

Chin J Plant Ecol ›› 2023, Vol. 47 ›› Issue (6): 782-791.DOI: 10.17521/cjpe.2022.0185
Special Issue: 稳定同位素生态学; 根系生态学; 菌根真菌
• Research Articles • Previous Articles Next Articles
HE Fei1,*( ), LI Chuan1, Faisal SHAH2, LU Xie-Min1, WANG Ying1, WANG Meng1, RUAN Jia1, WEI Meng-Lin1, MA Xing-Guang1, WANG Zhuo1, JIANG Hao1
), LI Chuan1, Faisal SHAH2, LU Xie-Min1, WANG Ying1, WANG Meng1, RUAN Jia1, WEI Meng-Lin1, MA Xing-Guang1, WANG Zhuo1, JIANG Hao1
Received:2022-05-09
Accepted:2022-10-10
Online:2023-06-20
Published:2022-10-10
Supported by:HE Fei, LI Chuan, Faisal SHAH, LU Xie-Min, WANG Ying, WANG Meng, RUAN Jia, WEI Meng-Lin, MA Xing-Guang, WANG Zhuo, JIANG Hao. Carbon transport and phosphorus uptake in an intercropping system of Robinia pseudoacacia and Amorphophallus konjac mediated by arbuscular mycorrhizal hyphal networks[J]. Chin J Plant Ecol, 2023, 47(6): 782-791.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2022.0185
| 处理 Treatment | A室植物 Plant in compartment A | A室处理 Treatment in compartment A | B室植物 Plant in compartment B | 种植方式 Cropping pattern |
|---|---|---|---|---|
| M-AMF | 刺槐 Robinia pseudoacacia | 未接种 Non-inoculated | 刺槐 R. pseudoacacia | 单作 Monocropping |
| I-AMF | 刺槐 R. pseudoacacia | 未接种 Non-inoculated | 魔芋 Amorphophallus konjac | 间作 Intercropping |
| M+AMF | 刺槐 R. pseudoacacia | 接种 Inoculated | 刺槐 R. pseudoacacia | 单作 Monocropping |
| I+AMF | 刺槐 R. pseudoacacia | 接种 Inoculated | 魔芋 A. konjac | 间作 Intercropping |
Table 1 Treatments in the two-compartment root system of Robinia pseudoacacia and Amorphophallus konjac
