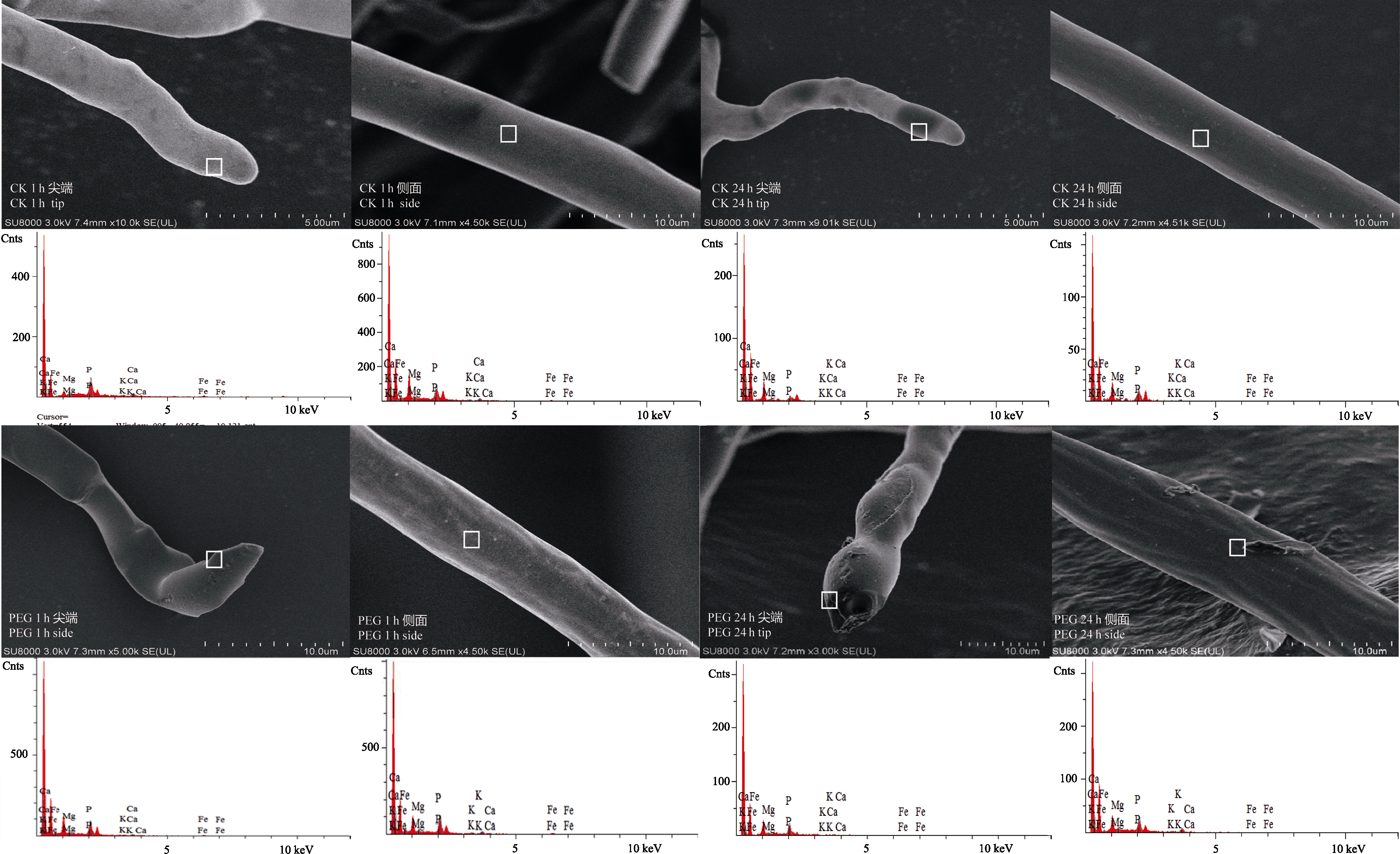
Fig. 3 FE-SEM images and selective elemental analysis (by EDS) of arbuscular mycorrhizal fungi hyphae after treatment by PEG for 1 h (PEG 1 h) and 24 h (PEG 24 h). CK 1 h and CK 24 h are corresponding controls.