| 处理 Treatment | A室植物 Plant in compartment A | A室处理 Treatment in compartment A | B室植物 Plant in compartment B | 种植方式 Cropping pattern |
|---|---|---|---|---|
| M-AMF | 刺槐 Robinia pseudoacacia | 未接种 Non-inoculated | 刺槐 R. pseudoacacia | 单作 Monocropping |
| I-AMF | 刺槐 R. pseudoacacia | 未接种 Non-inoculated | 魔芋 Amorphophallus konjac | 间作 Intercropping |
| M+AMF | 刺槐 R. pseudoacacia | 接种 Inoculated | 刺槐 R. pseudoacacia | 单作 Monocropping |
| I+AMF | 刺槐 R. pseudoacacia | 接种 Inoculated | 魔芋 A. konjac | 间作 Intercropping |
| 分室 Compartment | 植物 Plant | 处理 Treatment | AMF侵染率 AMF infection rate (%) | 丛枝丰度 Arbuscular abundance (%) | 侵染强度 Infection intensity (%) |
|---|---|---|---|---|---|
| A室 Compartment A | 刺槐 R. pseudoacacia | M+AMF | 54.8 ± 3.2b | 2.9 ± 0.2b | 6.8 ± 0.7b |
| 刺槐 R. pseudoacacia | I+AMF | 62.0 ± 0.3a | 4.8 ± 0.2a | 10.0 ± 0.2a | |
| B室 Compartment B | 刺槐 R. pseudoacacia | M+AMF | 47.1 ± 2.0c | 1.0 ± 0.1c | 2.2 ± 0.2c |
| 魔芋 A. konjac | I+AMF | 60.4 ± 6.6a | 4.6 ± 0.2a | 9.5 ± 0.6a |
Table 2 Infection status of Robinia pseudoacacia and Amorphophallus konjac plants after inoculation with arbuscular mycorrhizal fungi (AMF) (mean ± SD)
| 分室 Compartment | 植物 Plant | 处理 Treatment | AMF侵染率 AMF infection rate (%) | 丛枝丰度 Arbuscular abundance (%) | 侵染强度 Infection intensity (%) |
|---|---|---|---|---|---|
| A室 Compartment A | 刺槐 R. pseudoacacia | M+AMF | 54.8 ± 3.2b | 2.9 ± 0.2b | 6.8 ± 0.7b |
| 刺槐 R. pseudoacacia | I+AMF | 62.0 ± 0.3a | 4.8 ± 0.2a | 10.0 ± 0.2a | |
| B室 Compartment B | 刺槐 R. pseudoacacia | M+AMF | 47.1 ± 2.0c | 1.0 ± 0.1c | 2.2 ± 0.2c |
| 魔芋 A. konjac | I+AMF | 60.4 ± 6.6a | 4.6 ± 0.2a | 9.5 ± 0.6a |