| P | Ca | Fe | ||||||
|---|---|---|---|---|---|---|---|---|
| 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | |||
| 尖端 Tip | CK | 1 h | 29.53 ± 2.03cd | 30.03 ± 0.52d | 5.97 ± 0.68c | 6.14 ± 0.72c | 15.86 ± 0.26c | 24.64 ± 2.07cd |
| 24 h | 31.34 ± 2.34c | 32.85 ± 0.61d | 4.49 ± 0.86c | 5.39 ± 1.27c | 14.60 ± 1.26c | 26.53 ± 1.91c | ||
| PEG | 1 h | 38.80 ± 1.07b | 39.62 ± 0.41c | 16.15 ± 0.84a | 18.00 ± 0.87a | 37.15 ± 0.69a | 37.44 ± 1.13b | |
| 24 h | 51.49 ± 1.63a | 50.46 ± 0.39a | 5.32 ± 1.45c | 4.91 ± 0.66c | 32.48 ± 1.70ab | 52.43 ± 0.49a | ||
| 侧面 Side | CK | 1 h | 23.51 ± 1.12d | 39.01 ± 0.34c | 7.83 ± 1.3bc | 7.88 ± 1.40bc | 12.03 ± 1.11d | 19.51 ± 1.39d |
| 24 h | 28.00 ± 1.67cd | 43.47 ± 1.07bc | 9.84 ± 0.83b | 10.32 ± 0.92b | 12.07 ± 0.78d | 19.17 ± 0.87d | ||
| PEG | 1 h | 37.69 ± 0.84b | 37.51 ± 1.43c | 10.34 ± 1.12b | 12.03 ± 0.75b | 28.54 ± 0.76b | 33.28 ± 1.13b | |
| 24 h | 56.12 ± 2.21a | 45.04 ± 0.47b | 14.99 ± 0.73a | 16.21 ± 0.40a | 35.09 ± 1.43a | 41.70 ± 0.60ab | ||
Table 2 Concentrations of P, Ca and Fe at the tip and side of arbuscular mycorrhizal fungi hyphae after treatment by PEG for 1 h and 24 h (determined by energy-dispersive X-ray spectroscopy) (mean ± SD, n = 6)
| P | Ca | Fe | ||||||
|---|---|---|---|---|---|---|---|---|
| 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | |||
| 尖端 Tip | CK | 1 h | 29.53 ± 2.03cd | 30.03 ± 0.52d | 5.97 ± 0.68c | 6.14 ± 0.72c | 15.86 ± 0.26c | 24.64 ± 2.07cd |
| 24 h | 31.34 ± 2.34c | 32.85 ± 0.61d | 4.49 ± 0.86c | 5.39 ± 1.27c | 14.60 ± 1.26c | 26.53 ± 1.91c | ||
| PEG | 1 h | 38.80 ± 1.07b | 39.62 ± 0.41c | 16.15 ± 0.84a | 18.00 ± 0.87a | 37.15 ± 0.69a | 37.44 ± 1.13b | |
| 24 h | 51.49 ± 1.63a | 50.46 ± 0.39a | 5.32 ± 1.45c | 4.91 ± 0.66c | 32.48 ± 1.70ab | 52.43 ± 0.49a | ||
| 侧面 Side | CK | 1 h | 23.51 ± 1.12d | 39.01 ± 0.34c | 7.83 ± 1.3bc | 7.88 ± 1.40bc | 12.03 ± 1.11d | 19.51 ± 1.39d |
| 24 h | 28.00 ± 1.67cd | 43.47 ± 1.07bc | 9.84 ± 0.83b | 10.32 ± 0.92b | 12.07 ± 0.78d | 19.17 ± 0.87d | ||
| PEG | 1 h | 37.69 ± 0.84b | 37.51 ± 1.43c | 10.34 ± 1.12b | 12.03 ± 0.75b | 28.54 ± 0.76b | 33.28 ± 1.13b | |
| 24 h | 56.12 ± 2.21a | 45.04 ± 0.47b | 14.99 ± 0.73a | 16.21 ± 0.40a | 35.09 ± 1.43a | 41.70 ± 0.60ab | ||

Fig. 4 The effect of PEG treatment for 1 h and 24 h on the pH value at the tip and side of arbuscular mycorrhizal fungi hypha. Fluorescence image at 488 nm excitation shows the pH variation in the hyphal cell.
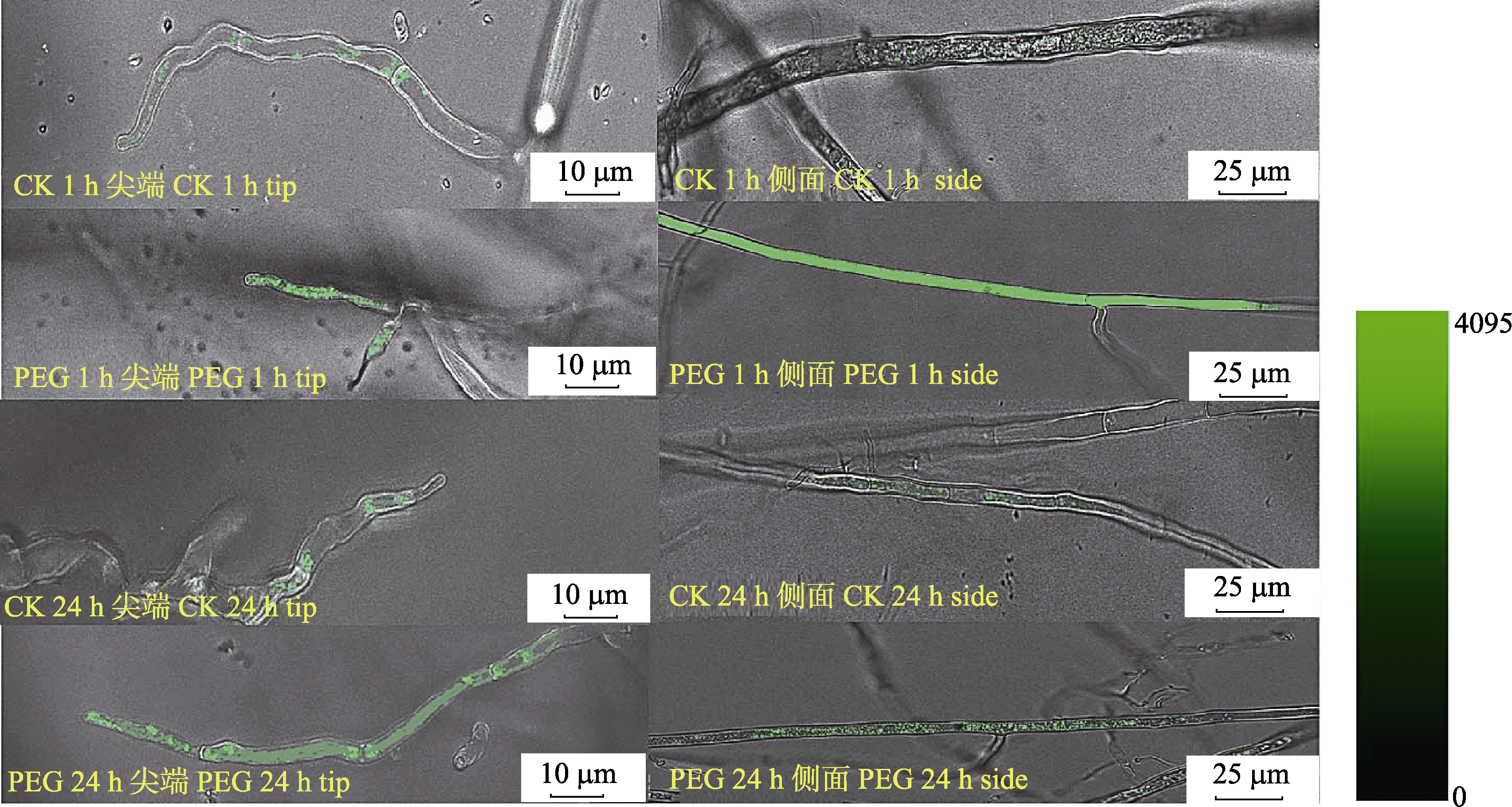
Fig. 5 The effect of PEG treatment for 1 h and 24 h on the Ca2+ concentration at the tip and side of arbuscular mycorrhizal fungi hypha. Fluorescence image at 488 nm excitation shows the Ca2+ variation in the hyphal cell.