| 分室 Compartment | 植物 Plant | 处理 Treatment | 株高 Plant height (cm) | 地径 Ground diameter (mm) | 地上干质量 Aboveground dry mass (g·plant-1) | 根系干质量 Root dry mass (g·plant-1) |
|---|---|---|---|---|---|---|
| A室 Compartment A | 刺槐 R. pseudoacacia | M-AMF | 6.67 ± 0.03e | 1.71 ± 0.02c | 1.15 ± 0.06f | 0.10 ± 0.01g |
| 刺槐 R. pseudoacacia | I-AMF | 6.94 ± 0.14e | 1.72 ± 0.02c | 1.42 ± 0.08e | 0.21 ± 0.03ef | |
| 刺槐 R. pseudoacacia | M+AMF | 10.83 ± 0.09cd | 1.81 ± 0.01c | 2.78 ± 0.14c | 0.32 ± 0.05d | |
| 刺槐 R. pseudoacacia | I+AMF | 11.83 ± 0.09c | 1.81 ± 0.02c | 2.82 ± 0.18c | 1.04 ± 0.05c | |
| B室 Compartment B | 刺槐 R. pseudoacacia | M-AMF | 6.83 ± 0.11e | 1.71 ± 0.01c | 1.12 ± 0.12f | 0.15 ± 0.04fg |
| 魔芋 A. konjac | I-AMF | 23.80 ± 1.10b | 13.30 ± 0.40b | 13.54 ± 0.14b | 1.99 ± 0.02b | |
| 刺槐 R. pseudoacacia | M+AMF | 10.61 ± 0.12d | 1.80 ± 0.00c | 2.20 ± 0.05d | 0.23 ± 0.01e | |
| 魔芋 A. konjac | I+AMF | 26.72 ± 1.33a | 15.11 ± 0.62a | 14.85 ± 0.11a | 2.71 ± 0.08a |
Table 3 Effects of intercropping and arbuscular mycorrhizal fungi (AMF) inoculation on agronomic traits in Robinia pseudoacacia and Amorphophallus konjac (mean ± SD)
| 分室 Compartment | 植物 Plant | 处理 Treatment | 株高 Plant height (cm) | 地径 Ground diameter (mm) | 地上干质量 Aboveground dry mass (g·plant-1) | 根系干质量 Root dry mass (g·plant-1) |
|---|---|---|---|---|---|---|
| A室 Compartment A | 刺槐 R. pseudoacacia | M-AMF | 6.67 ± 0.03e | 1.71 ± 0.02c | 1.15 ± 0.06f | 0.10 ± 0.01g |
| 刺槐 R. pseudoacacia | I-AMF | 6.94 ± 0.14e | 1.72 ± 0.02c | 1.42 ± 0.08e | 0.21 ± 0.03ef | |
| 刺槐 R. pseudoacacia | M+AMF | 10.83 ± 0.09cd | 1.81 ± 0.01c | 2.78 ± 0.14c | 0.32 ± 0.05d | |