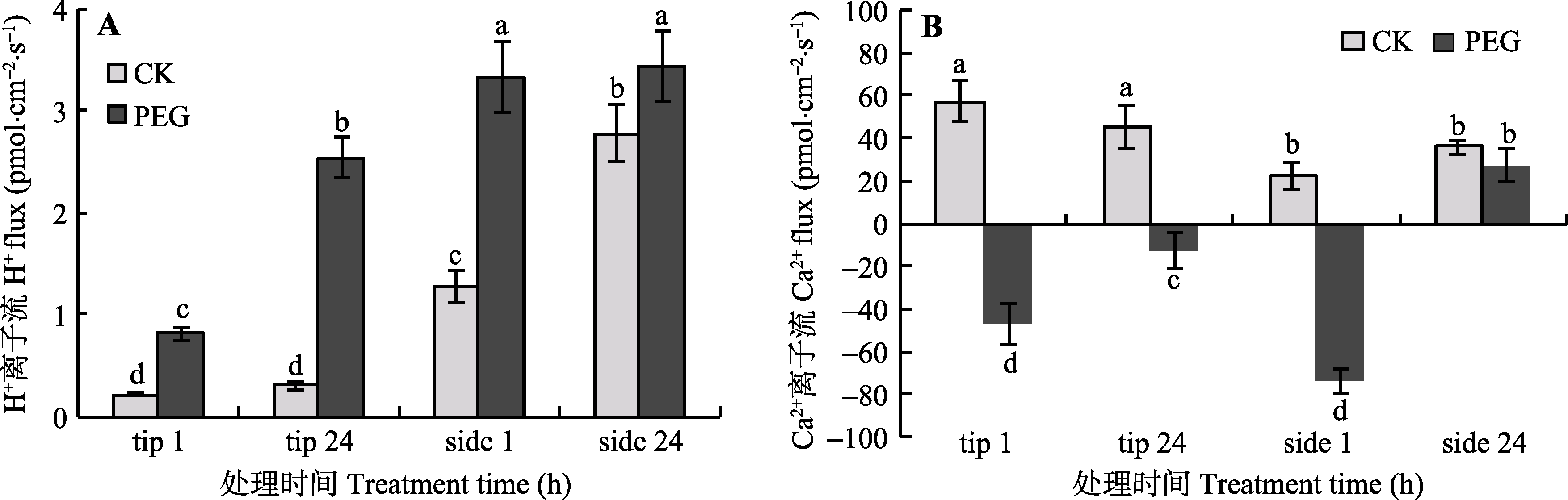
Fig. 6 The effects of PEG treatment on H+ (A) and Ca2+ (B) flux at the tip and side of arbuscular mycorrhizal hyphae (mean ± SD, n = 6). tip stands for hyphal tip, and side stands for lateral hypha. Columns marked by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
| [1] |
Augé RM, Duan X ( 1991). Mycorrhizal fungi and nonhydraulic root signals of soil drying. Plant Physiology, 97, 821-824.
DOI URL |
| [2] |
Augé RM, Toler HD, Saxton AM ( 2015). Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza, 25, 13-24.
DOI URL PMID |
| [3] |
Ayling SM, Smith SE, Smith FA ( 2000). Transmembrane electric potential difference of germ tubes of arbuscular mycorrhizal fungi responds to external stimuli. New Phytologist, 147, 631-639.
DOI URL |
| [4] |
Azad AK, Sawa Y, Ishikawa T, Shibata H ( 2004). Phosphorylation of plasma membrane aquaporin regulates temperature-?dependent opening of tulip petals. Plant and Cell Physiology, 45, 608-617.
DOI URL PMID |
| [5] |
Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM ( 2012). Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Annals of Botany, 109, 1009-1017.
DOI URL PMID |
| [6] |
Bartnicki-Garcia S, Bracker CE, Gierz G, López-Franco R, Lu H ( 2000). Mapping the growth of fungal hyphae: Orthogonal cell wall expansion during tip growth and the role of turgor. Biophysical Journal, 79, 2382-2390.
DOI URL PMID |
| [7] |
Bécard G, Fortin JA ( 1988). Early events of vesicular?-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytologist, 108, 211-218.
DOI URL |
| [8] |
Bunney TD, Shaw PJ, Watkins PA, Taylor JP, Beven AF, Wells B, Calder GM, Dr?bak BK ( 2000). ATP-dependent regulation of nuclear Ca 2+ levels in plant cells . FEBS Letters, 476, 145-149.
DOI URL PMID |
| [9] |
Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, San Segundo B ( 2014). Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiology, 165, 688-704.
DOI URL PMID |
| [10] |
Chitarra W, Pagliarani C, Maserti B, Lumini E, Siciliano I, Cascone P, Schubert A, Gambino G, Balestrini R, Guerrieri E ( 2016). Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiology, 171, 1009-1023.
DOI URL PMID |
| [11] | Duncan DB ( 1955). Multiple range and multiple F tests. Biometrics, 11, 1-42. |
| [12] | Ferrol N, Barea JM, Azcón-Aguilar C ( 2000). The plasma membrane H +-ATPase gene family in the arbuscular mycorrhizal fungus Glomus mosseae. Current Genetics, 37, 112-118. |
| [13] |
Fromm J, Lautner S ( 2007). Electrical signals and their physiological significance in plants. Plant, Cell & Environment, 30, 249-257.
DOI URL PMID |
| [14] |
Gaxiola RA, Palmgren MG, Schumacher K ( 2007). Plant proton pumps. FEBS Letters, 581, 2204-2214.
DOI URL |
| [15] |
Gévaudant F, Duby G, von Stedingk E, Zhao R, Morsomme P, Boutry M ( 2007). Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance . Plant Physiology, 144, 1763-1776.
DOI URL |
| [16] |
Gianinazzi-Pearson V, Smith SE, Gianinazzi S, Smith FA ( 1991). Enzymatic studies on the metabolism of vesicular-?arbuscular mycorrhizas. New Phytologist, 117, 61-74.
DOI URL |
| [17] | Gong DS, Xiong YC, Ma BL, Wang TM, Ge JP, Qin XL, Li PF, Kong HY, Li ZZ, Li FM ( 2010). Early activation of plasma membrane H +-ATPase and its relation to drought adaptation in two contrasting oat ( Avena sativa L.) genotypes. Environmental and Experimental Botany, 69, 1-8. |
| [18] |
Harrison MJ ( 2005). Signaling in the arbuscular mycorrhizal symbiosis. Annual Review Microbiology, 59, 19-42.
DOI URL |
| [19] |
Hijikata N, Murase M, Tani C, Ohtomo R, Osaki M, Ezawa T ( 2010). Polyphosphate has a central role in the rapid and massive accumulation of phosphorus in extraradical mycelium of an arbuscular mycorrhizal fungus. New Phytologist, 186, 285-289.
DOI URL PMID |
| [20] | Isfort RJ, Cody DB, Asquith TN, Ridder GM, Stuard SB, Leboeuf RA ( 1993). Induction of protein phosphorylation, protein synthesis, immediate-early-gene expression and cellular proliferation by intracellular pH modulation. FEBS Journal, 213, 349-357. |
| [21] |
Kühtreiber WM, Jaffe LF ( 1990). Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. The Journal of Cell Biology, 110, 1565-1573.
DOI URL |
| [22] |
Li T, Du J, Hao ZP, Zhang X, Chen BD ( 2012). Molecular basis for enhancement of plant drought tolerance by arbuscular mycorrhizal symbiosis: A mini-review. Acta Ecologica Sinica, 32, 7169-7176.
DOI URL |
|
[ 李涛, 杜娟, 郝志鹏, 张莘, 陈保冬 ( 2012). 丛枝菌根提高宿主植物抗旱性分子机制研究进展. 生态学报, 32, 7169-7176.]
DOI URL |
|
| [23] |
Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD ( 2013). First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist, 197, 617-630.