| 刺槐 R. pseudoacacia | I+AMF | 11.83 ± 0.09c | 1.81 ± 0.02c | 2.82 ± 0.18c | 1.04 ± 0.05c | |
| B室 Compartment B | 刺槐 R. pseudoacacia | M-AMF | 6.83 ± 0.11e | 1.71 ± 0.01c | 1.12 ± 0.12f | 0.15 ± 0.04fg |
| 魔芋 A. konjac | I-AMF | 23.80 ± 1.10b | 13.30 ± 0.40b | 13.54 ± 0.14b | 1.99 ± 0.02b | |
| 刺槐 R. pseudoacacia | M+AMF | 10.61 ± 0.12d | 1.80 ± 0.00c | 2.20 ± 0.05d | 0.23 ± 0.01e | |
| 魔芋 A. konjac | I+AMF | 26.72 ± 1.33a | 15.11 ± 0.62a | 14.85 ± 0.11a | 2.71 ± 0.08a |
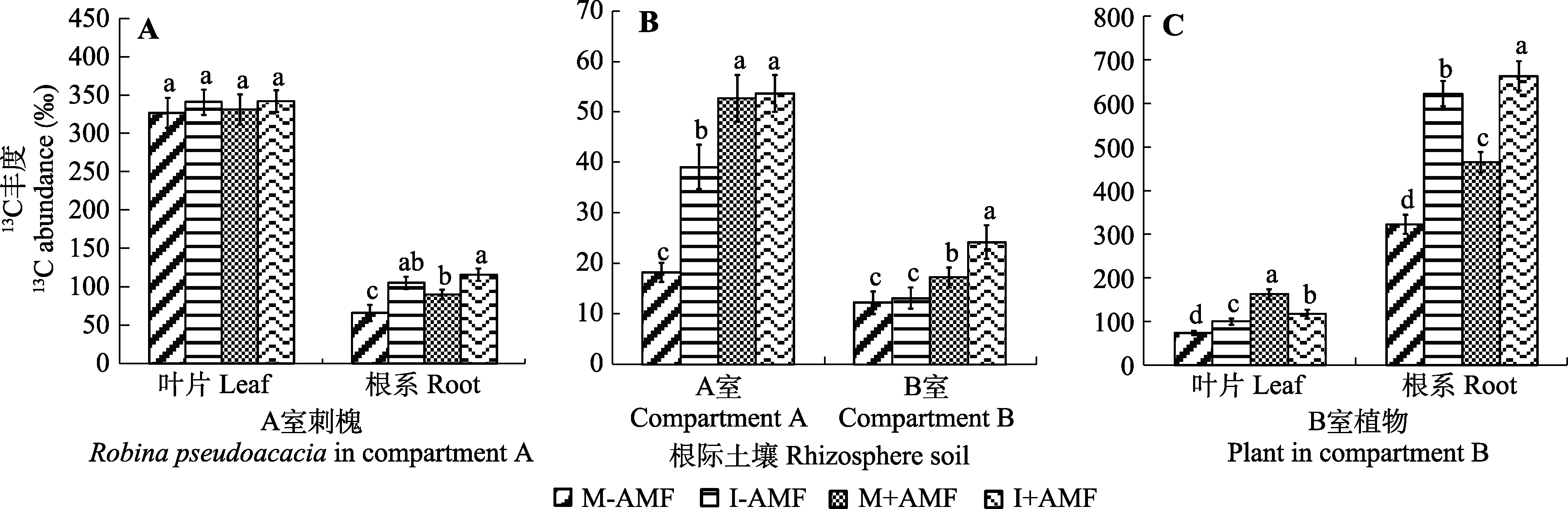
Fig. 2 13C transport in the Robinia pseudoacacia-soil-Amorphophallus konjac system under different treatments (mean ± SD). See Table 1 for treatments. Different lowercase letters indicate significant differences among treatments (p < 0.05).
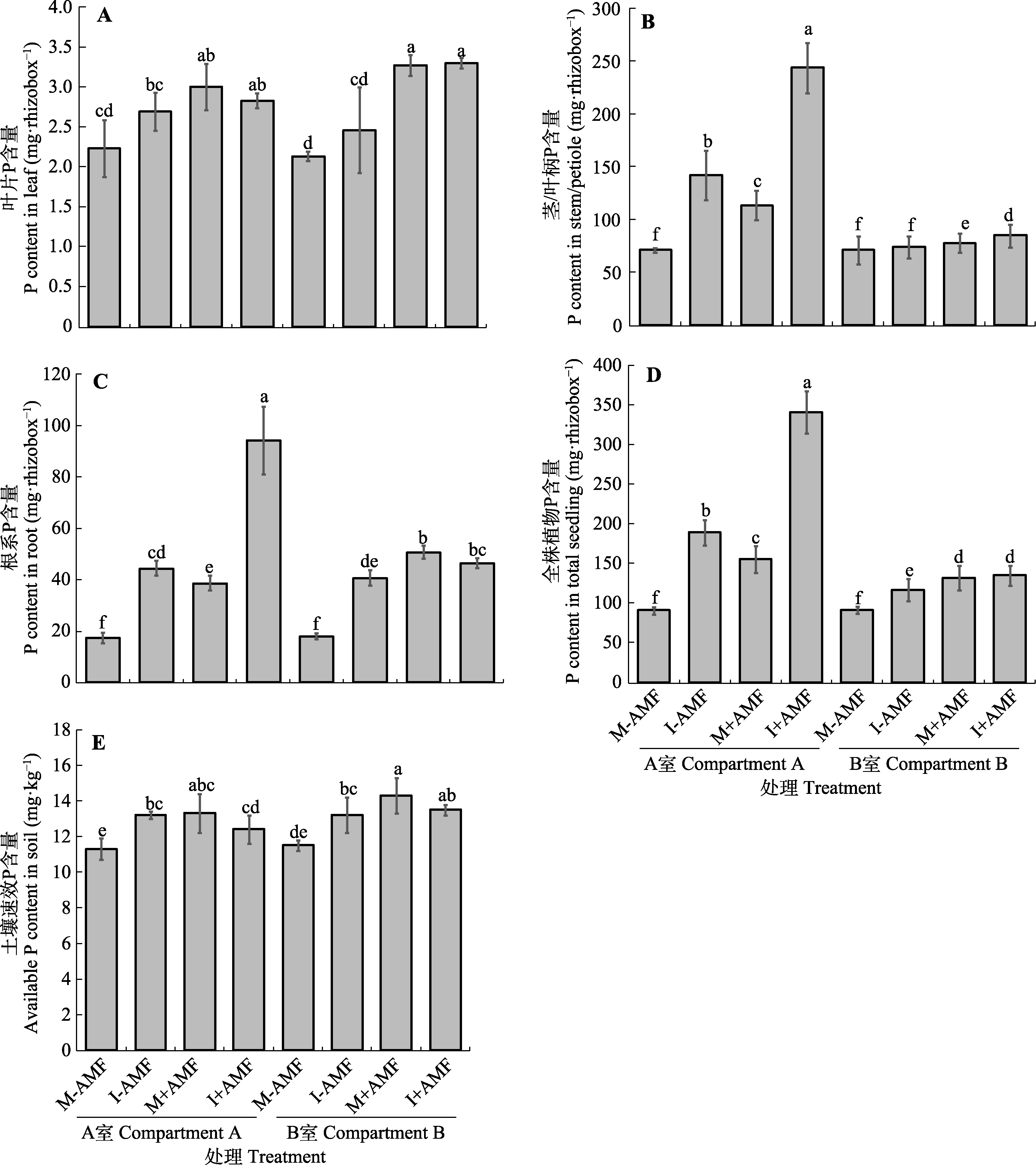
Fig. 3 Phosphorus (P) content in the leaves (A), stems or petioles (B), roots (C), and total plants (D), and available P concentration in the rhizosphere soils (E) of Robinia pseudoacacia and Amorphophallus konjac (mean ± SD). See Table 1 for treatments. Different lowercase letters indicate significant differences among treatments (p < 0.05). rhizobox, single compartment in rhizobox.
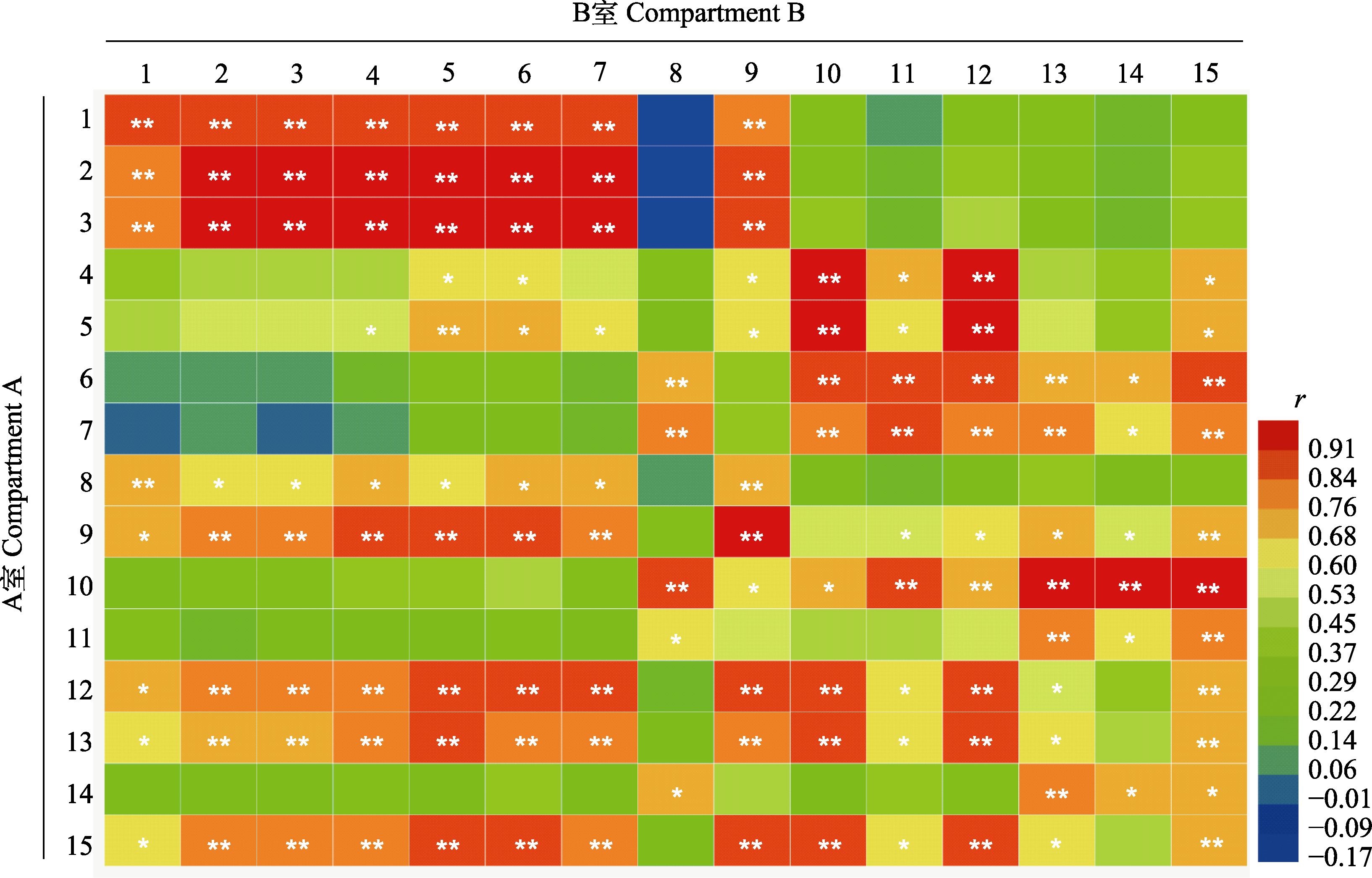
Fig. 4 Pearson’s correlation coefficients (r) between arbuscular mycorrhizal fungi (AMF) infection, plant agronomic traits, 13C abundance, and phosphorus (P) content of Robinia pseudoacacia and Amorphophallus konjac. 1, AMF infection rate; 2, arbuscular abundance; 3, AMF infection intensity; 4, aboveground dry mass; 5, root dry mass; 6, plant height; 7, ground diameter; 8, leaf 13C abundance; 9, root 13C abundance; 10, soil 13C abundance; 11, leaf P content; 12, stem or petiole P content; 13, root P content; 14, soil available P content; 15, total plant P content. *, p < 0.05; **, p < 0.01.