DOI URL PMID |
| [24] | Liu T, Li Z, Hui C, Tang M, Zhang H ( 2016). Effect of Rhizophagus irregularis on osmotic adjustment, antioxidation and aquaporin PIP genes expression of Populus × canadensis ‘Neva’ under drought stress. Acta Physiologiae Plantarum, 38, 191. DOI: 10.1007/s11738-016-2207-6. |
| [25] | Liu RJ, Chen YL ( 2007). Mycorrhizology. Science Press, Beijing. 447-448. |
| [26] |
Ludwig AA, Romeis T, Jones JD ( 2004). CDPK-mediated signalling pathways: Specificity and cross-talk. Journal of Experimental Botany, 55, 181-188.
DOI URL PMID |
| [27] |
Ma R, Zhang M, Li B, Du G, Wang J, Chen J ( 2005). The effects of exogenous Ca 2+ on endogenous polyamine levels and drought-resistant traits of spring wheat grown under arid conditions . Journal of Arid Environments, 63, 177-190.
DOI URL |
| [28] |
Mak M, Babla M, Xu SC, O’Carrigan A, Liu XH, Gong YM, Holford P, Chen ZH ( 2014). Leaf mesophyll K+, H+and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean . Environmental and Experimental Botany, 98, 1-12.
DOI URL |
| [29] |
Porcel R, Ruiz-Lozano JM ( 2004). Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany, 55, 1743-1750.
DOI URL PMID |
| [30] |
Querejeta J, Egerton-Warburton LM, Allen MF ( 2003). Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia, 134, 55-64.
DOI URL |
| [31] |
Ramos AC, Fa?anha AR, Feijó JA ( 2008a). Ion dynamics during the polarized growth of arbuscular mycorrhizal fungi: From presymbiosis to symbiosis. In: Varma A ed. Mycorrhiza. Springer, Berlin, Heidelberg.
DOI URL |
| [32] |
Ramos AC, Fa?anha AR, Feijó JA ( 2008b). Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi . New Phytologist, 178, 177-188.
DOI URL |
| [33] |
Requena N, Breuninger M, Franken P, Ocón A ( 2003). Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiology, 132, 1540-1549.
DOI URL PMID |
| [34] | Riquelme M, Bartnicki-Garcia S ( 2004). Key differences between lateral and apical branching in hyphae of Neurospora crassa. Fungal Genetics and Biology, 41, 842-851. |
| [35] |
Rudd JJ, Franklin-Tong VE ( 2001). Unravelling response-?specificity in Ca 2+ signalling pathways in plant cells . New Phytologist, 151, 7-33.
DOI URL |
| [36] |
Sánchez-Romera B, Ruiz-Lozano JM, Zamarre?o áM, García-?Mina JM, Aroca R ( 2016). Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza, 26, 111-122.
DOI URL PMID |
| [37] |
Santi S, Locci G, Monte R, Pinton R, Varanini Z ( 2003). Induction of nitrate uptake in maize roots: Expression of a putative high-affinity nitrate transporter and plasma membrane H+- ATPase isoforms . Journal of Experimental Botany, 54, 1851-1864.
DOI URL PMID |
| [38] | Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H ( 2016). Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Frontiers in Plant Science, 7, 196. DOI: 10.3389/fpls.?2016.?00196. |
| [39] | Smith S, Read D ( 2008). Mycorrhizal Symbiosis. Academic Press, New York. |
| [40] | St-Arnaud M, Hamel C, Vimard B, Caron M, Fortin JA ( 1996). Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycological Research, 100, 328-332. |
| [41] |
Sun YP, Unestam T, Lucas SD, Johanson KJ, Kenne L, Finlay R ( 1999). Exudation-reabsorption in a mycorrhizal fungus, the dynamic interface for interaction with soil and soil microorganisms. Mycorrhiza, 9, 137-144.
DOI URL |
| [42] |
Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frey NF, Gianinazzi-?Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, Kawaguchi M, Krajinski F, Lammers PJ, Masclaux FG, Murat C, Morin E, Ndikumana S, Pagni M, Petitpierre D, Requena N, Rosikiewicz P, Riley R, Saito K, Clemente HS, Shapiro H, van Tuinen D, Bécard G, Bonfante P, Paszkowski U, Shachar-Hill YY, Tuskan GA, Young JW, Sanders IR, Henrissat B, Rensing SA, Grigoriev IV, Corradi N, Roux C, Martin F ( 2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 110, 20117-20122.
DOI URL PMID |
| [43] | van Hees PA, Rosling A, Essén S, Godbold DL, Jones DL, Finlay RD ( 2006). Oxalate and ferricrocin exudation by the extramatrical mycelium of an ectomycorrhizal fungus in symbiosis withPinus sylvestris. New Phytologist, 169, 367-378. |
| [44] | Wu S, Zhang X, Sun Y, Wu Z, Li T, Hu Y, Su D, Lü J, Li G, Zhang Z, Zheng L, Zhang J, Chen B ( 2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environmental Science & Technology, 49, 14036-14047. |
| [45] |
Xiong YC, Li FM, Zhang T, Xia C ( 2007). Evolution mechanism of non-hydraulic root-to-shoot signal during the anti-drought genetic breeding of spring wheat. Environmental and Experimental Botany, 59, 193-205.
DOI URL |
| [46] |
Xu L, Li T, Wu Z, Feng H, Yu M, Zhang X, Chen B ( 2018). Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Applied Soil Ecology, 125, 213-221.
DOI URL |
| [47] |
Yan F, Zhu Y, Müller C, Z?rb C, Schubert S ( 2002). Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency . Plant Physiology, 129, 50-63.
DOI URL |
| [48] | Yang PZ ( 2012). Mechanism Involved in Drought/Salt Tolerance Improvement in Alfalfa Due to Symbiotic Interaction with Rhizobium. PhD dissertation, Northwest A&F University,Yangling, Shaanxi. 4-6. |
| [ 杨培志 ( 2012). 紫花苜蓿根瘤菌共生对干旱及盐胁迫的响应机制研究. 博士学位论文, 西北农林科技大学, 陕西杨凌. 4-6.] | |
| [49] |
Yoshida S ( 1991). Chilling-induced inactivation and its recovery of tonoplast H+-ATPase in mung bean cell suspension cultures . Plant Physiology, 95, 456-460.
DOI URL |
| [50] |
Zhao R, Guo W, Bi N, Guo J, Wang L, Zhao J, Zhang J ( 2015a). Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Applied Soil Ecology, 88, 41-49.
DOI URL |
| [51] |
Zhao X, Xu M, Wei R, Liu Y ( 2015b). Expression OsCAS (calcium-sensing receptor) in an Arabidopsis mutant increases drought tolerance. PLOS ONE, 10, e0131272. DOI: 10.1371/journal.pone.0131272.
DOI URL PMID |
| [52] | Zou JJ, Li XD, Ratnasekera D, Wang C, Liu WX, Song LF, Zhang WZ, Wu WH ( 2015). Arabidopsis calcium-dependent protein kinase 8 and catalase 3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. The Plant Cell, 27, 1445-1460. |
| [53] |
Zhou S, Han YY, Chen Y, Kong X, Wang W ( 2015). The involvement of expansins in response to water stress during leaf development in wheat. Journal of Plant Physiology, 183, 64-74.