| [1] | Bao SD (2007). Soil and Agricultural Chemistry Analysis. 3rd ed. China Agriculture Press, Beijing. |
| 鲍士旦 (2007). 土壤农化分析. 3版. 中国农业出版社, 北京.] | |
| [2] |
Bicharanloo B, Shirvan MB, Keitel C, Dijkstra FA (2020). Nitrogen and phosphorus availability affect wheat carbon allocation pathways: rhizodeposition and mycorrhizal symbiosis. Soil Research, 58, 125-136.
DOI URL |
| [3] |
Cameron DD, Leake JR, Read DJ (2006). Mutualistic mycorrhiza in orchids: evidence from plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytologist, 171, 405-416.
DOI PMID |
| [4] |
Carey EV, Marler MJ, Callaway RM (2004). Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecology, 172, 133-141.
DOI URL |
| [5] | Chang YF (2016). Response of the Rhizosphere Microenvironment of Robinia pseudoacacia L. Seedlings to Elevated CO2Combined with Cd- and Pb-Contaminated Soils. Master degree dissertation, Chang’an University, Xi’an. |
| [常亚飞 (2016). 大气CO2浓度升高和土壤Cd/Pb污染耦合对刺槐幼苗根际土壤微生态环境的影响. 硕士学位论文, 长安大学, 西安.] | |
| [6] |
Costa NR, Crusciol CAC, Trivelin PCO, Pariz CM, Costa C, Castilhos AM, Souza DM, Bossolani JW, Andreotti M, Meirelles PRL, Moretti LG, Mariano E (2021). Recovery of 15N fertilizer in intercropped maize, grass and legume and residual effect in black oat under tropical conditions. Agriculture, Ecosystems & Environment, 310, 107226. DOI: 10.1016/j.agee.2020.107226.
DOI |
| [7] |
Cui L, Guo F, Zhang JL, Yang S, Wang JG, Meng JJ, Geng Y, Li XG, Wan SB (2019). Improvement of continuous microbial environment in peanut rhizosphere soil by Funneliformis mosseae. Chinese Journal of Plant Ecology, 43, 718-728.
DOI URL |
|
[崔利, 郭峰, 张佳蕾, 杨莎, 王建国, 孟静静, 耿耘, 李新国, 万书波 (2019). 摩西斗管囊霉改善连作花生根际土壤的微环境. 植物生态学报, 43, 718-728.]
DOI |
|
| [8] |
Francis R, Read DJ (1984). Direct transfer of carbon between plants connected by vesicular-arbuscular mycorrhizal mycelium. Nature, 307, 53-56.
DOI |
| [9] |
Gao X, Wu M, Xu RN, Wang XR, Pan RQ, Kim HJ, Liao H (2014). Root interactions in a maize/soybean intercropping system control soybean soil-borne disease, red crown rot. PLoS ONE, 9, e95031. DOI: 10.1371/journal.pone.0095031.
DOI |
| [10] |
He F (2021). Response of root-associated bacterial communities to different degrees of soft rot damage in Amorphophallus konjac under a Robinia pseudoacacia plantation. Frontiers in Microbiology, 12, 652758. DOI: 10.3389/fmicb.2021.652758.
DOI |
| [11] | He F, Zhang ZL, Liu LP, Cui M, Xue QH (2015). Microecological mechanism for healthy growth and higher yield of Amorphophallus konjac under acacia forest. Acta Botanica Boreali-Occidentalia Sinica, 35, 364-372. |
| [何斐, 张忠良, 刘列平, 崔鸣, 薛泉宏 (2015). 刺槐林魔芋健康高产的土壤微生态机制. 西北植物学报, 35, 364-372.] | |
| [12] | He J, Wei LY, Zhou LJ (2012). Effect of intercropping pattern and woodland type on konjac disease and yield. Shaanxi Forest Science and Technology, (3), 37-38. |
| [和军, 魏凌云, 周立军 (2012). 间作方式与林地类型对魔芋病害及产量的影响. 陕西林业科技, (3), 37-38.] | |
| [13] |
He Y, Ding N, Shi JC, Wu M, Liao H, Xu JM (2013). Profiling of microbial PLFAs: implications for interspecific interactions due to intercropping which increase phosphorus uptake in phosphorus limited acidic soils. Soil Biology & Biochemistry, 57, 625-634.
DOI URL |
| [14] | He YQ, Wang XX, Zhang C, Zhang RF, Lin ZH, Wang H (2021). Research progress on the effects of intercropping and interplanting modes in the cultivation of Chinese medicinal materials. Jiangsu Journal of Agricultural Sciences, 37, 1077-1083. |
| [何雅祺, 王鑫鑫, 张弛, 张瑞芳, 林智慧, 王红 (2021). 间作、套种模式在中药材栽培中的效应研究进展. 江苏农业学报, 37, 1077-1083.] | |
| [15] | Hoagland DR, Arnon DI (1950). The Water-Culture Method for Growing Plants Without Soil. California Agricultural Experiment Station, Berkeley. 347. |
| [16] |
Kang LL, He YJ, Zang LP, Si JP, Yang Y, Shen KP, Xia TT, Tan QY, Wu BL, Guo Y, Wang W, Liang Q (2021). Mycorrhizal networks interacting with litter improves nutrients and growth for one plant through the vary of N/P ratio under karst soil. Phyton, 90, 701-717.
DOI URL |
| [17] | Li L, Li SM, Sun JH, Zhou LL, Bao XG, Zhang HG, Zhang FS (2007). Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus- deficient soils. Proceedings of the National Academy of Sciences of the United States of America, 104, 11192-11196. |
| [18] |
Li XQ, Zhang MX, Li Y, Yu XY, Nie JF (2021). Effect of neonicotinoid dinotefuran on root exudates of Brassica rapa var. chinensis. Chemosphere, 266, 129020. DOI: 10.1016/j.chemosphere.2020.129020.
DOI |
| [19] |
Li YF, Ran W, Zhang RP, Sun SB, Xu GH (2009). Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant and Soil, 315, 285-296.
DOI URL |
| [20] | Liang WQ, Hu YJ, Pu JB, Yu YF, Wang P, Chen RB, Chen ZL, Xu P (2021). Correlation analysis between active ingredients contents and soil factors in Radix peucedani. Chinese Journal of Modern Applied Pharmacy, 38, 1302-1308. |
| [梁卫青, 胡轶娟, 浦锦宝, 俞叶飞, 王盼, 陈如兵, 陈子林, 徐攀 (2021). 前胡有效成分含量与土壤因子的相关性分析. 中国现代应用药学, 38, 1302-1308.] | |
| [21] | Lin YL (2019). Environmental Behavior and Microbial Response of DBP in Rhizosphere Interface of Brassica chinensis L. PhD dissertation, Northeast Agricultural University, Harbin. 75-77. |
| 林宇龙 (2019). 酞酸酯DBP在小白菜根际界面的环境行为及微生物响应的机制. 博士学位论文, 东北农业大学, 哈尔滨. 75-77.] | |
| [22] | Mao DR (2004). Research Methods of Plant Nutrition. China Agricultural University Press, Beijing. |
| 毛达如 (2004). 植物营养研究方法. 中国农业大学出版社, 北京.] | |
| [23] | Mao MX, Zhu F (2021). Progress and perspective in research on plant resistance mediated by root exudates. Chinese Journal of Eco-Agriculture, 29, 1649-1657. |
| [毛梦雪, 朱峰 (2021). 根系分泌物介导植物抗逆性研究进展与展望. 中国生态农业学报, 29, 1649-1657.] | |
| [24] | More SS, Shinde SE, Kasture MC (2019). Root exudates a key factor for soil and plant: an overview. The Pharma Innovation Journal, 8, 449-459. |
| [25] |
Pfeffer PE, Douds DD Jr, Bücking H, Schwartz DP, Shachar-Hill Y (2004). The fungus does not transfer carbon to or between roots in an arbuscular mycorrhizal symbiosis. New Phytologist, 163, 617-627.
DOI PMID |
| [26] |
Phillips JM, Hayman DS (1970). Improved procedures for clearing roots and staining parasitic and vesicular- arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 55, 158-161.
DOI URL |
| [27] | Prasojo YS, Niimi M, Rahman MM, Ishigaki G, Akashi R (2022). Effect of soybean (Glycine max L. Merr) intercropping into rhodesgrass (Chloris gayana Kunth.) on dry matter yield, crude protein, and silage characteristics grown in southwest Japan. Journal of Animal and Plant Sciences, 32, 460-465. |
| [28] |
Ren LX, Lou YS, Zhang N, Zhu XD, Hao WY, Sun SB, Shen QR, Xu GH (2013). Role of arbuscular mycorrhizal network in carbon and phosphorus transfer between plants. Biology and Fertility of Soils, 49, 3-11.
DOI URL |
| [29] |
Schütz L, Saharan K, Mäder P, Boller T, Mathimaran N (2022). Rate of hyphal spread of arbuscular mycorrhizal fungi from pigeon pea to finger millet and their contribution to plant growth and nutrient uptake in experimental microcosms. Applied Soil Ecology, 169, 104156. DOI: 10.1016/j.apsoil.2021.104156.
DOI |
| [30] |
Smith SE, Smith FA (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual Review of Plant Biology, 62, 227-250.
DOI PMID |
| [31] |
Stock SC, Koester M, Boy J, Godoy R, Nájera F, Matus F, Merino C, Abdallah K, Leuschner C, Spielvogel S, Gorbushina AA, Kuzyakov Y, Dippold MA (2021). Plant carbon investment in fine roots and arbuscular mycorrhizal fungi: a cross-biome study on nutrient acquisition strategies. Science of the Total Environment, 781, 146748. DOI: 10.1016/j.scitotenv.2021.146748.
DOI |
| [32] |
Talas-Oğraş T, İpekçi Z, Bajroviç K, Gözükırmızı N (2005). Antibacterial activity of seed proteins of Robinia pseudoacacia. Fitoterapia, 76, 67-72.
PMID |
| [33] |
Voets L, Goubau I, Olsson PA, Merckx R, Declerck S (2008). Absence of carbon transfer between Medicago truncatula plants linked by a mycorrhizal network, demonstrated in an experimental microcosm. FEMS Microbiology Ecology, 65, 350-360.
DOI URL |
| [34] | Wang QJ, Guo DJ, Ma Y (2021). Stoichiometric characteristics of soil carbon, nitrogen and phosphorus and their relationship with soil available phosphorus under continuous application of organic fertilizer for vegetable cultivation in greenhouse. Jiangsu Journal of Agricultural Sciences, 37, 893-901. |
| [王秋君, 郭德杰, 马艳 (2021). 连续施用有机肥下设施土壤碳氮磷化学计量学特征及其与土壤有效磷的关系. 江苏农业学报, 37, 893-901.] | |
| [35] |
Wang ZH, Song Y (2022). Toward understanding the genetic bases underlying plant-mediated “cry for help” to the microbiota. iMeta, 1, e8. DOI: 10.1002/imt2.8.
DOI |
| [36] | Wu JP, Diao Y, Gu YC, Hu ZL (2010). Infection pathways of soft rot pathogens on Amorphophallus konjac. African Journal of Microbiology Research, 4, 1495-1499. |
| [37] | Yang Y, Jiang CH, He YJ, Ou J, Wang PP, Si JP, He MH, Lin Y (2017). Effects of arbuscular mycorrhizal networks on the N and P contents and stoichiometry of three plants species from karst area. Plant Physiology Journal, 53, 2078-2090. |
| [杨应, 蒋长洪, 何跃军, 欧静, 王鹏鹏, 司建朋, 何敏红, 林艳 (2017). 丛枝菌根网对喀斯特适生植物氮、磷化学计量特征的影响. 植物生理学报, 53, 2078-2090.] | |
| [38] |
Yang Y, Ou XH, Yang G, Xia YS, Chen ML, Guo LP, Liu DH (2017). Arbuscular mycorrhizal fungi regulate the growth and phyto-active compound of Salvia miltiorrhiza seedlings. Applied Sciences, 7, 68. DOI: 10.3390/app7010068.
DOI |
| [39] |
Yin HJ, Zhang ZL, Liu Q (2018). Root exudates and their ecological consequences in forest ecosystems: problems and perspective. Chinese Journal of Plant Ecology, 42, 1055-1070.
DOI URL |
|
[尹华军, 张子良, 刘庆 (2018). 森林根系分泌物生态学研究: 问题与展望. 植物生态学报, 42, 1055-1070.]
DOI |
|
| [40] | Zhang BB, Shi CY, Liu HJ, Ren GB, Sun Z (2017). Basal application of potassium benefits the yield formation of sweet potato. Journal of Plant Nutrition and Fertilizer, 23, 208-216. |
| [张彬彬, 史春余, 柳洪鹃, 任国博, 孙哲 (2017). 钾肥基施利于甘薯块根产量的形成. 植物营养与肥料学报, 23, 208-216.] | |
| [41] | Zhang DS, Wang YY, Tang L, Zheng Y, Zuo JH (2013). Effects of wheat and fababean intercropping on available phosphorus of red soils and its relationship with rhizosphere soil pH. Plant Nutrition and Fertilizer Science, 19, 127-133. |
| [张德闪, 王宇蕴, 汤利, 郑毅, 左建花 (2013). 小麦蚕豆间作对红壤有效磷的影响及其与根际pH值的关系. 植物营养与肥料学报, 19, 127-133.] | |
| [42] | Zhang ZL, Liu LP, Zheng M (2012). Cultivation techniques of Amorphophallus rivieri under Robinia pseudoacacia forest. Northern Horticulture, (13), 148-150. |
| [张忠良, 刘列平, 郑敏 (2012). 刺槐林下魔芋抗病栽培技术. 北方园艺, (13), 148-150.] | |
| [43] | Zuo YM, Chen Q, Zhang FS (2004). The mechanisms of root exudates of maize in improvement of iron nutrition of peanut in peanut maize intercropping system by 14C tracer technique. Acta Agriculturae Nucleatae Sinica, 18(1), 43-46. |
| [左元梅, 陈清, 张福锁 (2004). 利用14C示踪研究玉米/花生间作玉米根系分泌物对花生铁营养影响的机制. 核农学报, 18(1), 43-46.] | |
| [44] |
Zuo YM, Zhang FS (2008). Effect of peanut mixed cropping with gramineous species on micronutrient concentrations and iron chlorosis of peanut plants grown in a calcareous soil. Plant and Soil, 306, 23-36.
DOI URL |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2026 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
![]()