DOI URL PMID |
| [1] | Ke-Yu CHEN Sen Xing Yu Tang Sun JiaHui Shijie Ren Bao-Ming JI. Arbuscular mycorrhizal fungal community characteristics and driving factors in different grassland types [J]. Chin J Plant Ecol, 2024, 48(5): 660-674. |
| [2] | Die Hu Xinqi Jiang DAI Zhicong Daiyi Chen Yu Zhang Shan-Shan Qi. Arbuscular mycorrhizal fungi enhance the herbicide tolerance of an invasive weed Sphagneticola trilobata [J]. Chin J Plant Ecol, 2024, 48(5): 651-659. |
| [3] | CHEN Bao-Dong, FU Wei, WU Song-Lin, ZHU Yong-Guan. Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling [J]. Chin J Plant Ecol, 2024, 48(1): 1-20. |
| [4] | YANG Jia-Rong, DAI Dong, CHEN Jun-Fang, WU Xian, LIU Xiao-Lin, LIU Yu. Insight into recent studies on the diversity of arbuscular mycorrhizal fungi in shaping plant community assembly and maintaining rare species [J]. Chin J Plant Ecol, 2023, 47(6): 745-755. |
| [5] | HE Fei, LI Chuan, Faisal SHAH, LU Xie-Min, WANG Ying, WANG Meng, RUAN Jia, WEI Meng-Lin, MA Xing-Guang, WANG Zhuo, JIANG Hao. Carbon transport and phosphorus uptake in an intercropping system of Robinia pseudoacacia and Amorphophallus konjac mediated by arbuscular mycorrhizal hyphal networks [J]. Chin J Plant Ecol, 2023, 47(6): 782-791. |
| [6] | CHEN Tu-Qiang, XU Gui-Qing, LIU Shen-Si, LI Yan. Hydraulic traits adjustments and nonstructural carbohydrate dynamics of Haloxylon ammodendron under drought stress [J]. Chin J Plant Ecol, 2023, 47(10): 1407-1421. |
| [7] | ZHOU Jie, YANG Xiao-Dong, WANG Ya-Yun, LONG Yan-Xin, WANG Yan, LI Bo-Rui, SUN Qi-Xing, SUN Nan. Difference in adaptation strategy between Haloxylon ammodendron and Alhagi sparsifolia to drought [J]. Chin J Plant Ecol, 2022, 46(9): 1064-1076. |
| [8] | XIE Wei, HAO Zhi-Peng, ZHANG Xin, CHEN Bao-Dong. Research progress and prospect of signal transfer among plants mediated by arbuscular mycorrhizal networks [J]. Chin J Plant Ecol, 2022, 46(5): 493-515. |
| [9] | MA Ju-Feng, XIN Min, XU Chen-Chao, ZHU Wan-Ying, MAO Chuan-Zao, CHEN Xin, CHENG Lei. Effects of arbuscular mycorrhizal fungi and nitrogen addition on nitrogen uptake of rice genotypes with different root morphologies [J]. Chin J Plant Ecol, 2021, 45(7): 728-737. |
| [10] | PANG Fang, XIA Wei-Kang, HE Min, QI Shan-Shan, DAI Zhi-Cong, DU Dao-Lin. Nitrogen-fixing bacteria alleviates competition between arbuscular mycorrhizal fungi and Solidago canadensis for nutrients under nitrogen limitation [J]. Chin J Plant Ecol, 2020, 44(7): 782-790. |
| [11] | LIU Li-Yan, FENG Jin-Xia, LIU Wen-Xin, WAN Xian-Chong. Effects of drought stress on photosynthesis, growth and root structure of transgenic PtPIP2;8 poplar 84K (Populus alba × P. glandulosa) [J]. Chin J Plant Ecol, 2020, 44(6): 677-686. |
| [12] | LUO Jin-Huan, TAN Zhao-Yuan, CHEN Bin, CHEN Guang-Wu, JIANG Kai, HEI Qi-Fang, ZHANG Hui. Key characteristics for facilitating Leucaena leucocephala to successfully invade pioneer communities of tropical rain forests [J]. Chin J Plant Ecol, 2020, 44(12): 1215-1223. |
| [13] | CUI Li, GUO Feng, ZHANG Jia-Lei, YANG Sha, WANG Jian-Guo, MENG Jing-Jing, GENG Yun, LI Xin-Guo, WAN Shu-Bo. Improvement of continuous microbial environment in peanut rhizosphere soil by Funneliformis mosseae [J]. Chin J Plant Ecol, 2019, 43(8): 718-728. |
| [14] | GAO Wen-Tong, ZHANG Chun-Yan, DONG Ting-Fa, XU Xiao. Effects of arbuscular mycorrhizal fungi on the root growth of male and female Populus cathayana individuals grown under different sexual combination patterns [J]. Chin J Plant Ecol, 2019, 43(1): 37-45. |
| [15] | Xi WANG,Hong-Ling HU,Ting-Xing HU,Cheng-Hao ZHANG,Xin WANG,Dan LIU. Effects of drought stress on the osmotic adjustment and active oxygen metabolism of Phoebe zhennan seedlings and its alleviation by nitrogen application [J]. Chin J Plan Ecolo, 2018, 42(2): 240-251. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn